Abstract
Many patients who undergo hematopoietic cell transplantation (HCT) present with anemia and have received red blood cell transfusions before HCT. As a result, iron overload is frequent and appears to be particularly prominent in patients with myelodysplastic syndromes (MDS). There is evidence that peritransplant events contribute to further iron accumulation, although the mechanism that disrupts normal iron homeostasis remains to be determined. Recent studies suggest that iron overload, as determined by ferritin levels, a surrogate marker for iron, is a risk factor for increased non-relapse mortality after HCT. Iron overload is associated with an increased rate of infections, in particular with fungal organisms. Furthermore. anecdotal data suggest that increased hepatic iron may mimic the clinical picture of (chronic) graft-versus-host-disease (GVHD). Whether excess iron contributes to GVHD and whether iron depletion, be it by phlebotomy or chelation, reduces the post-transplantation complication rate and improves transplant outcome has yet to be determined.
Keywords: Allogeneic transplantation, iron overload, hepcidin, GVHD
Introduction
Body iron stores are regulated at the level of intestinal absorption. While various aspects of the regulation are still being uncovered, the following model has emerged [1]. Non-heme iron is absorbed primarily in the duodenum where it is reduced from the ferric to ferrous form via a ferroreductase (DCYTB) and transported across the brush border by the divalent metal transporter 1 (DMT1). Iron is then stored in ferritin or transported across the basolateral side of the enterocyte by ferroportin 1 (in conjunction with hephaestin, a ferroxidase that oxidizes iron back into the ferric form) for delivery to transferrin (Tf) and entry into the circulation. Expression of the iron transporters appears to be regulated in response to body iron stores. Hepcidin, a peptide hormone secreted by the liver, has an inhibitory effect on iron absorption (via interaction with ferroportin) [2]. The complex regulation of hepcidin expression is incompletely understood, but appears to involve ‘sensing’ by Tf receptors [3], oxygen tension [4], the growth and differentiation factor 15 (GDF15) [5], and the serine protease TMPRSS6 [6] among others.
Many patients undergoing hematopoietic cell transplantation (HCT) for marrow disorders, particularly patients with myelodysplastic syndromes (MDS), show iron overload, with hepatic iron concentrations reaching levels as observed in hereditary hemochromatosis [7]. Iron overload and ensuing liver pathology have also been described in patients with thalassemia [8]. Contributing factors include enhanced intestinal iron absorption secondary to anemia, and the administration of red blood cell (RBC) transfusions [9].
Recent data show a significantly shortened survival of MDS patients who are transfusion dependent [10], and several reports suggest that transfusion dependence, iron overload or both are also reflected in inferior outcome after HCT [11,12]. In fact, there is evidence that peri-and post-transplant events further enhance iron accumulation. For example, cytotoxic conditioning (in preparation for HCT), and the resulting arrest of erythropoiesis cause a period of hyperferremia, leading to Tf saturation and appearance of potentially toxic non-Tf-bound iron (NTBI) in the circulation [13,14].
The relationship between iron and post-transplant toxicity and mortality, related in particular to graft-versus-host disease (GVHD) and infections, is not clear. However, both the liver and the intestinal mucosa, which express essential iron regulatory genes including HAMP, the gene that encodes hepcidin, and ferroportin 1, are targets of conditioning-related toxicity as well as GVHD, initiated by donor-derived T lymphocytes [15]. The ensuing release of cytokines, including interleukin (IL)-6, might directly affect expression of hepcidin, since IL-6 is a potent inducer of hepcidin (via STAT 3) [1]. GVHD also involves the interaction of Fas-ligand expressed on activated donor T lymphocytes with host tissue, including enterocytes and hepatocytes [16]. It is conceivable, therefore, that T lymphocyte-inflicted tissue damage disrupts iron homeostasis, thereby leading to uncontrolled iron accumulation, which in turn may aggravate tissue damage related to the development of GVHD and infections.
Hepatic Iron Overload in Patients Undergoing HCT
Severe iron overload occurs in ptients with hemochromatosis and other genetically determined disorders [7,17]. However, iron overload also occurs with acquired hematopoietic disorders [8,18]. In patients with ineffective hematopoiesis and anemia, intestinal iron absorption is increased, hepcidin is down-regulated, and binding to and internalization of ferroportin 1 is reduced, resulting in increased influx of iron into the system [19]. Secondly, since marrow function is impaired, iron is not effectively utilized. Thus, thirdly, patients require RBC transfusions, adding exogenous iron loading at a rate of 200–250 mg per unit of RBC [9].
