Abstract
The production of polysaccharide-derivatized surfaces, polymers, and biomaterials has been shown to be a useful strategy for mediating the biological properties of materials, owing to the importance of polysaccharides for the sequestration and protection of bioactive proteins in vivo. We have therefore sought to combine the benefits of polysaccharide derivatization of polymers with unique opportunities to use these polymers for the production of bioactive, noncovalently assembled hydrogels. Accordingly, we report the synthesis of a heparin-modified poly(ethylene glycol) (PEG) star copolymer that can be used in the assembly of bioactive hydrogel networks via multiple strategies and that is also competent for the delivery of bioactive growth factors. A heparin-decorated polymer, synthesized by the reaction of thiol end-terminated four-arm star PEG (Mn = 10 000) with maleimide functionalized low molecular weight heparin (LMWH, Mr = 3000), has been characterized via 1H NMR spectroscopy and size-exclusion chromatography; results indicate attachment of the LMWH with at least 73% efficiency. Both covalently and noncovalently assembled hydrogels can be produced from the PEG–LMWH conjugate. Viscoelastic noncovalently assembled hydrogels have been formed on the basis of the interaction of the PEG–LMWH with a PEG polymer bearing multiple heparin-binding peptide motifs. The binding and release of therapeutically important proteins from the assembled hydrogels have also been demonstrated via immunochemical assays, which demonstrate the slow release of basic fibroblast growth factor (bFGF) as a function of matrix erosion. The combination of these results suggests the opportunities for producing polymer–polysaccharide conjugates that can assemble into novel hydrogel networks on the basis of peptide–saccharide interactions and for employing these materials in delivery applications.
Introduction
The production of polysaccharide-derivatized polymeric materials has gained increasing attention owing to the importance of polysaccharide–protein interactions in applications such as wound healing, tissue engineering, protein therapeutics, and molecular diagnostics, coupled with the increasing understanding of and synthetic control over polysaccharide and glycopolymer structures.1–3 The wide-spread use of polysaccharides in biological applications finds its origins in the generally low immunological response of the highly hydrated polysaccharides, the prevalence of polysaccharides in mediating biological processes in vivo, and the availability of hydrogel-forming polysaccharides such as hyaluronic acid, modified celluloses, alginates, and dextran. Heparin, a highly sulfated glycosaminoglycan, has also received an enormous amount of research attention because of its potent and important activities.4 These include binding to antithrombin III to mediate thrombosis, as well as binding to growth factors to stabilize their conformations, potentiate their activities, and protect them from degradation and thermal inactivation.
Heparin-containing delivery systems for the controlled release of growth factors have been reported by many investigators and have permitted delivery of growth factors for wound healing and tissue engineering applications. These delivery systems can rely on passive inclusion of heparin in hydrogel matrices,5,6 noncovalent immobilization of heparin in the matrix via affinity interactions with heparin-binding peptides,7–11 or covalent attachment of heparin to a drug delivery matrix (such as alginate, collagen, or hyaluronate).12–16 Results from multiple investigations suggest that matrices that contain immobilized heparin are most successful for the delivery of bioactive growth factors over extended periods of time. For example, recent reports demonstrate the copolymerization of styrylated heparin with albumin to form surfaces capable of the delivery of basic fibroblast growth factor (bFGF)17 or to form three-dimensional structures.18 Silyl-heparin has also been produced to mediate adsorption of heparin to surfaces for delivery of bFGF.19 In other investigations, heparin and bFGF have been transiently immobilized in a fibrin matrix via noncovalent interactions between the growth factor, heparin, and covalently incorporated heparin-binding peptides.8 The release rate of the growth factor may be controlled by varying the affinity between heparin and the heparin-binding peptides and is also promoted by cell-mediated degradation of the fibrin matrix.
In addition to polymeric, heparinized delivery systems such as those above, noncovalently associated hydrogels have been increasingly studied as a route to the production of responsive, reversible, and injectable delivery systems.10,20–22 Although protein–protein, peptide–peptide, and ionic cross-linking interactions have been employed widely as an assembly mechanism for polymeric materials, there are only limited reports of the use of peptide–polysaccharide interactions for assembly,11 despite the prevalence of specific and high affinity protein–polysaccharide interactions in biology. The objective of this work was therefore the synthesis of a new polymer–heparin conjugate with an architecture that permits assembly with other macromolecules via specific heparin–peptide interactions and that might therefore permit manipulation of hydrogel mechanical and release properties via variations in heparin–peptide binding affinity and kinetics. Such polymer conjugates would be useful in the production, via multiple strategies, of bioactive surfaces, hydrogels, and fibers with potential applications in wound healing and tissue engineering.
We report the synthesis of a multifunctional PEG–heparin star copolymer and its incorporation into hydrogel networks that are capable of growth factor delivery. Our initial studies have focused on the use of a PEG-based star polymer (Mn = 10 000) because of PEG’s widely reported low immunogenicity, biocompatibility, lack of protein fouling, and hydrophilicity.23 The multifunctional nature of the star polymer architecture permits the formation of noncovalently cross-linked hydrogels from this conjugate and may have additional uses where multivalent presentation of polysaccharides is relevant. Heparin has been used to modify the termini of the PEG star polymer owing to its uses in antithrombogenic coatings and its ability to bind to a variety of biologically relevant proteins such as antithrombin III, laminin, fibronectin, and growth factors. Low molecular weight heparin (LMWH), which has been suggested in limited reports to bind to growth factors,24,25 has been employed in order to minimize the number of reactive (amine) groups on the heparin and therefore minimize intermolecular cross-linking reactions between PEG termini and LMWH upon conjugation of LMWH to the star PEG polymer. In contrast to the many previous reports of the covalent modification of polymers with heparin, the multifunctional PEG–LMWH star copolymer reported here has the capability to mediate the noncovalent assembly of hydrogel networks with varying physical properties via interactions with multiple heparin-binding molecules; furthermore, such hydrogel networks are erodible and capable of binding and releasing growth factors. Specifically, the binding and release of bFGF from hydrogels containing the PEG–LMWH copolymer is reported.
Experimental Procedures
Materials and Methods
Hydroxy terminated and thiol terminated four-arm star poly(ethylene glycol)s (Mn = 10 300; Mw = 11 300 and Mn = 10 000; Mw = 10 800, respectively) were purchased from Polymer Source (Dorval, QC, Canada). A low molecular weight heparin sodium salt (porcine intestinal mucosa, Mr = 3000) was obtained from Sigma (St. Louis, MO). For peptide synthesis and HPLC, the amino acids and HBTU (2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) were received from Protein Technologies, Inc. (Tucson, AZ). Rink amide MBHA (4-methylbenzhydrylamine) resin was purchased from EMD Biosciences (San Diego, CA). DMF and acetonitrile (HPLC grade) were obtained from Fisher (Fairlawn, NJ). All other chemicals were obtained from Sigma- Aldrich unless otherwise specified. Thionyl chloride was gently stirred with Linde type 4A molecular sieves and distilled prior to use. Triethylamine was distilled from CaH2. All other reagents were used as received.
1H NMR spectra were acquired, under standard quantitative conditions at ambient temperature, on a Bruker DRX-400 NMR spectrometer. All spectra were recorded in DMSO-d6 and deuterium oxide and were referenced to TMS (tetramethylsilane) and DSS (sodium 2,2-dimethyl-2-silapentane-5-sulfonate), respectively. Mass spectra were obtained on a Bruker Biflex III matrix assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometer operated in the reflectron mode using delayed extraction. Spectra were acquired with a minimum of 50 laser shots per spectrum. Calibration was performed with Sequazyme Peptide Mass Standards Kit from Applied Biosystems. All MALDI analytes were dissolved in a 50% acetonitrile, 0.3% TFA containing matrix (α-cyano-4-hydroxycinnamic acid), at an analyte concentration of 5 mg/mL.
