Abstract
Objective
Cognitive impairments in schizophrenia are associated with lower expression of markers of γ-aminobutyric acid (GABA) synthesis in the prefrontal cortex. The effects of GABA are mediated by GABAA receptors that mediate either phasic or tonic inhibition. The authors assessed the expression of GABAA receptor α4 and δ subunits, which coassemble to form receptors mediating tonic inhibition, in schizophrenia.
Method
The authors used in situ hybridization to quantify expression patterns of GABAA receptor α4 and δ subunits in pre-frontal cortex from 23 matched pairs of schizophrenia and comparison subjects.
Results
Levels of δ mRNA were significantly lower in schizophrenia subjects regardless of medication use, whereas α4 mRNA levels were lower only in subjects with schizophrenia receiving certain medications at the time of death. To understand the nature of this unexpected dissociation between α 4 and δ subunit expression in schizophrenia, the authors used similar methods to quantify α4 and δ mRNA levels in multiple animal models. During postnatal development of monkey prefrontal cortex, levels of α4 mRNA decreased, whereas δ mRNA levels increased. In addition, δ mRNA levels, but not α4 mRNA levels, were lower in the medial frontal cortex of mice with a genetic deletion of the GABAA receptor α1 subunit, and neither δ nor α4 mRNA levels were altered in rodent models of altered excitatory neurotransmission.
Conclusions
Since GABAA receptor α1 subunits also have lower mRNA levels in schizophrenia, show increased expression with age in monkey prefrontal cortex, and can coassemble with δ subunits to form functional GABAA receptors, lower δ mRNA levels in schizophrenia might reflect a reduced number of α1βxδ GABAA receptors that could contribute to deficient tonic inhibition and prefrontal cortical dysfunction in schizophrenia.
Deficits in certain cognitive functions, such as working memory, are core features of schizophrenia (1). Alterations in the inhibitory circuitry of the dorsolateral pre-frontal cortex may contribute to the impairments in working memory since optimal levels of γ-aminobutyric acid (GABA) neurotransmission in this cortical region are essential for normal working memory performance (2, 3). Consistent with this idea, postmortem studies have consistently shown lower expression of the mRNA for the 67-kDa isoform of glutamate decarboxylase (GAD67), the principal enzyme responsible for the synthesis of GABA, and for the GABA membrane transporter 1 (GAT-1), in the dorsolateral prefrontal cortex of subjects with schizophrenia (see reference 4 for a review).
Understanding the functional significance of these pre-synaptic alterations requires knowledge of the expression levels of postsynaptic GABAA receptors. GABAA receptors are ligand-gated chloride ion channels assembled from different subunit classes that most commonly include 2α, 2β, and 1γ or 1δ subunits (5). Different combinations of subunits form GABAA receptors with unique properties. For instance, GABAA receptors containing a γ2 subunit predominantly mediate phasic inhibition, defined as the rapid and synchronous opening of synaptic receptors that result in an inhibitory postsynaptic potential (6). In contrast, δ-containing GABAA receptors mediate tonic inhibition, defined as the constant activation of extrasynaptic receptors that, by increasing input conductance, reduces the probability of generating an action potential (6). Tonic inhibition mediated by δ-containing receptors has been described in many cell types, including neocortical pyramidal cells (7, 8).
Expression of GABAA δ subunit mRNA was reported to be significantly lower in the dorsolateral prefrontal cortex of subjects with schizophrenia in two microarray studies (9, 10). Because δ subunits are thought to preferentially coassemble with α4 subunits in forebrain GABAA receptors (11, 12), lower δ mRNA levels in subjects with schizophrenia could represent a reduced complement of α4βxδ GABAA receptors in the illness. However, although α4 subunit mRNA levels were also lower by microarray in the dorsolateral prefrontal cortex of the same subjects with schizophrenia, the deficit was not correlated with that of the δ subunit (10). Furthermore, it is unclear what pathogenetic mechanisms give rise to lower levels of these subunits in schizophrenia.