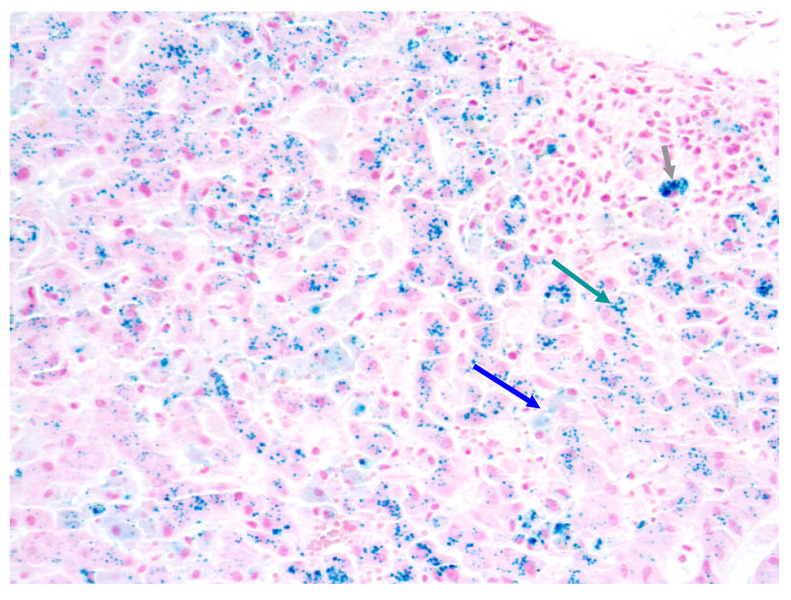
Gordon et al. were the first to show a remarkable increase in serum iron following high intensity transplant conditioning, resulting in Tf saturation and appearance of NTBI in plasma [20]. Numerous reports suggesting dysregulation of iron homeostasis after HCT followed [8,11,21–25]. Altes et al. studied 59 patients, 19–66 years of age with hematologic diagnoses, who died at a median of 5 months after autologous (n=24) or allogeneic (n=35) HCT [25]. The median HIC was 7,700 µg/g dry weight (138 µmol/g; range 31–631 µmol/g). Strasser et al. [22] determined the iron content in marrow and liver in 10 consecutive allogeneic HCT recipients, 1–59 years of age, who died 0.5–8.7 (median 2.2) years after HCT. Patients had received 48 ± 26 units of RBC, 30 ± 17 of those during the peri- and post-transplant period. The median HIC was 4,307 µg/g dry weight (range 1,832–13,120) (illustrated in Figure 1). The biochemically determined marrow iron was 932–3,942 (median 1,999) µg/g dry weight. There was a strong correlation between morphometric marrow iron content and biochemical hepatic iron index [µmol Fe/g dry tissue/patient age in years] (r=0.82; p=0.004).
Figure 1. Liver biopsy 7 months after allogeneic HCT.
Iron stain (20x). Arrows indicate iron in hepatocytes, Kupffer cells and macrophages, respectively.