Synthesis of the PEG–LMWH Conjugate
N-Deacetylation of LMWH
Low molecular weight heparin (LMWH, Mr = 3000) was first separated, via anionic exchange chromatography on DEAE resin, into low- and high-affinity fractions (Supporting Information). LMWH was eluted from the column via gradient elution in Tris buffer (pH 7.5, 20 mM) with sodium chloride concentrations ranging from 0 to 2 M. The high-affinity (later-eluting) fraction of LMWH was collected, dialyzed against several changes of deionized water (SpectraPor, MWCO 1000), and lyophilized prior to use. All experiments reported were conducted with the high-affinity fraction of LMWH. The fractionated LMWH (250 mg), hydrazine (5.3 g), hydrazine sulfate (53 mg), and water (2.3 mL) were added to a heavy duty reaction vessel. The reaction vessel was tightly capped, and the mixture was stirred at 95 °C for 16 h.26 After the solvents were removed in vacuo, the product was dialyzed, using a SpectraPor dialysis membrane (MWCO 1000), first against 1 M NaCl and finally against water, and lyophilized to afford a slightly yellowish solid, 153 mg (61% yield). 1H NMR (400 MHz, D2O, 29 °C): δ = 3.38–5.43 (72H, heparin, m). The disappearance of the acetyl group resonance at 2.13 ppm confirmed N-deacetylation; a ninhydrin assay also confirmed the presence of free amine groups after the hydrazinolysis reaction.27 It should be noted that SEC analysis of the purified N-deacetylated LMWH indicated that there was no significant reduction in the molecular weight of LMWH as a result of hydrazinolysis.
Synthesis of Maleimide Functionalized LMWH (1)
To a 10 mL vial were added N-deacetylated LMWH (60 mg), dry dowex 50WX4’200 ion-exchange resin (Aldrich, strongly acidic cation), and anhydrous DMF (4 mL). The reaction mixture was stirred at room temperature until complete dissolution of LMWH was observed. The resin was filtered and to the filtrate were added a few drops of anhydrous triethylamine and a solution of 4-(N-maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester (SMCC, 4 equiv. to each free amine functionality in N-deactylated LMWH) (Sigma, St. Louis, MO) in anhydrous DMF (0.3 mL), and the reaction mixture was stirred at 60 °C for 20 h. The solvent was removed in vacuo to yield a slightly yellowish solid, which was redissolved in PBS (pH 6.5, 0.10 M, 0.15 M NaCl). After the insoluble particles were filtered, the filtrate was dialyzed using a SpectraPor membrane (MWCO 1000) first against 1 M NaCl and finally against water and was then lyophilized to give a slightly yellowish solid, 57 mg (89% yield); 1H NMR (400 MHz, D2O, 29 °C): δ = 1.05–2.29 (10H, cyclohexyl, m), 3.38–5.43 (72H, heparin, m), and 6.83 ppm (2H, −COCH=CHCO−, s).
Synthesis of the PEG–LMWH (2)
To a 100 mL round-bottom flask equipped with a N2 inlet were added 1 (120 mg) and PBS (10 mL, pH 6.5, 0.10 M, 0.15 M NaCl), and the solution was degassed by bubbling with N2 for 15 min. To this solution was slowly added degassed PBS containing thiol terminated poly(ethylene glycol) (65 mg in 10 mL), and the reaction mixture was stirred at room temperature for 2 h. Complete reaction of the thiol was confirmed via analysis with Ellman’s reagent.28 The reaction mixture was quenched by adding a solution of N-ethylmaleimide (12 mg) in DMF (0.4 mL). The crude product was purified via FPLC with a Superdex 200 gel filtration column (AKTA Explorer 10 FPLC,Amersham, Piscataway, NJ) at 0.5 mL/min using 0.2 M NaCl as eluting buffer (Supporting Information). The product isolated via FPLC was purified via dialysis against water and lyophilized to give a slightly yellowish solid, 150 mg (81% yield); 1H NMR (400 MHz, D2O, 29 °C): δ = 1.05–2.29 (40H, cyclohexyl, m), 2.74–3.03 (12H, −COCH2CHCO−, m), 3.38–5.43 (288H, heparin, m), and 3.70 ppm (908H, PEG backbone, −CH2CH2O−, s) (Supporting Information).
Synthesis of the PEG–HIP Conjugate
Peptide Synthesis
The peptide CRPKAKAKAKAKDQTK was used in these investigations; this sequence is derived from the heparin-binding domain of heparin interacting protein (HIP).29–35 The peptide was prepared via standard Fmoc solid-phase peptide chemistry with HBTU activation on a PS3 Automated Solid-Phase Peptide Synthesizer (Protein Technologies, Inc., Tucson, AZ). The peptide was cleaved from the resin with TFA/water/EDT/TIS (94:2.5:2.5:1) and precipitated in cold diethyl ether. Peptide purification was performed via reverse-phase chromatography on a Delta600 HPLC (Waters) equipped with a preparative Symmetry300 C18 column (Waters; 5µm particle size, 3.9 × 150 mm). The peptide was eluted from the column via the application of a linear gradient from 5% to 70% solvent B over 49 min at 5 mL/min, where solvent A is 0.1% TFA in water and solvent B is 0.1% TFA in acetonitrile. The HIP peptide was purified to a minimum of 99.5% purity as confirmed via HPLC (Supporting Information). The mass of the purified peptide was confirmed via MALDI-TOF mass spectrometry (single peak observed), m/z = 1771.9 [(M+H)+, calcd 1772.0].
Synthesis of the Vinyl Sulfone Terminated Four-Arm Star PEG (3)
The vinyl sulfone terminated four-arm PEG was synthesized via protocols reported by Harris36 with some modifications, or via those reported by Lutolf and Hubbell.37 The slight modifications to these procedures are detailed in the Supporting Information, along with the NMR spectrum of the resulting product. Conversion of the termini of four-arm PEG to vinyl sulfone groups proceeded at yields similar to those previously reported (67–78% and 90–98%, respectively); the ultimate functionalization of the four-arm star PEG with peptides was the same regardless of the initial method used to generate the vinyl sulfone terminated star PEG (see below).
Synthesis of the PEG–HIP (4)
The HIP peptide carrying an N-terminal cysteine was reacted via Michael addition with vinyl sulfone modified star PEG according to procedures reported by Lutolf and Hubbell.37 To a 10 mL vial were added 3 (80 mg) and degassed PBS (pH 6.5, 0.10 M, 0.15 M NaCl). To this solution was added a degassed PBS solution (pH 6.5, 0.10 M, 0.15 M NaCl) of the HIP peptide (2 equiv. to each vinyl sulfone functionality in 3). The reaction mixture was quickly sealed and stirred at room temperature for 16 h. The crude product was purified via FPLC with a Superdex 200 gel filtration column (Amersham, Piscataway, NJ) with isocratic elution, employing 0.2 M NaCl at a flow rate of 0.5 mL/min as the eluting buffer. The fractions containing the PEG-HIP were combined, dialyzed using a SpectraPor dialysis membrane (MWCO 1000) against water to remove NaCl, and lyophilized to yield the PEG–HIP as a white solid, 69 mg (86% yield) (Supporting Information shows the 1H NMR spectrum).
Covalent Cross-Linking of Hydrogel Networks
To a graduated polypropylene tube were added potassium per-sulfate (12 mg), poly(ethylene glycol) methyl ether acrylate (Aldrich, designated Mn ~454, 1.2 mL), and a solution of 2 (0.2–20 mg/mL, 600 µL), and the solution was degassed with N2 for 15 min prior to the cross-linking reaction. The degassed solution was added to a 96-well microplate (55 µL in each well), and the plate was covered with a lid. The plate was then placed on a preheated hot plate set at 65 °C for 15 min. After the plate was cooled to room temperature, 200 mL of PBS was added to each well, and the plate was placed in a 4 °C refrigerator for 16 h. Hydrogels were isolated as transparent disks with dimensions of 12.23 ± 0.05 mm in diameter and 2.58 ± 0.12 mm thickness and were determined gravimetrically to contain 90 ± 0.4 wt % water.