In this study, in order to understand the nature of the apparent dissociation between lower levels of α4 and δ subunits in schizophrenia, we 1) evaluated the expression patterns of α4 and δ mRNAs in the dorsolateral prefrontal cortex of a larger cohort of subjects with schizophrenia and 2) explored the relationship between, and the determinants of, α4 and δ subunit mRNA expression in multiple animal models.
Method
Human Subjects
With the consent of the next of kin, brain tissue specimens were obtained from the Allegheny County Medical Examiner’s Office in Pittsburgh at the time of autopsy. Twenty-three subjects with schizophrenia were each matched with one comparison subject for sex, and as closely as possible for age and postmortem interval. (Details on all subjects are provided in Table S1 of the data supplement that accompanies the online edition of this article.) Subjects with schizophrenia did not differ significantly from comparison subjects in age, postmortem interval, brain pH, RNA integrity number (RIN), or tissue storage time at −80°C (Table 1). An independent panel of experienced research clinicians made consensus DSM-IV diagnoses for each subject using medical records and structured interviews conducted with one or more surviving family members (13). All procedures were approved by the Committee for Oversight of Research Involving the Dead and the Institutional Review Board for Biomedical Research at University of Pittsburgh.
TABLE 1.
Characteristics of Comparison Subjects and Subjects With Schizophrenia Matched for Age, Sex, and Postmortem Interval
| Characteristic | Comparison Subjects (N=23) | Subjects With Schizophrenia (N=23) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Male | 17 | 74 | 17 | 74 |
| White | 18 | 78 | 18 | 78 |
| Mean | SD | Mean | SD | |
| Age (years) | 48.0 | 15.5 | 47.9 | 14.1 |
| Postmortem interval (hours) | 18.0 | 5.5 | 17.8 | 9.3 |
| Brain pH | 6.9 | 0.2 | 6.8 | 0.3 |
| RNA integrity number | 8.7 | 0.4 | 8.4 | 0.7 |
| Storage time (months at −80°C) | 92.4 | 23.5 | 96.6 | 23.6 |
In Situ Hybridization
The right prefrontal cortex of each human brain was blocked and frozen, serial sections (20 μm) were thaw-mounted onto glass slides, and sections containing area 9 were identified in Nissl-stained sections as previously described (14). Templates for the synthesis of α4 and δ subunit riboprobes were obtained by polymerase chain reaction with specific primer sets. A 516-bp fragment for the α4 subunit corresponding to bases 1231–1746 of the human gene (GenBank NM_000809) and a 607-bp DNA fragment for the δ subunit corresponding to bases 419–1025 of the human gene (GenBank BC033801) were amplified. Nucleotide sequencing revealed 100% homologies for the amplified fragments to the previously reported sequences. DNA fragments were subcloned into the plasmid pSTBlue-1 (Novagen, Madison, Wisc.). Sense and antisense riboprobes were transcribed in vitro in the presence of (35)S-CTP (Amersham Biosciences, Piscataway, N.J.), digested with DNase I, and purified by centrifugation through RNeasy mini spin columns (Qiagen, Valencia, Calif.).
For each transcript, we used three tissue sections per subject, spaced at approximately 560 μm. Sections from a given pair were always processed together; six runs were performed for each transcript. In situ hybridization was performed as previously described (15; see also the Supplemental Method section in the online data supplement). Briefly, radioactivity of hybridized probes was detected by autoradiographic films and then by nuclear emulsion. Film optical density measures were obtained within area 9 and expressed as nanocuries per gram of tissue by reference to radioactive carbon-14 standards (ARC Inc., St. Louis) exposed on the same film. To assess mRNA levels in each cortical layer, three cortical traverses, 1.5 mm in width, were sampled from each section (nine traverses per subject). Each cortical traverse was located in portions of the tissue section cut perpendicular to the pial surface, as determined by the presence of pyramidal neurons with vertically oriented apical dendrites on adjacent Nissl-stained sections. The average mRNA level within each layer was determined by measuring optical density in zones located 10%–20% (layer 2), 20%–50% (layer 3), 50%–60% (layer 4), 60%–80% (layer 5), and 80%–100% (layer 6) from the pial surface to the white matter border (14). All cortical optical density measures were corrected by subtracting optical density measures in the white matter.