These data could have been biased as only autopsy samples were studied. However, Armand et al. showed that 47% of 600 patients who received HCT after high-dose conditioning had serum ferritin levels (used as a surrogate measure for iron) of ≥ 1,000 ng/ml pre-transplant, which was associated with significantly increased non-relapse mortality (p<0.0001) [11]. The greatest impact was observed in 103 patients with a diagnosis of MDS with a hazard ratio (HR) for non-relapse mortality of 3.0 (p=0.001). Our own data in 172 patients with MDS transplanted with peripheral blood progenitor cells from HLA-identical donors also showed inferior survival in patients with ferritin levels ≥1,000 ng/ml pre-HCT (p=0.03); the incidence of GHVD increased with increasing pre-transplant ferritin levels, although differences did not reach statistical significance [12]. However, this study revealed a strong correlation between serum ferritin levels and the previously reported HCT-comorbidity index (HCT-CI), which has been shown to correlate inversely with the probability of transplant success [26]. Alessandrino et al. [27] presented data showing that the WHO Prognostic Scoring System (WPSS) classification of patients with MDS, one parameter of which is transfusion dependence, significantly impacted transplant outcome, with a probability of survival of 80% in low risk patients, but only 60% in intermediate risk patients; as intermediate risk patients have a score only one point higher than low risk, this could be accounted for by transfusion dependence (which is scored as one point). Further, Pullarkat et al. [28] and Mahindra et al. [29] reported in abstract form that patients with ferritin levels of >1000 and >1615 ng/ml, respectively, were at higher risk of death both from relapse and non-relapse causes, and had a higher incidence of GVHD than patients with lower ferritin levels. Thus, these data support the hypothesis that iron overload in transplant patients can be severe, is associated with toxicity, and may interfere with transplant success. However, none of the above studies were conducted in a prospective fashion, and even the data reported by Platzbecker et al. [12] mentioned could not link transplant outcome directly to transfusion dependence or iron, but only to ferritin levels. Ferritin, however, is an acute phase reactant and might be elevated for various reasons. Also, there is recent evidence that ferritin itself has pro-inflammatory effects and thereby could contribute to complications, such as sinusoidal obstruction syndrome, after HCT [30].
Iron and GVHD
Liver and intestinal mucosa (in addition to skin) are the major target organs of GVHD [31–33]. GVHD is initiated by alloreactive donor T lymphocytes, but also involves host components, in particular, host antigen presenting cells (reviewed in [33]). A histologic hallmark of GVHD is apoptosis. Liver GVHD typically presents with a rise in serum transaminases and bilirubin, with Fas/Fas-Ligand induced hepatocyte apoptosis, and cell mediated bile duct injury [34]. Intestinal GVHD usually causes diarrhea related to inflammation and edema of the bowel mucosa caused by enterocyte apoptosis, crypt abscesses, ulceration, and denudation [33]. Donor T lymphocytes, and TNFα and Fas-mediated signals play a central role in inflicting tissue injury [35]. In murine models, signaling via the death receptor Fas (CD95) is essential for GVHD, particularly in the liver [36,37]. Following cross-linking of Fas receptors on hepatocytes by Fas-ligand (CD178) expressed on activated T lymphocytes, hepatocytes undergo apoptosis, and our studies indicate that this may be associated with iron deposition [38,39]. Endothelial injury induced by T cells and cytokines may precede hepatocyte injury [40,41], and both inflammatory/necrotic and apoptotic changes (necrapoptosis) may be present [16,40]. How those cellular injuries affect the expression of iron regulatory signals is currently under study (see below).
One report [42] described six patients suspected of having hepatic GVHD who were found to have severe iron overload with serum ferritin concentrations of 2,398–11,159 ng/ml (four patients also had liver biopsies showing high HIC). Liver functions normalized with phlebotomy and iron depletion without continuation of immunosuppressive therapy. Since, as discussed, many transplant recipients are multiply transfused, it is to be expected that liver biopsies show iron overload (even with autologous or syngeneic transplants) in addition to signs of GVHD (with allogeneic transplants). Iron accumulation beyond that present before HCT is presumably related to continued transfusion need until complete recovery of marrow function after HCT. However, additional data suggest that other factors in the peri-transplant period contribute to iron deposition and hepatic injury [38].
A relationship of GVHD and iron has also been suggested by Gerbitz et al. in a murine model [43]. These investigators showed that treatment of mice with cobalt-protoporphyrin (CoPP) to induce haem oxygenase 1 (HO1, also known as heat shock protein 32, the rate limiting enzyme for the breakdown of haem into biliverdin IX, carbon monoxide [CO] and iron) alleviated or prevented GVHD after transplantation of allogeneic T lymphocytes [43]. Further, Ferris et al. observed that deletion of HO1 in HEK-293 fibroblasts resulted in iron accumulation and apoptosis, while overexpression of HO1, which enhanced iron efflux from the cells, was cytoprotective [44]. Deletion of HO1, which is constitutively expressed in hepatocytes, resulted in iron accumulation in the liver. Conceivably, therefore, HO1 may have a downstream scavenging function in maintaining iron homeostasis and cellular integrity.