Noncovalent Assembly of Networks
For the noncovalent assembly of hydrogel networks, solutions of 2 and 4 in PBS were mixed (by pipetting) in various ligand mole ratios of LMWH:HIP (the degrees of LMWH and the HIP peptide functionalities in 2 and 4 were estimated to be 73 and 77%, respectively, via 1H NMR spectroscopy). Changes in viscoelastic properties upon mixing were immediately apparent for LMWH:HIP ratios of 9:1, 8:2, and 6:4. For experiments to investigate growth factor binding and delivery, hydrogel formed at a ligand mole ratio of 8:2 (LMWH:HIP) was used. bFGF (10 ng, R&D Systems, Minneapolis, MN) was added to a solution of 2 in PBS (2.5 wt %) with a final volume of 291 µL. The resulting solution was incubated for 2 h to allow complete association of bFGF with LMWH as well as to accomplish uniform distribution of bFGF. To this solution was added a 10 wt % solution of 4 to achieve a total volume of 300 µL; a hydrogel formed instantaneously. The hydrogel was briefly agitated to provide even mixing. The assembled hydrogel was stored no longer than 2 h at 4 °C prior to use.
Toluidine Blue Assay
Toluidine blue assays were employed to determine the amount of LMWH in the hydrogels and were performed as previously reported,38 with slight modifications. Specific details of the assay are provided in the Supporting Information.
Binding and Release of bFGF
Covalently Cross-Linked, Nondegradable Hydrogels
All hydrogels were stored in PBS at 4 °C prior to use. All binding and delivery experiments for covalently cross-linked hydrogels were performed at 4 °C in 24-well polystyrene assay plates (Corning Inc., Corning, NY), blocked with 3% BSA in PBS. For growth factor binding experiments, covalent hydrogels were placed in the wells and incubated with 500 µL of bFGF (R&D Systems, Minneapolis, MN, 100 ng/mL in PBS/BSA) for 16 h. After five washes with 0.05% Tween20 in PBS, the amount of bFGF bound to the hydrogels was determined via a standard ELISA assay protocol39 employing biotinylated anti-human bFGF (R&D Systems), horseradish peroxidase conjugated NeutrAvidin, (Pierce, Rockford, IL), and a TMB substrate solution (Pierce).
For growth factor delivery experiments, covalently cross-linked hydrogels were placed in the wells of 24-well assay plates. Aliquots (4 µL) of bFGF (2.5 µg/mL) were carefully loaded on the hydrogels and the plates were incubated for 16 h. PBS (1 mL) was added to each well, incubated for specific measured times, and then removed and replaced with fresh PBS (1 mL). The removed saline solutions were analyzed via the bFGF Quantikine kit (R&D Systems, Minneapolis, MN) to determine cumulative release of bFGF as a function of time (based on the initial amount of bFGF loaded into the hydrogel). A PEG hydrogel lacking any heparin was used as a control, and the experiments were performed in duplicate.
Noncovalently Assembled Hydrogel
All bFGF delivery experiments for the noncovalently assembled hydrogel were performed under the same conditions as for the covalently cross-linked hydrogels, except that 24-well polystyrene assay plates equipped with microporous transwell inserts (8 µm pore size, Corning Inc., Corning, NY) were used. Both the wells and the inserts were blocked with 3% BSA in PBS. The wells and inserts were aspirated and washed 4 times with 0.05% Tween20 (Sigma, St. Louis, MO) in PBS prior to use. The assembled hydrogel was carefully placed in the insert and initially PBS (1 mL total) was added to the insert and well to level the saline solutions in both compartments. The saline solution from the well was removed at different times and replaced with fresh PBS (1 mL). The removed saline solutions were analyzed with a bFGF Quantikine kit (R&D Systems, Minneapolis, MN) to determine cumulative release of bFGF (based on the initial loading of bFGF in the gel). The experiments were performed in triplicate.
Erosion of Noncovalently Assembled Hydrogel
All erosion experiments for the noncovalently assembled hydrogel were performed at 4 °C in 24-well polystyrene assay plates equipped with 8 µm transwell inserts (Corning Inc., Corning, NY). The assembled hydrogel was carefully placed in the insert (by pipetting), and PBS (1 mL total) was initially added to the insert and well to level the saline solutions in both compartments. The saline solution from the well was removed at different times and replaced with fresh PBS (1 mL). The removed saline solutions were dialyzed using a SpectraPor membrane (MWCO 500) against deionized water at ambient temperature over 2 days with four water bath changes. The desalted solutions were then lyophilized to white solids, which were weighed to determine the mass of polymer liberated from the gel as a function of time. The experiments were performed in triplicate.
Results and Discussion
Synthesis of the PEG Conjugates
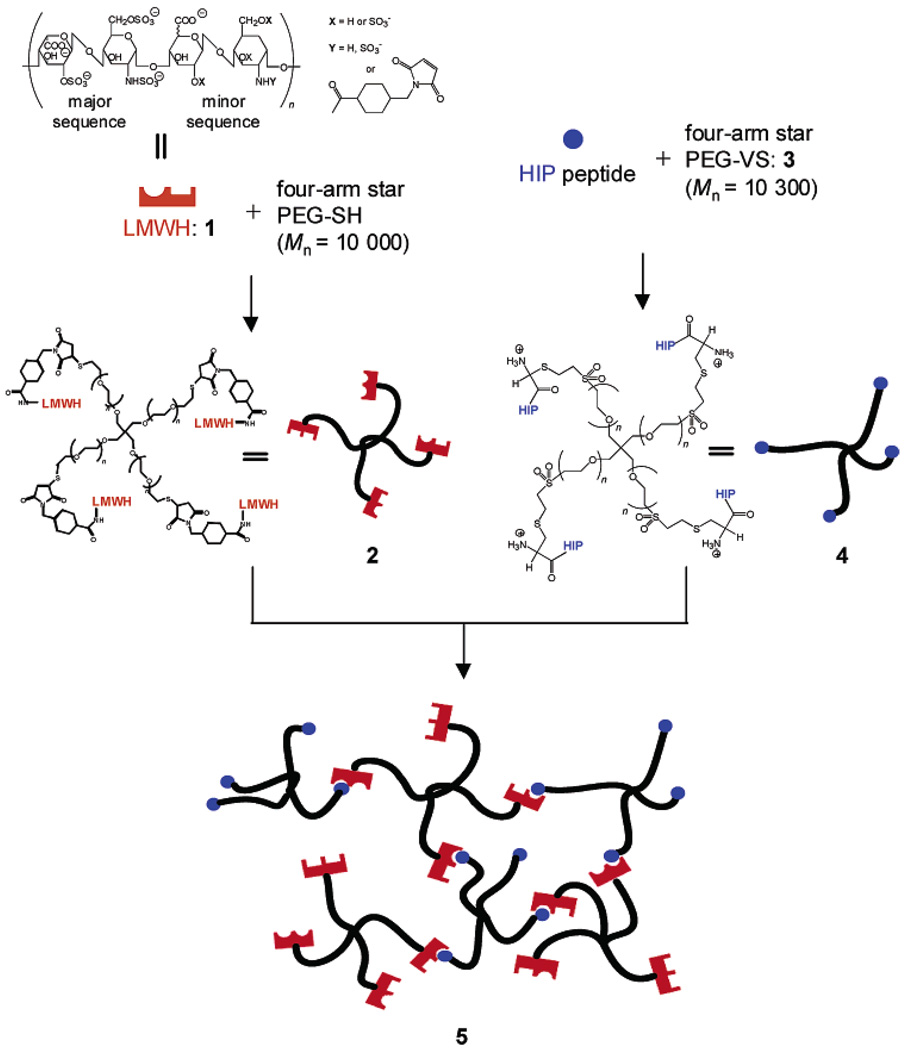
The synthesis of the PEG–LMWH star bioconjugate offers opportunities for the production of novel, noncovalently assembled, bioactive hydrogels capable of local and sustained release of therapeutically relevant proteins. Matrices comprising the PEG–LMWH copolymer are desirable targets owing to their hydrophilicity, biocompatibility, low immunogenicity, and ability to bind growth factors and other proteins and peptides. Figure 1 illustrates the synthetic scheme and use of the PEG–LMWH conjugate for the assembly of hydrogel matrix, 5, which may be useful for localized delivery of a variety of therapeutically relevant heparin-binding proteins and peptides. The individual constituent molecules, 2 and 4, were synthesized by modifying the chain ends of four-arm star poly(ethylene glycol) (PEG) with either low molecular weight heparin (LMWH, Mr = 3000) or a heparin-binding peptide derived from the heparin-binding domain of heparin interacting protein (HIP), respectively.40 The specific synthetic schemes followed to produce 2 and 4 are illustrated in the Supporting Information. Previous investigations,41 which indicate that heparin conjugated to PEG retains its ability to simultaneously bind antithrombin III (ATIII) and thrombin, suggest that 2 would remain competent for binding to heparin-binding peptides and proteins. The underlying structural criteria for 2 and 4 were established from this finding and a theoretical prerequisite in which a minimum of three functional groups on one assembly partner and two functional groups on the complementary partner is necessary to permit physical cross-linking into a hydrogel network.