Statistical Analyses
Two analysis of covariance (ANCOVA) models were performed to examine the expression differences in α4 and δ mRNAs between subject groups. The mean film optical density measures from the three sections per subject were used as the dependent variables. The first model had diagnostic group as main effect, pair as blocking effect (pair reflects the matching of individual subject pairs for sex, age, and postmortem interval), and pH, RIN, and freezer storage time as covariates. Because subject pairing may be considered an attempt to balance the two diagnostic groups with regard to the experimental factors instead of a true statistical paired design, a second ANCOVA model was performed with diagnostic group as main effect and sex, age, postmortem interval, pH, RIN, and storage time as covariates. The two models produced similar results for diagnostic group effect. Because the effect of age on δ mRNA expression was significant, the results of the second unpaired model are reported.
Two-sample t tests were used to assess the influence of sex, diagnosis of schizoaffective disorder, suicide, history of alcohol abuse or dependency, and presence of benzodiazepines, mood stabilizers, or antidepressants at the time of death on the within-subject pair differences in gene expression levels.
Nonhuman Primate Models
Expression levels of the mRNAs of interest were assessed in two cohorts of monkeys. The first cohort consisted of 18 experimentally naive, young adult, male long-tailed macaque monkeys (Macaca fascicularis) that were exposed to twice-daily oral doses of haloperidol, olanzapine, or placebo for 17–27 months and then euthanized (16). At steady state, trough serum levels were ~1.5 ng/ml for haloperidol and ~15 ng/ml for olanzapine. The second cohort consisted of 31 female rhesus macaque monkeys (Macaca mulatta) ranging in age from birth through adult (see Table S2 in the online data supplement). Details of the in situ hybridization procedures and analytic methods for both cohorts are provided in the Supplemental Method section of the online data supplement.
Rodent Models
We analyzed mRNA levels in mice with a targeted deletion of the GABAA α1 subunit or genetically reduced levels of the N-methyl-D-aspartate (NMDA) receptor Nr1 subunit (Nr1 hypomorphic mice) and in adult rats with neonatal hippocampal lesions or peripubertal lesions of the mediodorsal thalamic nucleus. Details of each model and experimental procedures are provided in the Supplemental Method section of the online data supplement.
Results
Specificity of Human Riboprobes
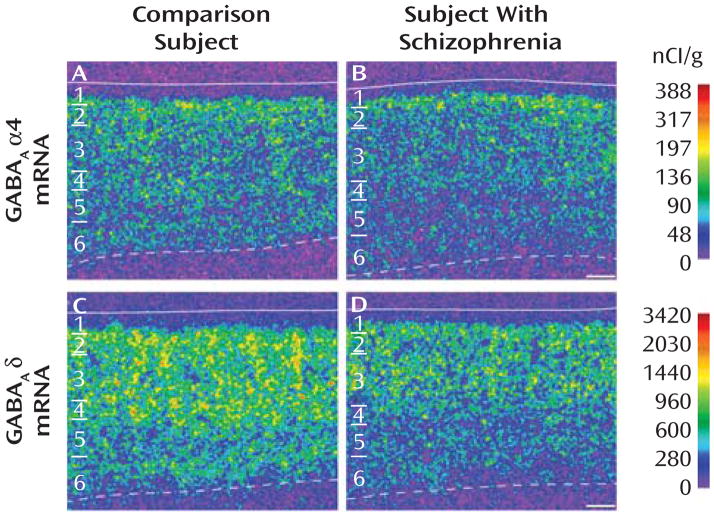
The specificity of the riboprobes for the α4 and δ subunits was confirmed by several findings. First, clusters of silver grains were present over neurons, but not over glia, which are known not to express these transcripts (see Figure S1A, C in the online data supplement). Second, signal above background was not detected in tissue processed with sense riboprobes for α4 or δ subunits (see Figure S2B, D in the online data supplement). Third, consistent with previous reports in primates and humans (17, 18), the expression of α4 mRNA was uniform across layers 2–5, lower in layer 6, and absent in layer 1 (Figure 1A). Similarly, the laminar distribution of δ mRNA was consistent with that previously described in human cortex (18): high and uniform across layer 2 to layer 4, low in layer 5, moderate in layer 6, and absent in layer 1 (Figure 1C).