Janin et al. documented endothelial apoptosis as identified by immunostaining for caspase 8 that was mediated by Fas-ligand expressed on donor T lymphocytes following the infusion of allogeneic C57BL/6 [H-2b] splenic lymphocytes into severe combined immunodeficiency (SCID) recipients (Balb/c background [H-2d]) [16]. We established a similar model in which we transplanted allogeneic C57Bl/6 [H-2b] T lymphocytes (splenocytes) into non-obese diabetic (NOD)/SCID mice (H-2d) in an attempt to determine whether Fas/Fas-Ligand mediated injury would affect iron homeostasis. The use of NOD/SCID mice allowed us to achieve engraftment without cytotoxic conditioning. In mice fed normal chow and infused with 3.0×107 histoincompatible, but not those infused with syngeneic T lymphocytes, liver sections (on day 14) showed iron deposition in hepatocytes. This was confirmed by quantitative analysis of liver tissue. These mice also showed histological evidence of injury of the gut mucosa and the liver, and serum transaminases were elevated. Control mice showed slight increases in body weight over 14 days, whereas in mice given histoincompatible T cells the weight declined progressively, as classically observed in murine GVHD [45]. The weight loss was prevented or reversed in mice pre-treated with apoTf [39]. Based on previous observations [35,46], we postulated that pre-treatment of mice with ApoTf would interfere with T lymphocyte-mediated hepatic injury and liver iron deposition. Results supported this hypothesis by showing substantial reduction in hepatocyte apoptosis and serum transaminase elevations [38].
We showed, in addition, that NOD/SCID mice fed diets with three different iron contents were able to regulate expression of hepcidin and ferroportin appropriately as long as no allogeneic T lymphocytes were infused. However, the infusion of histoincompatible T cells resulted in significant dysregulation of iron: serum iron levels increased in mice on all diets, most markedly in mice on high iron diet, and ferroportin expression in the duodenal mucosa increased 10–100-fold following the infusion of allogeneic cells, even in mice on high iron diet. Conversely, hepcidin expression in the liver declined consistently, including mice on high iron diet and documented liver iron overload. These observations provide support for the notion that allogeneic transplantation may contribute to iron overload.
Allogeneic GVHD, Iron Homeostasis, and the Effect of Transferrin
Hepcidin expression is typically decreased in patients with anemia. One factor involved in suppression of hepcidin is GDF15, expressed by erythroblasts, and recent data indicate that active erythropoiesis is required for anemia-mediated down-regulation of hepcidin to occur [47,48]. Since many patients who undergo HCT are anemic, hepcidin levels are expected to be low, and, as a consequence, iron uptake should be high. However, this notion does not consider the fact that conditioning for HCT results in transient arrest of erythropoiesis, and hepatic and intestinal injury and cytokine release which might be expected to lead to upregulation of hepcidin. Further, as indicated above, transplant conditioning is associated with rapid Tf saturation, changes in ferritin levels, and accumulation of NTBI [20,49,50], which may lead to oxidative stress, as also described by Nunez et al. [20,50,51]. Following up on the observation of transplant conditioning-related rises in plasma iron levels and increases of highly reactive NTBI [20], Parkkinen et al. observed that rapid Tf saturation and accumulation of NTBI after HCT was prevented by infusion of high doses of ApoTf [14].
The mechanism of a contribution of immunocompetent, alloreactive donor T lymphocytes to any alterations in iron homeostasis is not clear. However, donor T cells are activated by host antigen presenting cells and cytokines, which leads to expansion and cytotoxic effects on target organs with the histologic hallmark of apoptosis [7,16,52,53]. Fas-mediated signals (initiated by allogeneic cells) may result in changes in redox status, which could lead to alterations in the expression and function of hepcidin (and other regulatory factors). Inflammatory pathways, in particular IL-6 signaling, appear to be involved, as well as bone morphogenetic proteins 2/4 among other factors [54]. However, in our murine model in which no cytotoxic conditioning regimen is administered, primary inflammatory signals are unlikely to represent the essential trigger, although T-cell activation will lead to cytokine release, including IL-6 and TNFα. Inflammatory responses are likely to be more relevant in the clinical setting where cytotoxic conditioning is administered in preparation for HCT, although one would expect IL-6-induced upregulation of hepcidin to counteract iron uptake and accumulation. Thus, while there is little information on the expression and function of iron regulatory proteins in the setting of allogeneic HCT, our model suggests that hepatic iron deposition is enhanced by signals that are directly or indirectly related to alloreactive T lymphocytes.