Figure 1.
Schematic of the assembly of noncovalent hydrogel network, 5, from the individual constituent molecules 2 and 4. Potential HIP peptide and bFGF binding domains in LMWH, 1, are indicated by half circles and rectangles, respectively.
Synthesis of the PEG–LMWH (2)
There were several reasons for the choice of LMWH for conjugation to the four-arm star PEG. The biofunctionality of heparin was of interest for the intended applications of this bioconjugate, but the reaction of maleimide functionalized high molecular weight heparin with the four-arm star PEG via the conjugation strategies shown would lead to a cross-linked network because of the reaction of multiple PEG chain ends per heparin molecule. As a soluble bioconjugate was the synthetic target, LMWH was employed to minimize the potential for the attachment of multiple PEG chain ends to heparin. As N-deacetylated high affinity LMWH is estimated to have less than one randomly positioned free amino group per chain, the use of LMWH would satisfy necessary criteria. This estimate is based on the fact that high molecular weight heparin (Mr = 12 000), which is four times the average molecular weight of LMWH employed in these investigations, possesses an average of one free amino group as well as one N-acetylated group per chain.42 The heterogeneity of heparin precludes a precise determination of the exact number of free amino groups per chain, although our experimental estimation of the average number of free amine groups in the N-deacetylated LMWH (1.2, see below) is consistent with the above predictions.
Although LMWH employed in these investigations is heterogeneous, this heterogeneity provides a functional advantage by permitting binding of various and multiple proteins and peptides to 2. For example, the HIP binding sequence in heparin is reported to be similar or identical to the well-characterized pentasaccharide ATIII binding sequence, 33 so LMWH in 2, which is on average a dodeca- to didodecasaccharide, should be of sufficient length to facilitate network formation upon binding with 4. Furthermore, the binding of LMWH to growth factors, including bFGF, VEGF, and HGF, has been suggested in limited reports,24,25 which is consistent with the fact that the bFGF and VEGF binding sequences in heparin have been indicated to be penta- or hexasaccharide sequences.43–45 This bioconjugate should therefore serve a variety of functions including both network formation via interaction with 4, as well as growth factor binding.
To isolate LMWH that would display increased binding affinity for heparin-binding peptides, LMWH was fractionated on a DEAE ion exchange column. Two distinct peaks were observed (Supporting Information) and the higher-affinity fraction that eluted at higher salt concentrations was isolated and used in the present studies. According to previous reports, ion exchange fractions of heparin have similar charge densities and differ primarily in molecular weight, which increases monotonically as the salt concentration required for elution increases.46 The use of the high affinity fraction of LMWH should therefore provide a statistically greater number of potential binding sites for the HIP peptide and/or growth factors in 2; consequently, increased cross-linking density and improved growth factor loading may be realized in 5. The improved affinity of the fractionated LMWH, relative to unfractionated LMWH, for heparin-binding peptides was confirmed via surface plasmon resonance studies (SPR, data not shown). Unfractionated LMWH immobilized to a biosensor chip did not bind to any measurable amount of the HIP peptide, while the high affinity, fractionated LMWH was competent for peptide binding.
Reported methods for the conjugation of heparin to polymers and surfaces include three primary strategies, namely reaction at the reducing terminus, modification of free amine groups, or coupling to free carboxylic acid groups on the polysaccharide. Reaction at the reducing terminus of heparin is perhaps the most attractive strategy, as this chemical modification closely mimics the covalent linkage of the polysaccharide to proteoglycans in the ECM.47 Indeed, surface plasmon resonance (SPR) studies of the interaction of heparin with various proteins (antithrombin III, avidin, thrombin, and lactoferrin) have shown that protein affinity for heparin is the highest when heparin is immobilized on the biosensor chip via its reducing terminus.48 In contrast, a reduction in affinity is observed when heparin is immobilized via its free amine groups, with further reduction in affinity when heparin is immobilized via its carboxylic acid groups.48 Accordingly, we initially attempted to conjugate LMWH directly to an amine-terminated four-arm star PEG (Mn = 10 000) through LMWH’s reducing terminus via a Schiff base reaction. These reactions were unsuccessful, resulting in only 15–20% conversion; that is, fewer than one in four arms was functionalized. LMWH was therefore modified, via reaction of its free amines, with SMCC to produce maleimide-functionalized LMWH, 1, which was then conjugated to the thiol-terminated four-arm star PEG to yield 2. Although there are a variety of bifunctional cross-linking reagents that could be used for the conjugation, the stability of the maleimide group to hydrolysis coupled with its reactivity toward thiols suggested the utility of this bioconjugation strategy. Indeed, these reactions were both facile and proceeded at sufficient conversions. Furthermore, LMWH immobilized to a biosensor chip via its free amines was shown, via SPR assays, to bind to the HIP peptide employed in the synthesis of 4 (data not shown). The above results, coupled with the requirement for 75% functionalization of the four-arm star PEG with LMWH, provided the motivation for our synthetic approach.
Our initial attempts to obtain 1 using untreated LMWH (Mr = 3000) were unsuccessful due to the lack of free amine groups in LMWH. Hydrazinolysis of GlcNAc residues in LMWH afforded the necessary free amine groups, which was confirmed via the disappearance of the acetyl group resonance at 2.13 ppm via 1H NMR spectroscopy (in D2O) and via a positive ninhydrin assay result.27 The average number of free amine groups per LMWH chain after the N-deacetylation was estimated to be 1.2 based on the area of the acetyl peak that is lost. SEC analysis on the isolated LMWH after the hydrazinolysis showed no reduction in molecular weight. Subsequently, the N-deacetylated LMWH was treated with SMCC to introduce maleimide functionality. The reaction between this product (1) and the thiol-terminated four-arm star PEG was monitored via Ellman’s assay to ensure the highest degree of LMWH functionalization in 2. The test showed that 93% of the thiol groups had been consumed after 2 h. Thiol consumption was not significantly improved by increasing the reaction time or the amount of 1 added to the reaction, perhaps as a result of inaccessibility of the remaining thiol groups to the sterically bulky 1.