FIGURE 1. Representative Autoradiograms Illustrating Expression of α4 and δ mRNAs in Dorsolateral Prefrontal Cortex Area 9 of a Comparison Subject and a Subject With Schizophreniaa.
a Subjects were matched for age, sex, and postmortem interval. The intensity of hybridization signals is presented in a pseudocolor manner according to the calibration scales (nCi/g) for each mRNA. The solid lines represent the pial surface and the dotted lines represent the border between gray matter and white matter. The six cortical layers are identified on the left in each panel. Scale bars= 500 μm.
Levels of α4 and δ mRNAs in Schizophrenia
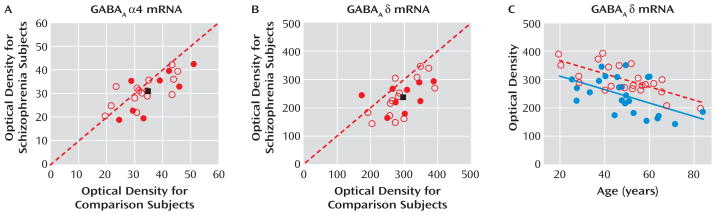
The mean α4 mRNA expression level in area 9 was only 9% lower in subjects with schizophrenia (31.2 nCi/g [SD= 7.0]) than in matched comparison subjects (34.6 nCi/g [SD=8.5]), and this difference did not reach statistical significance (F=3.42, df=1, 38, p=0.072) (Figure 2A). In contrast, the mean expression level of δ mRNA (Figure 2B) was significantly (F=15.95, df=1, 38, p<0.001) lower by 19% in the schizophrenia subjects (238.15 nCi/g [SD=63.29]) than in comparison subjects (295.13 nCi/g [SD=52.96]) (Figure 2B).
FIGURE 2. Film Autoradiogram Optical Density Measures (nCi/g) for α4 and δ mRNAs in Dorsolateral Prefrontal Cortex Area 9 for Comparison Subjects and Subjects With Schizophreniaa.
a In panels A and B, open red circles represent subject pairs with the mRNA level for the comparison subject denoted on the x-axis and the mRNA level for the subject with schizophrenia on the y-axis. Closed red circles represent pairs of a comparison subject and a subject with schizoaffective disorder. The closed black squares represent the group mean mRNA levels for comparison subjects and subjects with schizophrenia and schizoaffective disorder. Lower mRNA levels in subjects with schizophrenia or schizoaffective disorder are indicated by markers below the diagonal unity line. For the analysis in panel A, F=3.42, df=1, 38, p=0.072; in panel B, F=15.95, df=1, 38, p<0.001. As shown in panel C (open red circles represent comparison subjects, closed blue circles represent subjects with schizophrenia), the mRNA expression levels of δ were negatively correlated with age. The regression line for δ mRNA in subjects with schizophrenia (r=−30, p=0.007) is parallel to and shifted downward from that for comparison subjects (r=−0.48, p<0.001), suggesting that the decreased expression of δ mRNA in schizophrenia is similar in magnitude across adult life.
The ANCOVAs also revealed a significant effect of age (F=19.56, df=1, 38, p<0.0001) on δ mRNA expression levels. Interestingly, levels of δ mRNA were significantly negatively correlated with age in both comparison subjects (r= −0.48, p<0.001) and schizophrenia subjects (r=−0.30, p= 0.007), with the regression line in the schizophrenia subjects parallel to and shifted downward from that for comparison subjects (Figure 2C). This finding suggests that the disease-related reduction in δ mRNA levels is similar in magnitude across adult life.