Pierpaoli et al. showed that treatment of rodents before HCT with Tf (from the prospective donors) facilitated engraftment and attenuated or prevented the development of GVHD [55,56]. Similar protection was achieved with human Tf [55]. We confirmed those data in a canine model [57]. In vitro experiments showed that Tf, particularly in the form of ApoTf, suppressed alloreactivity as well as mitogen-induced proliferation [39,58,59], and had profound anti-apoptotic effects. For example, in non-transformed murine (NMH) and human (HH4) hepatocyte cell lines, Fas-mediated apoptosis was down-modulated or prevented by ApoTf [60]. While Fas-induced apoptosis was accompanied by a decrease in cellular glutathione (GSH) levels, consistent with the implied changes in cellular redox potential, GSH levels were preserved or restored in the presence of ApoTf [60], similar to the effects of N-acetylcysteine or glutamine as reported by others [49]. ApoTf was most effective when supplied 24 hours before Fas ligation [39,60]. Strikingly, in human HH4 hepatocytes the addition of iron saturated HoloTf (rather than ApoTf) to cultures 24 hours before the agonistic anti-human Fas antibody CH11 (mimicking the effect of Fas-ligand expressing T lymphocytes) in a dose-dependent fashion increased caspase-3 activation and the proportion of apoptotic cells.
Apoptosis is central to tissue damage in GVHD, and the present data appear pertinent to the observation of GVHD prevention by ApoTf [57,61]. Of note, the cytoprotective effect of ApoTf was absent in mice with a genetic inactivation of Tf receptor 2 (mice homozygous for the receptor inactivation also express low levels of hepcidin) [62], while, in contrast, neutralization of Tf receptor 1 (CD71) did not interfere with the cytoprotective effects of Tf [59].
Summary
Iron overload is frequent in patients with ineffective hematopoiesis who undergo HCT. Causes include enhanced iron absorption due to anemia, RBC transfusions and peri-transplant events. Infusion of allogeneic cells may contribute to the dysregulation of iron homeostasis by way of hepatic and intestinal injury. Fas-mediated signals appear to be involved, at least in preclinical models, where the administration of ApoTf provides a cytoprotective effect. This effect may be mediated via binding of NTBI or, possibly, via signals transmitted though Tf receptor 2. The exact pathway leading to inappropriate downregulation of hepcidin remains to be characterized.
Acknowledgments
We thank Helen Crawford for help with manuscript preparation.
Supported in part by NIH grants HL036444, CA 015704, Bethesda, MD, and a grant in aid from Novartis, Basel, Switzerland.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation (Review) Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 3.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110:2182–2189. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, Constante M, Layoun A, Santos MM. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113:3593–3599. doi: 10.1182/blood-2008-08-173641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakhal S, Talbot NP, Crosby A, Stoepker C, Townsend AR, Robbins PA, et al. Regulation of growth differentiation factor 15 expression by intracellular iron. Blood. 2009;113:1555–1563. doi: 10.1182/blood-2008-07-170431. [DOI] [PubMed] [Google Scholar]
- 6.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrangelo A, Trautwein C. Mechanisms of disease: The role of hepcidin in iron homeostasis--implications for hemochromatosis and other disorders (Review) Nature Clinical Practice Gastroenterology and Hepatology. 2004;1:39–45. doi: 10.1038/ncpgasthep0019. [DOI] [PubMed] [Google Scholar]
- 8.Lucarelli G, Clift RA. Marrow transplantation in thalassemia. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas' Hematopoietic Cell Transplantation. Third ed. Oxford, UK: Blackwell Publishing Ltd.; 2004. pp. 1409–1416. [Google Scholar]