Analysis of the reaction mixture (after quenching and dialysis) via SEC yielded two broad fractions of different sizes, which were identified as 2 and unreacted 1 after analysis of the collected fractions via 1H NMR spectroscopy (data not shown). SEC also showed the absence of a peak at the expected elution volume for the thiol-terminated four-arm star PEG, indicating that the star PEG was completely consumed during the reaction. Characterization of the fraction with the largest hydrodynamic volume via 1H NMR spectroscopy showed the expected resonance from the ethylene oxide protons of the star PEG backbone at 3.70 ppm, along with resonances from LMWH at 3.38–5.43 ppm in D2O (Supporting Information). Integration of the relevant peaks (PEG backbone vs. LMWH) in the NMR spectrum indicated an average of 73% functionalization in 2, suggesting that on the average three out of four arms had been successfully functionalized with 1 (Supporting Information). It should be noted that the value of 73% functionalization is very likely underestimated due to overlapping of the PEG peak with the LMWH peaks. Additionally, it is possible that there is a small fraction of LMWH functionalized with two or more maleimide groups. Coupling of these LMWH with greater than one arm of thiol-functionalized PEG star polymer would reduce the number of LMWH molecules conjugated per PEG polymer and might therefore also contribute to the lower-than-expected functionalization values observed. Nevertheless, since on average, a minimum of three arms of the star PEG were functionalized, 2 carries a necessary number of functional arms for the formation of a cross-linked network and was used in the assembly of noncovalent hydrogels with 4.
Synthesis of the PEG–HIP (4)
The functionalization of hydroxy-terminated four-arm star PEG with vinyl sulfone groups was chosen for facile conjugation of the HIP peptide as previously reported, since the reaction of peptides flanked by cysteine with vinyl sulfone groups has been demonstrated to proceed readily and provides a non-hydrolyzable linkage between the PEG and peptide termini.11,37 The degree of vinyl sulfone functionalization in 3 prior to peptide conjugation was determined via 1H NMR spectroscopy. The expected vinyl resonances at 6.15–6.21 and 6.91–6.97 ppm were observed, along with the ethylene oxide resonances of the star PEG backbone at 3.49 ppm in DMSO-d6 (Supporting Information). Integration of the appropriate resonances indicated up to 98% functionalization in 3 with vinyl sulfone; the degree of functionalization depended on the synthetic strategy employed (Experimental Section).
A variety of heparin-binding peptides could be chosen for attachment to 3. The heparin-binding domain of the heparin interacting protein (HIP), CRPKAKAKAKAKDQTK, was chosen for conjugation to 3 to yield 4 based not only on its heparin binding affinity,34 but also on the following observations. (1) The HIP peptide and bFGF binding sequences in heparin are reported to be structurally distinct,33 suggesting that they may not compete for the same binding sequence in heparin. (2) The proposed binding sequences of the HIP peptide and bFGF in heparin are hexa- or pentasaccharides, 33,49 so LMWH employed would likely accommodate at least one molecule of the HIP peptide and/or bFGF (see above).
Accordingly, the HIP peptide sequence above was prepared via standard Fmoc solid-phase peptide chemistry and was purified to > 99.5% purity via HPLC. An N-terminal cysteine residue was included in the sequence to permit coupling to 3 via Michael-type addition as previously reported.11,37 The reaction mixture was easily purified via SEC, as described above, and the NMR spectrum of the purified conjugate showed the ethylene oxide resonances of the PEG at 3.70 ppm in D2O, as well as the aliphatic resonances of the peptide backbone (Supporting Information). Additionally, the vinyl resonances at 6.80–7.36 ppm were of extremely low intensity, indicating that the majority of the vinyl sulfone group (ca. 95%) in 3 had reacted. Integration of different HIP peptide resonances between 1.00 and 2.50 ppm with the PEG backbone resonance revealed an average functionalization of the star PEG with the HIP peptide in 4 to be 77% (regardless of the mode of preparation of 3), indicating that an average of three out of four arms had been functionalized with the HIP peptide (Supporting Information). This degree of functionalization is likely underestimated due to errors in integration of the overlapping peptide and PEG peaks in the NMR spectrum, as described above in the characterization of 2. Despite the heterogeneity in architecture of the resulting product, a sufficient number of arms in 4 were functionalized with the HIP peptide, on average, to support hydrogel formation upon interaction with 2. The PEG–HIP was therefore employed in the assembly of noncovalent hydrogels (see below).
Hydrogel Formation and Growth Factor Binding/Release
Covalently Cross-Linked Hydrogels
The PEG–LMWH conjugate was chemically cross-linked into hydrogels in order to facilitate ELISA assays that would determine if LMWH, after a series of chemical reactions, is capable of binding/sequestering growth factors. The conjugate 2 was chemically cross-linked with PEG methyl ether acrylate via free radical polymerization initiated by potassium persulfate. Radical chain transfer from propagating radicals to ethylene glycol units of both the macromonomer PEG side chain and 2 by abstraction of hydrogen atoms generates PEG radicals which are prone to couple bimolecularly to form an insoluble gel, 6.50,51 Such a hydrogel matrix is covalently modified with LMWH and is therefore an ideal substrate to analyze the ability of modified LMWH to bind a variety of heparin binding proteins. In these investigations, the binding and delivery of basic fibroblast growth factor (bFGF), which is known to induce the proliferation of a wide range of cells including endothelial cells, fibroblasts, smooth muscle cells, and chondrocytes,16 was investigated.
After the polymerization mixture was cured, the resulting gel was washed thoroughly with PBS and then incubated in PBS to fully hydrate the gels prior to growth factor binding and release experiments. The amount of immobilized LMWH (ca. 22 µg per gel), determined via toluidine blue assay, was unchanged after repeated washing and incubation. The growth factor binding of 6, as well as that of a control hydrogel lacking 2, but containing the same amount of free LMWH (22 µg), 7, was evaluated via a sandwich ELISA assay. As seen in Figure 2, 6 exhibited significant bFGF binding relative to 7, indicating that the passively encapsulated LMWH diffused out of 7 during the experiment. This observation is in good agreement with results of toluidine blue assays, which failed to detect any LMWH in 7 after washing with PBS overnight. Although the exact effect of the chemical modification on the binding capacity and binding affinity of LMWH may not be known, this ELISA experiment unambiguously demonstrates that 2 is capable of binding and sequestering bFGF. The hydrogels were also capable of binding VEGF (data not shown), suggesting the general utility of 2 for sequestering heparin-binding proteins.
Figure 2.
bFGF binding to covalently cross-linked PEG hydrogels. Absorbance values reflect the amount of bFGF detected in the hydrogel via a standard ELISA assay. Average values from two experiments are shown for hydrogels containing 21.8 µg of covalently immobilized LMWH (6) and merely passively encapsulated LMWH (7).