Influence of Confounding Factors on α4 and δ mRNA Expression
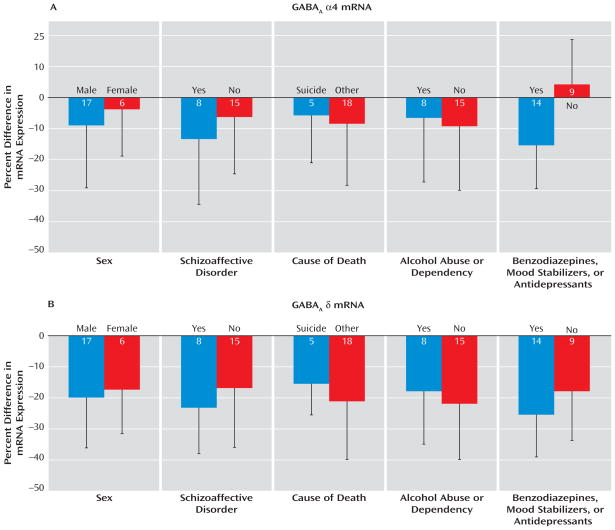
The within-subject pair differences in α4 mRNA expression were not influenced by sex, diagnosis of schizoaffective disorder, cause of death, or history of alcohol abuse or dependency (Figure 3A). However, the levels of α4 mRNA were decreased only in subjects with schizophrenia receiving benzodiazepines, mood stabilizers, or antidepressants at the time of death. In these subjects, the levels of α4 mRNA subunit were 16% lower relative to their matched comparison subjects. This within-subject pair difference was significantly different (t=−2.93, df=21, p=0.008) from the 4% increase in α4 mRNA levels in the subjects with schizophrenia not receiving these medications at time of death (Figure 3A). These findings suggest that the lower α4 mRNA levels in some subjects with schizophrenia represent a medication effect and not the disease process.
FIGURE 3. Effect of Confounding Factors on the Within-Subject Pair Percent Differences in Levels of α4 and δ mRNAs in Subjects With Schizophrenia and Matched Comparison Subjectsa.
a Whereas the mean within-subject pair percent differences in α4 mRNA were not affected by sex, diagnosis of schizoaffective disorder, cause of death, or a history of alcohol abuse or dependency (no significant differences), we found a significant effect of treatment with benzodiazepines, mood stabilizers, or antidepressants at time of death (t=−2.93, df=21, p=0.008). In contrast, subjects with schizophrenia showed a similar decrease in δ mRNA levels relative to their matched comparison subjects, independent of the presence or absence of each factor analyzed (no significant differences).
In contrast, the mean within-subject pair difference in δ mRNA expression was not influenced by sex, diagnosis of schizoaffective disorder, cause of death, alcohol abuse or dependency, or use of benzodiazepines, mood stabilizers, or antidepressants (Figure 3B). In addition, levels of δ mRNA in the dorsolateral prefrontal cortex did not differ among monkeys chronically exposed to haloperidol, olanzapine, or placebo (see Figure S2 in the online data supplement). Together, these findings suggest that the reduction in δ mRNA expression in subjects with schizophrenia reflects the underlying disease process.
Laminar Analysis of δ mRNA Expression in Schizophrenia
Because the lower levels of δ mRNA in subjects with schizophrenia did not seem to be a consequence of confounding factors, we assessed the expression of this transcript across cortical layers. Levels of δ mRNA were significantly lower in layer 3 (F=9.35, df=1, 38, p=0.004), layer 4 (F= 10.84, df=1, 38, p=0.002), layer 5 (F=23.52, df=1, 38, p<0.0001), and layer 6 (F=10.56, df=1, 38, p=0.003), but not in layer 2 (F=1.84, df=1, 38, p=0.184), in subjects with schizophrenia (see Figure S3 in the online data supplement).