- 9.Gattermann N. Assessing the guidelines on iron chelation in MDS - where are we? Leukemia Research. 2009;33 Suppl. 1:S22–S23. #27. [Google Scholar]
- 10.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 11.Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platzbecker U, Bornhäuser M, Germing U, Stumpf J, Scott BL, Kröger N, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS) Biol Blood Marrow Transplant. 2008;14:1217–1225. doi: 10.1016/j.bbmt.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102:2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 14.Parkkinen J, Sahlstedt L, von Bonsdorff L, Salo H, Ebeling F, Ruutu T. Effect of repeated apotransferrin administrations on serum iron parameters in patients undergoing myeloablative conditioning and allogeneic stem cell transplantation. Br J Haematol. 2006;135:228–234. doi: 10.1111/j.1365-2141.2006.06273.x. [DOI] [PubMed] [Google Scholar]
- 15.Zaucha JM, Gooley T, Heimfeld S, Bensinger W, Flowers M, Storb R, et al. The ratio of CD3:CD14 cells and the number of CD34 cells in G-CSF mobilized peripheral blood mononuclear cell (G-PBMC) products are significantly associated with clinical outcome in HLA-identical sibling transplantation. Blood. 2000;96((Part 1)[11]):205a. #874. [Google Scholar]
- 16.Janin A, Deschaumes C, Daneshpouy M, Estaquier J, Micic-Polianski J, Rajagopalan-Levasseur P, et al. CD95 engagement induces disseminated endothelial cell apoptosis in vivo: immunopathologic implications. Blood. 2002;99:2940–2947. doi: 10.1182/blood.v99.8.2940. [DOI] [PubMed] [Google Scholar]
- 17.Kowdley KV, Trainer TD, Saltzman JR, Pedrosa M, Krawitt EL, Knox TA, et al. Utility of hepatic iron index in American patients with hereditary hemochromatosis: a multicenter study. Gastroenterology. 1997;113:1270–1277. doi: 10.1053/gast.1997.v113.pm9322522. [DOI] [PubMed] [Google Scholar]
- 18.Beutler E, Hoffbrand AV, Cook JD. Iron deficiency and overload (Review) Hematology. 2003:40–61. doi: 10.1182/asheducation-2003.1.40. [DOI] [PubMed] [Google Scholar]
- 19.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 20.Gordon LI, Brown SG, Tallman MS, Rademaker AW, Weitzman SA, Lazarus HM, et al. Sequential changes in serum iron and ferritin in patients undergoing high-dose chemotherapy and radiation with autologous bone marrow transplantation: possible implications for treatment related toxicity. Free Radic Biol Med. 1995;18:383–389. doi: 10.1016/0891-5849(94)e0145-9. [DOI] [PubMed] [Google Scholar]
- 21.Armand P, Cutler CS, Kim HT, Ho VT, Koreth J, Alyea EP, et al. Prognostic impact of elevated serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2006;108((Part 1)[11]):179a. doi: 10.1182/blood-2006-10-054924. #595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strasser SI, Kowdley KV, Sale GE, McDonald GB. Iron overload in bone marrow transplant recipients. Bone Marrow Transplant. 1998;22:167–173. doi: 10.1038/sj.bmt.1701301. [DOI] [PubMed] [Google Scholar]
- 23.Strasser SI, McDonald SJ, Schoch HG, McDonald GB. Severe hepatocellular injury after hematopoietic cell transplant: incidence and etiology in 2136 consecutive patients. Hepatology. 2000;32((Pt. 2)[4]):299A. doi: 10.1038/bmt.2009.56. #557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho G-T, Parker A, MacKenzie JF, Morris AJ, Stanley AJ. Abnormal liver function tests following bone marrow transplantation: aetiology and role of liver biopsy. Eur J Gastroenterol Hepatol. 2004;16:157–162. doi: 10.1097/00042737-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Altes A, Remacha AF, Sarda P, Sancho FJ, Sureda A, Martino R, et al. Frequent severe liver iron overload after stem cell transplantation and its possible association with invasive aspergillosis. Bone Marrow Transplant. 2004;34:505–509. doi: 10.1038/sj.bmt.1704628. [DOI] [PubMed] [Google Scholar]
- 26.Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status-based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 27.Alessandrino EP, Della Porta MG, Bacigalupo A, van Lint MT, Falda M, Onida F, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood. 2008;112:895–902. doi: 10.1182/blood-2008-03-143735. [DOI] [PubMed] [Google Scholar]