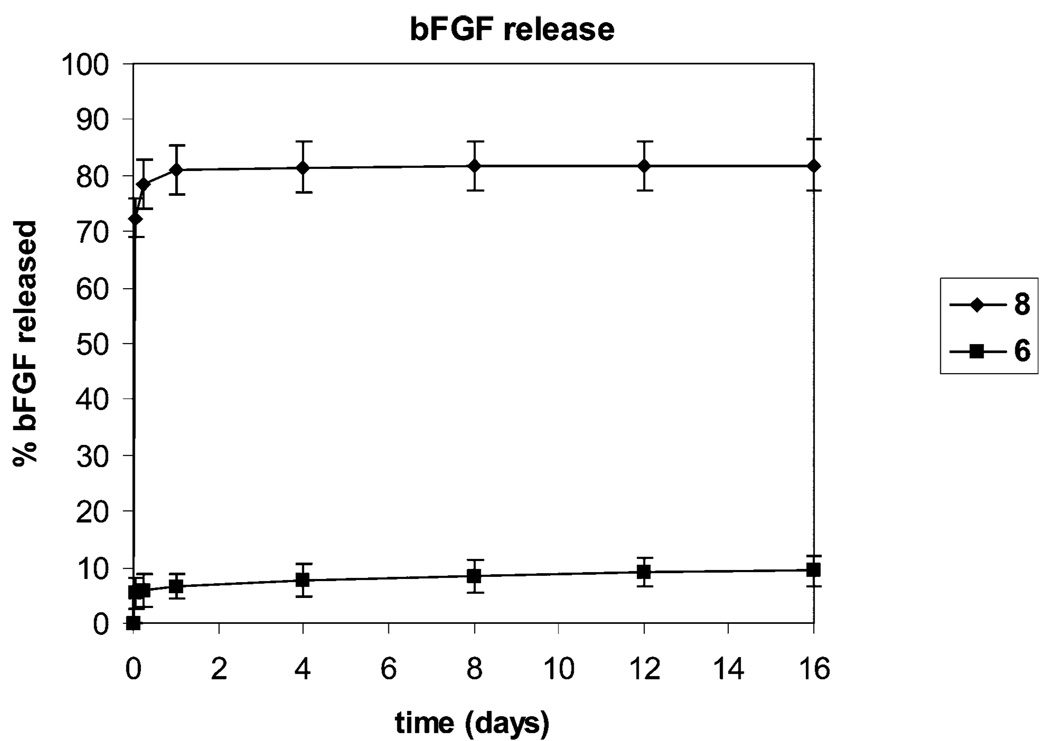
The release profile of bFGF from 6, which has a mole ratio of LMWH:bFGF of ca. 13,000:1, was also measured. The data, shown in Figure 3, show an essentially negligible burst release, after which the release was slowly sustained for at least 16 days with a cumulative release of approximately 8%; a nonheparin-containing control (8) releases nearly 80% of the initially loaded bFGF over the same time period. The results are consistent with previous computational and experimental results which suggest that heparin:bFGF ratios of only 100:1 and 1000:1 are necessary to reduce passive diffusion of bFGF, from materials with a cylindrical geometry, to 4% and 1.4% relative to that due to simple diffusion.8 The binding and slow release of bFGF from gels formed with 2 also indicates the potential for the production of growth-factor releasing hydrogels via additional strategies. The ability of 2 to support formation of noncovalently assembled, erodible hydrogels was therefore investigated to determine how broadly the conjugate could be used in materials design.
Figure 3.
Profile of bFGF release from the covalent hydrogels 6 and 8 (a control gel containing no heparin) as a function of time. Values shown are an average of three separate experiments.
Noncovalently Assembled Hydrogels
Experiments were conducted to determine if 2 was capable of noncovalent association with 4 to form a hydrogel network, 5, that could deliver growth factors. As mentioned above, the heterogeneity of the LMWH termini of 2 provides a functional advantage in these types of investigations, as assembly of hydrogel networks via association of 2 with 4 will not preclude the association of 2 with other heparin-binding peptides and proteins (such as growth factors). Accordingly, solutions containing 2 and 4 were mixed at varying ratios to test their ability to support hydrogel formation (Figure 1). Previous reports of hydrogel formation based on the assembly of similar PEG–peptide conjugates with high molecular weight heparin11 suggested the success of hydrogel formation upon the assembly of 2 and 4. Mixing of a 10 wt % PBS solution of 2 with a 10 wt % PBS solution of 4 at a 1:1 ligand mole ratio of LMWH:HIP immediately resulted in the production of a self-supporting hydrogel that excluded a significant fraction of water. Solutions with 2.5 and 10 wt % of 2 and 4, respectively, were also mixed to yield a final polymer concentration of 2.7 wt % and a ligand mole ratio of 8:2 (LMWH:HIP), to test the feasibility of producing a hydrogel at a much lower concentration and with excess LMWH functionality. Even at the low concentration of 2.7 wt %, the assembly of these molecules resulted in the formation of a self-supporting hydrogel (Supporting Information); water exclusion from this gel was not observed for the small volume gels employed in these experiments. The hydrogel behavior of mixtures of 2 and 4 was also confirmed via rheological measurements; the hydrogels exhibited moderate storage moduli (ca. 200 Pa) that exceed the loss moduli across all measured frequencies52 and at temperatures up to 37 °C. In contrast, mixtures of LMWH with 4 did not exhibit any detectable hydrogel behavior, confirming that the attachment of LMWH to the star PEG is required for hydrogel formation. The details of the rheological characterization of this and a variety of other hydrogels are not included here but are detailed in a companion manuscript. Based on the observed and measured hydrogel properties of the 8:2 mixture, and its excess heparin functionality, the 8:2 hydrogel preparation was used in all subsequent experiments.
The experimental results shown in Figure 2 indicate the successful binding of bFGF to 2, which supports the premise that bFGF could also be delivered from noncovalently assembled matrices that contain 2. Accordingly, a noncovalently associated network, 5, was also prepared in the presence of bFGF. Given that 2 is not covalently immobilized in this network, the release of 2 via erosion of 5 is likely; a very high ratio of LMWH:bFGF was therefore employed in order to fully sequester the bFGF. In 5 the mole ratio of LMWH to HIP peptide to bFGF (10 ng) is 1 800 000: 460 000:1. The HIP peptide is limiting in the assembly process, and therefore excess LMWH should be available for binding to bFGF. With the assumption that there exists one HIP peptide binding sequence per LMWH chain, the theoretical mole ratio of free LMWH to bFGF in 5 is 1 340 000:1, which represents a more than a 100-fold increase in mole ratio of free LMWH to bFGF when compared to 6.