Postnatal Development of α 4 and δ mRNAs in Monkey Dorsolateral Prefrontal Cortex
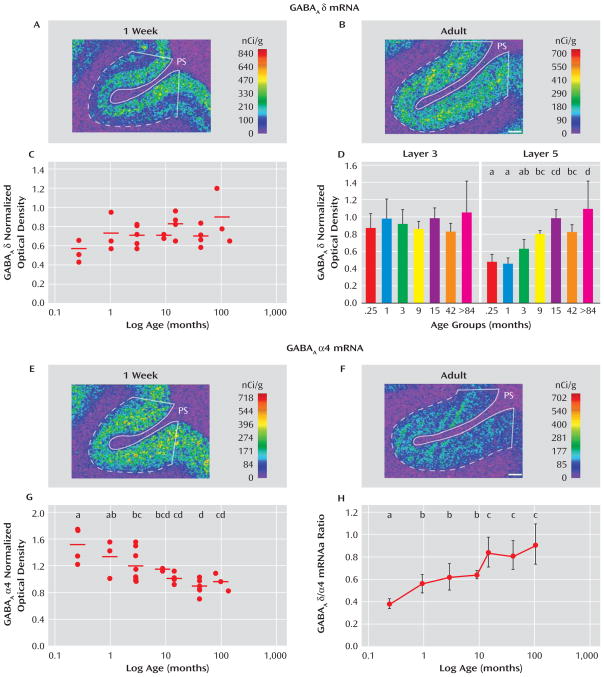
In order to understand the dissociation in expression of α4 and δ mRNAs in subjects with schizophrenia, we compared the postnatal developmental trajectories of these two subunits in monkey dorsolateral prefrontal cortex. The expression of δ mRNA increased during postnatal development (Figure 4A, B), with the mean overall cortical levels of δ mRNA 56% greater in the adult animals than those 1 week of age. However, the effect of age did not quite achieve statistical significance (F=2.45, df=6, 21, p= 0.059, Figure 4C), perhaps because δ mRNA levels appeared to increase only in the deep layers (Figure 4A, B). Consistent with this interpretation, δ mRNA levels did not change with age in layer 3 but significantly (F=7.32, df=6, 20, p<0.001) increased by 116% between 1 week of age and adulthood in layer 5 (Figure 4D).
FIGURE 4. Expression Patterns of δ and α4 mRNAs Across Postnatal Development in the Monkey Dorsolateral Prefrontal Cortexa.
a The overall gray matter levels of δ mRNA increased from 1 week of age to adulthood (panels A and B), although these changes did not quite reach statistical significance (panel C; F=2.45, df=6, 21, p=0.059). However, the levels significantly increased with age in layer 5 (panel D; F= 7.32, df=6, 20, p<0.001) but did not change in layer 3 (F=2.41, df=6, 20, p=0.065). In contrast, the expression levels of α4 decreased significantly with age (panels E, F, and G). The opposing developmental trajectories of δ and α4 mRNAs resulted in a significant increase in the δ to α4 ratio with age (panel H). In panels A, B, E, and F, the density of hybridization signals are presented in a pseudocolor manner according to the calibration scales (nCi/g). The solid white lines represent the pial surface (PS), and the dotted lines represent the border between gray matter and white matter. In panels C and G, the mean values for each subject group are represented by horizontal bars. Within each panel, age groups not sharing the same letter are significantly different at p<0.05; panel G, F=5.64, df=6, 21, p=0.0012; panel H, F=4.80, df=6, 21, p= 0.003. Scale bars=1 mm.
In contrast, the mRNA expression levels of α4 decreased during postnatal development across all cortical layers (Figure 4E, F). Between 1 week of age and adulthood, the mRNA levels of α4 significantly (F=5.64, df=6, 21, p= 0.0012) decreased by 36% (Figure 4G). The opposing trajectories of α4 and δ mRNAs resulted in a marked change in the ratio of δ to α4 mRNA levels across development. Between 1 week of age and adulthood, the ratio of δ to α4 mRNA levels significantly (F=4.80, df=6, 21, p=0.003) increased by 199% (Figure 4H).
The mRNA expression levels of δ and α4 subunits in the sexually mature monkeys did not seem to be affected by levels of gonadal steroids or stage of menstrual cycle since among the 42-month-old monkeys, the two animals in luteal phase at the time of euthanasia had mean optical density measures for δ (0.68) and α4 (0.83) that were very similar to those of the four animals in the follicular phase (δ, 0.69; α4, 0.89).