- 28.Pullarkat V, Blanchard S, Tegtmeier B, Dagis A, Patane K, Ito J, et al. Iron overload adversely affects survival and increases risk of graft-versus-host disease and blood stream infections after allogeneic hematopoietic stem cell transplantation. Blood. 2007;110((Part 1)[11]):875a–876a. #2981. [Google Scholar]
- 29.Mahindra A, Sobecks R, Rybicki L, Brown S, Pohlman B, Kalaycio M, et al. Elevated ferritin is associated with poorer survival following nonablative allogeneic transplantation. Blood. 2007;110((Part 1)[11]):591a. #1986. [Google Scholar]
- 30.Maradei C, Maiolino A, Mello de Azevedo A, Colares M, Bouzas LF, Nucci M. Serum ferritin as risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood 9999. 2009 doi: 10.1182/blood-2009-03-212282. prepublished online April 28, DOI:10.1182/blood-2009-03-212282. [DOI] [PubMed] [Google Scholar]
- 31.Sale GE, Shulman HM, Hackman RC. Pathology of hematopoietic cell transplantation. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas' Hematopoietic Cell Transplantation. Third ed. Oxford, UK: Blackwell Publishing Ltd.; 2004. pp. 286–299. [Google Scholar]
- 32.Deeg HJ, Antin JH. The clinical spectrum of acute graft-versus-host disease. Semin Hematol. 2006;43:24–31. doi: 10.1053/j.seminhematol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Graft-vs.-Host Disease. New York, NY: Marcel Dekker; 2005. [Google Scholar]
- 34.Shulman HM, McDonald GB. Hepatic complications of hematopoietic cell transplantation. In: Gershwin ME, Vierling JM, Manns MP, editors. Liver Immunology: Principles and Practice. Totowa, NJ: Human Press Inc.; 2007. pp. 409–421. [Google Scholar]
- 35.Yada S, Takamura N, Inagaki-Ohara K, O'leary MK, Wasem C, Brunner T, et al. The role of p53 and Fas in a model of acute murine graft-versus-host disease. J Immunol. 2005;174:1291–1297. doi: 10.4049/jimmunol.174.3.1291. [DOI] [PubMed] [Google Scholar]
- 36.Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miwa K, Hashimoto H, Yatomi T, Nakamura N, Nagata S, Suda T. Therapeutic effect of an anti-Fas ligand mAb on lethal graft-versus-host disease. Int Immunol. 1999;11:925–931. doi: 10.1093/intimm/11.6.925. [DOI] [PubMed] [Google Scholar]
- 38.Bair S, Spaulding E, Parkkinen J, Shulman HM, Lesnikov V, Beauchamp M, et al. Transplantation of allogeneic T cells alters iron homeostasis in NOD/SCID mice. Blood. 2009;113:1841–1844. doi: 10.1182/blood-2008-09-178517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesnikov VA, Lesnikova MP, Shulman HM, Wilson H-M, Hockenbery DM, Kocher M, et al. Prevention of Fas-mediated hepatic failure by transferrin. Lab Invest. 2004;84:342–352. doi: 10.1038/labinvest.3700035. [DOI] [PubMed] [Google Scholar]
- 40.Gores GJ, Kaufmann SH. Is TRAIL hepatotoxic? [Review] Hepatology. 2001;34:3–6. doi: 10.1053/jhep.2001.25173. [DOI] [PubMed] [Google Scholar]
- 41.Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Escolar G, et al. The release of soluble factors contributing to endothelial activation and damage after hematopoietic stem cell transplantation is not limited to the allogeneic setting and involves several pathogenic mechanisms. Biol Blood Marrow Transplant. 2009;15:537–546. doi: 10.1016/j.bbmt.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Kamble RT, Selby GB, Mims M, Kharfan-Dabaja MA, Ozer H, George JN. Iron overload manifesting as apparent exacerbation of hepatic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:506–510. doi: 10.1016/j.bbmt.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Gerbitz A, Ewing P, Wilke A, Schubert T, Eissner G, Dietl B, et al. Induction of heme oxygenase-1 before conditioning results in improved survival and reduced graft-versus-host disease after experimental allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2004;10:461–472. doi: 10.1016/j.bbmt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nature Cell Biology. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 45.van Bekkum DW, de Vries MJ. Radiation chimaeras. London: Logos Press Limited; 1967. [Google Scholar]