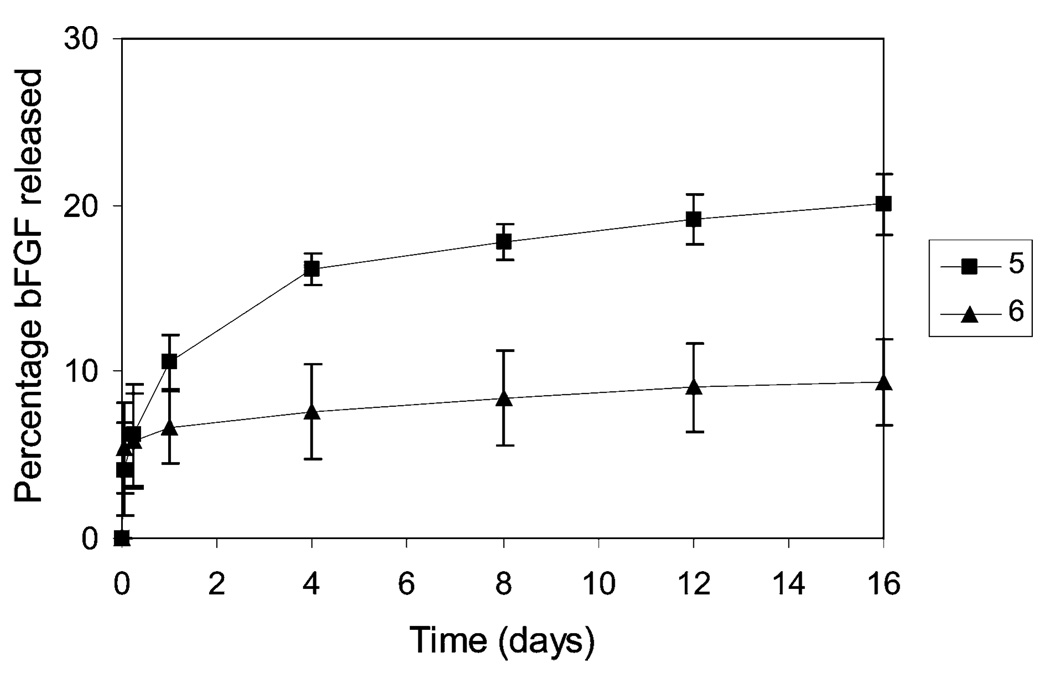
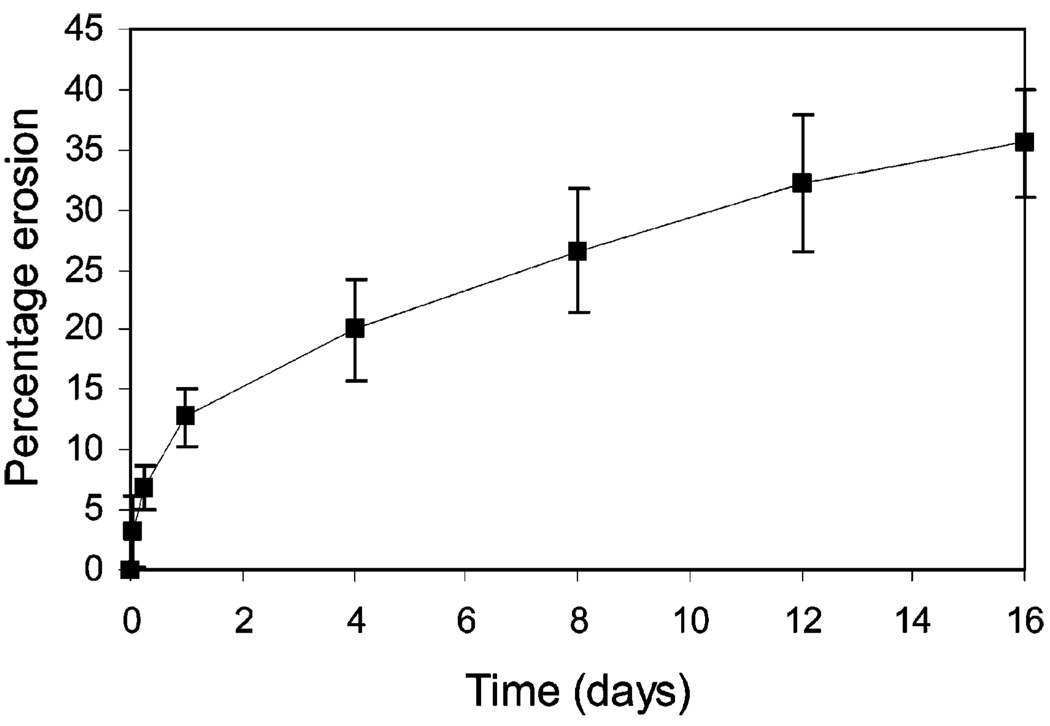
The release kinetics, if governed primarily by the LMWH: bFGF ratio, should be correspondingly slower for bFGF release from 5 relative to those from hydrogel 6 with LMWH:bFGF ratios of 13,000:1. As shown in Figure 4, however, bFGF release rates from 5 are higher than those from hydrogel 6, suggesting that bFGF release from this hydrogel is associated with matrix deterioration of the noncovalently associated system. The matrix erosion kinetics were examined to confirm the correlation between matrix erosion and the bFGF release trends observed for 5; Figure 5 shows the erosion profile for 5. A comparison of the data in Figures 4 and 5 indicates that bFGF release is closely associated with matrix erosion as a function of time. For example, matrix erosion resulted in the net weight loss of approximately 13% at 24 h. This net weight loss represents 10.9 wt % of 2 and 1.8 wt % of 4 based on the assumption that the assembled hydrogel erodes uniformly to give the individual molecules 2 and 4 at mole ratio of 8:2 (LMWH:HIP). Provided that bFGF is evenly sequestered in 5, 10.9 wt % of the initially loaded bFGF may be released in the form of the bFGF/2 complex solely due to matrix erosion. This analysis appears plausible, as the cumulative release of bFGF from 5 at 24 h is 10.6% (Figure 4) and diffusion-based release of bFGF from the highly heparinized hydrogel 6 at 24 h was shown to be insignificant (Supporting Information). After 4 days, the net losses of 2 (16.8 wt %) due to matrix erosion still closely correlate with the cumulative release of bFGF (16.1%). Although no concise explanation can be offered for the less than expected cumulative release of bFGF from 5 after 8 days, a significant amount of bFGF continues to be released even after 8 days due to matrix erosion.
Figure 4.
Profiles of bFGF release from the hydrogels 5 and 6 as a function of time. Values shown are an average of three separate experiments.
Figure 5.
Matrix erosion profile of the assembled hydrogel 5 as a function of time. The average values from three separate experiments are shown.
On the basis of these results, bFGF delivery from the assembled hydrogel appears to be primarily a matrix erosion controlled process in which the growth factor is released in conjunction with 2. The co-release of bFGF and 2 may have beneficial consequences on the bioactivity of the growth factor via stabilization of bFGF in solution. The rates of release of bFGF from the assembled hydrogel networks are similar to those reported for other heparin-containing delivery systems that have demonstrated utility for stimulation of endothelial cell growth in vitro and neovascularization in vivo.6,15,16 These binding and release results, coupled with the successful noncovalent assembly of hydrogels upon interaction of 2 with 4, indicate that 2 is capable of binding multiple types of heparin-binding molecules, and suggests that the bioconjugate may be generally useful for hydrogel assembly mediated by other heparin-binding peptides and proteins. The use of 2 in noncovalently assembled hydrogels therefore offers additional opportunities for the production of fully erodible matrices with erosion and delivery profiles that can be engineered based on designed peptide-heparin interactions. The combination of growth factor binding and opportunities for both covalent and noncovalent hydrogel formation suggests the potential for 2 in a variety of biomedically relevant applications.
Conclusions
These studies demonstrate that the synthesis of multifunctional PEG–LMWH bioconjugate provides multiple routes to bioactive materials. First, LMWH-decorated four-arm star PEG was prepared and was shown to be capable of a high level of binding to the heparin binding growth factor bFGF. The PEG–LMWH conjugate was shown to be competent for the assembly of erodible hydrogels via interactions with the PEG–HIP conjugate. Finally, the assembled hydrogel formed between the two constituent molecules was demonstrated to sequester and deliver bFGF in a controlled manner. The release kinetics of bFGF closely matched the matrix erosion kinetics mediated by dissociation of the constituent molecules, indicating matrix erosion as a primary mechanism by which bFGF is released and suggesting the potential for controlling hydrogel assembly, erosion, and delivery on the basis of specific peptide-heparin interactions. This approach may also be extended to delivery of other therapeutically important heparin binding proteins.
Supplementary Material
Acknowledgment
This work was supported by grants from the National Institutes of Health (5 P20 RR15588 and 1 R01 EB003172-01) and the Arnold and Mabel Beckman Foundation Young Investigator program. Alyssa Panitch and Brandon Seal are thanked for their helpful discussions. Cindy Farach-Carson and Weidong Yang are acknowledged for their assistance with sandwich ELISA assays. Le Zhang is thanked for collection of SPR data.