Expression of δ mRNA in Medial Frontal Cortex of GABAA α 1 Subunit Knockout Mice
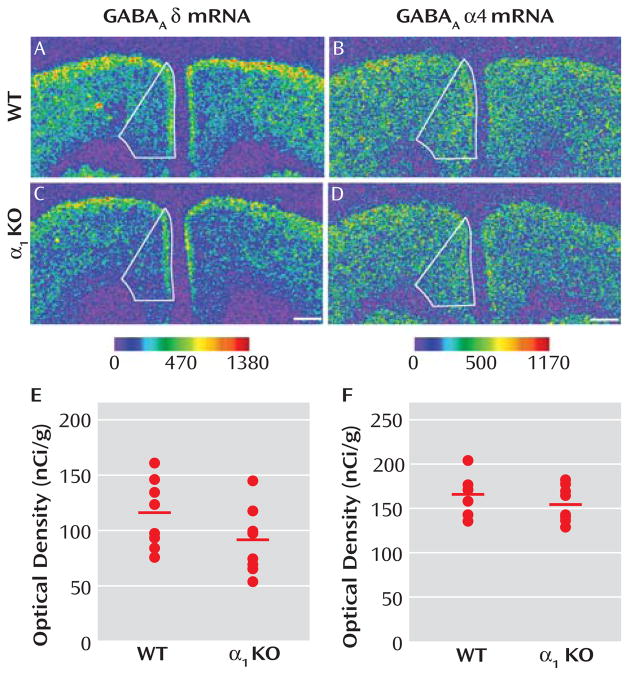
Reports that GABAA receptor α1 subunit mRNA levels are significantly lower in the dorsolateral prefrontal cortex of subjects with schizophrenia (10) and that variants in the α 1 subunit gene are associated with both schizophrenia and altered expression levels of GABAA receptor subunits (19) suggest that lower expression of the δ mRNA in schizophrenia could be a consequence of lower expression of the α1 subunit. As a proof-of-concept test of this hypothesis, we asked whether δ subunit mRNA levels were significantly reduced in the medial frontal cortex of GABAA α1 knockout mice. Mean δ mRNA levels in this region (Figure 5E) were significantly (F=10.50, df=1, 13, p= 0.006) decreased by 21% in α1 knockout (104.79 nCi/g [SD=19.85]) compared with wild-type mice (131.92 nCi/g [SD=16.75]). In contrast, the mean level of α4 mRNA expression (Figure 5F) was not significantly different in knockout mice (155.50 nCi/g [SD=21.10]) compared with wild-type mice (166.45 nCi/g [SD=21.46]).
FIGURE 5. Expression Levels of δ and α4 mRNAs Across the Medial Frontal Cortex of Wild-Type (WT) and GABAA α1 Knockout (KO) Micea.
a In panels A–D, medial frontal cortex is represented in the autoradiograms by solid lines. The mean δ mRNA levels were significantly (panel E; F=10.50, df=1, 13, p=0.006) reduced by 21% in the medial frontal cortex of α1 knockout mice. In contrast, the mRNA expression levels of α4 subunit were not changed (panel F; t=1.03, df=14, p=0.321) in the medial frontal cortex of α1 knockout mice compared with wild-type mice. The mean values for each subject group are represented by horizontal bars. Scale bars=500 μm.
These findings suggest that lower δ mRNA expression in schizophrenia might be due to lower levels of α1 subunits. Consistent with this idea, the mean within-pair percent change of δ mRNA in subjects with schizophrenia as measured in our study was significantly correlated with that of α1 mRNA recently measured in the same subjects (r=0.74, p<0.0001) (20).
Expression of δ mRNA in Animal Models of Reduced Excitatory Neurotransmission
The expression of δ subunit also appears to be modulated by levels of excitatory neurotransmission, at least in some brain regions (21, 22). These findings are of interest given the evidence suggesting that schizophrenia is associated with a reduction in excitatory neurotransmission through NMDA receptors (23, 24). To test the hypothesis that chronically decreased excitatory signaling through NMDA receptors leads to lower δ mRNA levels, we measured the expression of δ mRNA in the medial frontal cortex of mice with reduced expression of the obligatory Nr1 subunit of the NMDA receptor (Nr1 hypomorphic mice). Genotype had a significant (F=13.44, df=2, 11, p=0.002) effect on levels of NMDA Nr1 mRNA in this brain region (see Figure S4A–C, G in the online data supplement). The mean expression levels of the NMDA Nr1 subunit were significantly (p<0.05) lower by 45% in the medial frontal cortex of mice with a Nr1neo/neo genotype (569.4 nCi/g [SD=50.9]) compared with wild-type mice (1043.1 nCi/g [SD=197.8]) (see Figure S4G in the online data supplement). As previously reported (25), Nr1 mRNA levels were not significantly decreased in mice with a Nr1neo/+ genotype (897.7 nCi/g [SD=106.0]). However, Nr1 genotype had no effect on the expression of δ mRNA (see Figure S4D–F, G in the online data supplement). Furthermore, the mRNA levels of δ and of NMDA Nr1 were not correlated across all animals.