- 46.Socie G, Mary JY, Lemann M, Daneshpouy M, Guardiola P, Meignin V, et al. Prognostic value of apoptotic cells and infiltrating neutrophils in graft-versus-host disease of the gastrointestinal tract in humans: TNF and Fas expression. Blood. 2004;103:50–57. doi: 10.1182/blood-2003-03-0909. [DOI] [PubMed] [Google Scholar]
- 47.De Domenico I, McVey WD, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders (Review) Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 48.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evens AM, Mehta J, Gordon LI. Rust and corrosion in hematopoietic stem cell transplantation: the problem of iron and oxidative stress. Bone Marrow Transplant. 2004;34:561–571. doi: 10.1038/sj.bmt.1704591. [DOI] [PubMed] [Google Scholar]
- 50.Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation (Review) Bone Marrow Transplant 9999. 2008 doi: 10.1038/bmt.2008.99. prepublished online 28 April; doi:10.1038/bmt.2008.99. [DOI] [PubMed] [Google Scholar]
- 51.Núñez MT, Núñez-Millacura C, Tapia V, Muñoz P, Mazariegos D, Arredondo M, et al. Iron-activated iron uptake: a positive feedback loop mediated by iron regulatory protein 1. BioMetals. 2003;16:83–90. doi: 10.1023/a:1020743405347. [DOI] [PubMed] [Google Scholar]
- 52.Bowlus CL. The role of iron in T cell development and autoimmunity (Review) Autoimmunity Reviews. 2003;2:73–78. doi: 10.1016/s1568-9972(02)00143-x. [DOI] [PubMed] [Google Scholar]
- 53.Cronje L, Bornman L. Iron overload and tuberculosis: a case for iron chelation therapy (Review) International Journal of Tuberculosis & Lung Disease. 2005;9:2–9. [PubMed] [Google Scholar]
- 54.Nemeth E. Iron regulation and erythropoiesis (Review) Curr Opin Hematol. 2008;15:169–175. doi: 10.1097/MOH.0b013e3282f73335. [DOI] [PubMed] [Google Scholar]
- 55.Pierpaoli W. Effect of human transferrin on the engraftment of allogeneic bone marrow in various strains of lethally irradiated mice. Nat Immun. 1992;11:356–365. [PubMed] [Google Scholar]
- 56.Gahring LC, Weigle WO. The regulatory effects of cytokines on the induction of a peripheral immunologic tolerance in mice. J Immunol. 1990;145:1318–1323. [PubMed] [Google Scholar]
- 57.Deeg HJ, Pierpaoli W, Arrighi S, Seidel K, Graham T, Huss R, et al. Facilitation of DLA-incompatible marrow grafts by donor-specific serum transferrin? Transpl Immunol. 1996;4:113–116. doi: 10.1016/s0966-3274(96)80004-9. [DOI] [PubMed] [Google Scholar]
- 58.Lesnikova M, Lesnikov V, Arrighi S, Kistler G, Pierpaoli W, Deeg HJ. Upregulation of interleukin-10 and inhibition of alloantigen responses by transferrin and transferrin-derived glycans. Journal of Hematotherapy and Stem Cell Research. 2000;9:381–392. doi: 10.1089/15258160050079498. [DOI] [PubMed] [Google Scholar]
- 59.Lesnikov V, Lesnikova M, Deeg HJ. Pro-apoptotic and anti-apoptotic effects of transferrin and transferrin-derived glycans on hematopoietic cells and lymphocytes. Exp Hematol. 2001;29:477–489. doi: 10.1016/s0301-472x(00)00687-1. [DOI] [PubMed] [Google Scholar]
- 60.Lesnikov VA, Abbasi N, Lesnikova MP, Lazaro CA, Campbell JS, Fausto N, et al. Protection of human and murine hepatocytes against Fas-induced death by transferrin and iron. Apoptosis. 2006;11:79–87. doi: 10.1007/s10495-005-3086-2. [DOI] [PubMed] [Google Scholar]
- 61.Pierpaoli W, Lesnikov VA, Lesnikova MP, Arrighi S, Bardotti A. Unresponsiveness to human leukocytes in immunosuppressed mice by combined donor-derived human transferrin and antigens. Transpl Immunol. 1996;4:301–308. doi: 10.1016/s0966-3274(96)80051-7. [DOI] [PubMed] [Google Scholar]
- 62.Lesnikov V, Gorden N, Fausto N, Spaulding E, Campbell J, Shulman H, et al. Transferrin fails to provide protection against Fas-induced hepatic injury in mice with deletion of functional transferrin-receptor type 2. Apoptosis. 2008;13:1005–1012. doi: 10.1007/s10495-008-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]