Footnotes
Supporting Information Available. Experimental procedures and results. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Grande D, Baskaran S, Baskaran C, Gnanou Y, Chaikof EL. Macromolecules. 2000;33:1123–1125. [Google Scholar]
- 2.Grande D, Baskaran S, Chaikof EL. Macromolecules. 2001;34:1640–1646. [Google Scholar]
- 3.Shu XZ, Liu YC, Palumbo FS, Lu Y, Prestwich GD. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Capila I, Linhardt RJ. Angew. Chem., Int. Ed. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, Yura H, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Biomaterials. 2004;25:699–706. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara M, Obara K, Ishizuka T, Fujita M, Sato M, Masuoka K, Saito Y, Yura H, Matsui T, Hattori H, Kikuchi M, Kurita A. J. Biomed. Mater. Res. Part A. 2003;64A:551–559. doi: 10.1002/jbm.a.10427. [DOI] [PubMed] [Google Scholar]
- 7.Schense JC, Hubbell JA. Bioconjugate Chem. 1999;10:75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 8.Sakiyama-Elbert SE, Hubbell JA. J. Controlled Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 9.Sakiyama-Elbert SE, Hubbell JA. Annu. Rev. Mater. Res. 2001;31:183–201. [Google Scholar]
- 10.Halstenberg S, Panitch A, Rizzi S, Hall H, Hubbell JA. Biomacromolecules. 2002;3:710–723. doi: 10.1021/bm015629o. [DOI] [PubMed] [Google Scholar]
- 11.Seal BL, Panitch A. Biomacromolecules. 2003;4:1572–1582. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 12.Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y. J. Biomed. Mater. Res. 2001;56:216–221. doi: 10.1002/1097-4636(200108)56:2<216::aid-jbm1086>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Wissink MJB, Beernink R, Pieper JS, Poot AA, Engbers GHM, Beugeling T, van Aken WG, Feijen J. Biomaterials. 2001;22:151–163. doi: 10.1016/s0142-9612(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 14.Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. J. Biomed. Mater. Res. 2002;62:128–135. doi: 10.1002/jbm.10238. [DOI] [PubMed] [Google Scholar]
- 15.Chinen N, Tanihara M, Nakagawa M, Shinozaki K, Yamamoto E, Mizushima Y, Suzuki Y. J. Biomed. Mater. Res. Part A. 2003;67A:61–68. doi: 10.1002/jbm.a.10061. [DOI] [PubMed] [Google Scholar]
- 16.Wissink MJB, Beernink R, Poot AA, Engbers GHM, Beugeling T, van Aken WG, Feijen J. J. Controlled Release. 2000;64:103–114. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 17.Magoshi T, Matsuda T. Biomacromolecules. 2002;3:976–983. doi: 10.1021/bm0200377. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda T, Magoshi T. Biomacromolecules. 2002;3:942–950. doi: 10.1021/bm0200229. [DOI] [PubMed] [Google Scholar]
- 19.Zamora PO, Tsang R, Pena LA, Osaki S, Som P. Bioconjugate Chem. 2002;13:920–926. doi: 10.1021/bc0155216. [DOI] [PubMed] [Google Scholar]
- 20.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Science. 1998;281:389–392. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 21.Kopecek J. Eur. J. Pharm. Sci. 2003;20:1–16. doi: 10.1016/s0928-0987(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 22.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Biomaterials. 2002;23:4203–4210. doi: 10.1016/s0142-9612(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 23.Harris JM, Chess RB. Nat. Rev. Drug Discovery. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara M, Ono K, Ishikawa K, Hattori H, Saito Y, Yura H, Akaike T, Ozeki Y, Tanaka S, Mochizuki H, Kurita A. J. Biochem. 2000;127:797–803. doi: 10.1093/oxfordjournals.jbchem.a022672. [DOI] [PubMed] [Google Scholar]
- 25.Ishihara M, Sato M, Hattori H, Saito Y, Yura H, Ono K, Masuoka K, Kikuchi M, Fujikawa K, Kurita A. J. Biomed. Mater. Res. 2001;56:536–544. doi: 10.1002/1097-4636(20010915)56:4<536::aid-jbm1125>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Kariya Y, Herrmann J, Suzuki K, Isomura T, Ishihara M. J. Biochem. 1998;123:240–246. doi: 10.1093/oxfordjournals.jbchem.a021928. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser E, Colescot RL, Bossinge CD, Cook PI. Anal. Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 28.Ellman GL. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Julian J, Rohde LH, Carson DD. FASEB J. 1994;8:A1405. [Google Scholar]
- 30.Liu S, Carson DD. FASEB J. 1994;8:A872. [Google Scholar]
- 31.Liu S, Smith SE, Rohde LH, Julian J, Carson DD. FASEB J. 1995;9:A1504. [Google Scholar]
- 32.Liu SC, Smith SE, Julian J, Rohde LH, Karin NJ, Carson DD. J. Biol. Chem. 1996;271:11817–11823. doi: 10.1074/jbc.271.20.11817. [DOI] [PubMed] [Google Scholar]
- 33.Liu SC, Zhou FY, Hook M, Carson DD. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1739–1744. doi: 10.1073/pnas.94.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu SC, Hoke D, Julian J, Carson DD. J. Biol. Chem. 1997;272:25856–25862. doi: 10.1074/jbc.272.41.25856. [DOI] [PubMed] [Google Scholar]
- 35.Liu SC, Julian J, Carson DD. J. Biol. Chem. 1998;273:9718–9726. doi: 10.1074/jbc.273.16.9718. [DOI] [PubMed] [Google Scholar]
- 36.Morpurgo M, Veronese FM, Kachensky D, Harris JM. Bioconjugate Chem. 1996;7:363–368. doi: 10.1021/bc9600224. [DOI] [PubMed] [Google Scholar]
- 37.Lutolf MP, Hubbell JA. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 38.Duncan AC, Boughner D, Campbell G, Wan WK. Eur. Polym. J. 2001;37:1821–1826. [Google Scholar]
- 39.Kemeny DM. A practical guide to ELISA. 1st ed. London, England: Pergamon Press; 1991. [Google Scholar]
- 40.Raboudi N, Julian J, Rohde LH, Carson DD. J. Biol. Chem. 1992;267:11930–11939. [PubMed] [Google Scholar]
- 41.Tay SW, Merrill EW, Salzman EW, Lindon J. Biomaterials. 1989;10:11–15. doi: 10.1016/0142-9612(89)90003-3. [DOI] [PubMed] [Google Scholar]
- 42.Nadkarni VD, Linhardt RJ. Biotechniques. 1997;23:382–385. doi: 10.2144/97233bm07. [DOI] [PubMed] [Google Scholar]
- 43.Turnbull JE, Fernig DG, Ke YQ, Wilkinson MC, Gallagher JT. J. Biol. Chem. 1992;267:10337–10341. [PubMed] [Google Scholar]
- 44.Maccarana M, Casu B, Lindahl U. J. Biol. Chem. 1993;268:23898–23905. [PubMed] [Google Scholar]
- 45.Fairbrother WJ, Champe MA, Christinger HW, Keyt BA, Starovasnik MA. Structure. 1998;6:637–648. doi: 10.1016/s0969-2126(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 46.Reantragoon S, Arrigo LM, Dweck HS, Rosenfeld L. Arch. Biochem. Biophys. 1996;327:234–238. doi: 10.1006/abbi.1996.0115. [DOI] [PubMed] [Google Scholar]
- 47.Nadkarni VD, Pervin A, Linhardt R. J. Anal. Biochem. 1994;222:59–67. doi: 10.1006/abio.1994.1454. [DOI] [PubMed] [Google Scholar]
- 48.Osmond RIW, Kett WC, Skett SE, Coombe DR. Anal. Biochem. 2002;310:199–207. doi: 10.1016/s0003-2697(02)00396-2. [DOI] [PubMed] [Google Scholar]
- 49.Faham S, Hileman RE, Fromm JR, Linhardt RJ, Rees DC. Science. 1996;271:1116–1120. doi: 10.1126/science.271.5252.1116. [DOI] [PubMed] [Google Scholar]
- 50.Bo G, Wesslen B, Wesslen KB. J. Polym. Sci. Part A-Polym. Chem. 1992;30:1799–1808. [Google Scholar]
- 51.Luo N, Hutchison JB, Anseth KS, Bowman CN. Macromolecules. 2002;35:2487–2493. [Google Scholar]
- 52.Yamaguchi N, Chae B-S, Zhang L, Kiick KL, Furst EM. Biomacromolecules. 2005;6:1931–1940. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.