We also assessed whether δ subunit mRNA levels were altered in the medial frontal cortex of two rat models of reduced excitatory synaptic inputs in this brain region: adult rats with peripubertal lesions of the mediodorsal thalamic nucleus (MDTNL) and adult rats with neonatal lesions of the ventral hippocampus (NVHL). The mean mRNA levels of δ subunit in the medial frontal cortex of MDTNL rats (right side: 76.3 nCi/g [SD=11.9]; left side: 75.1 nCi/g [SD=12.9]) did not differ significantly from those in animals with a sham lesion (right side: 73.8 nCi/g [SD=13.1]; left side: 72.6 nCi/g [SD=13.5]) (see Figure S5A in the online data supplement). Similarly, the mean mRNA levels of the δ subunit in the medial frontal cortex of NVHL animals (right side: 38.6 nCi/g [SD=13.5]; left side: 42.3 nCi/g [SD=8.4]) did not differ significantly from those of animals with a sham lesion (right side: 40.5 nCi/g [SD=5.6]; left side: 42.3 nCi/g [SD=6.6]) (see Figure S5B in the online data supplement).
Discussion
Our findings indicate that expression levels of the mRNA for the δ, but not for the α4, subunit of the GABAA receptor are significantly lower in the dorsolateral prefrontal cortex of subjects with schizophrenia. To the extent that α4 mRNA levels are lower in schizophrenia (10), the reduction appears to be due to an effect of treatment with benzodiazepines, mood stabilizers, or antidepressants at the time of death; however, we cannot exclude the possibility that lower levels of α4 mRNA reflect a particular disease process in a subtype of schizophrenia with clinical features that require the prescription of these medications.
In contrast, lower δ mRNA levels in schizophrenia, which are consistent with two microarray studies in smaller subject cohorts (9, 10), appear to reflect the disease process of schizophrenia and are not attributable to potential confounding factors such as sex, diagnosis of schizoaffective disorder, suicide, alcohol abuse or dependency, or treatment with benzodiazepines, mood stabilizers, or antidepressants at time of death. In addition, the lower level of δ mRNA does not appear to be a consequence of exposure to antipsychotic medication, since the levels of δ mRNA were unchanged in the dorsolateral prefrontal cortex of monkeys chronically exposed to typical or atypical antipsychotics. Consistent with this interpretation, the mean percentage decrease in δ mRNA levels in the four subjects with schizophrenia who were off medications at the time of death (−23%) did not differ significantly from that in subjects who were receiving anti-psychotic medications (−18%).
In contrast to rodents (26), δ subunit mRNA levels are higher than α4 levels in human neocortex (18). We found that, consistent with these observations, the mean levels of δ subunit mRNA measured using probes directed at different portions of the transcript by in situ hybridization or microarray (10) were four to nine times higher than those of α 4 mRNA. Although δ subunits are thought to preferentially coassemble with α4 subunits in forebrain GABAA receptors (8, 11), these findings, in concert with the differential disease and developmental effects on δ versus α4 subunits, converge on the idea that the lower mRNA levels of δ subunit in subjects with schizophrenia do not reflect a reduced complement of α4βxδ GABAA receptors in the illness.
The δ subunit can coassemble with α1 subunits to form functional recombinant receptors (27, 28), and immunoprecipitation studies have shown that δ subunits are associated with α1 subunits (29). GABAA α1 subunits have also been found extrasynaptically (30, 31), consistent with the typical localization of δ-containing receptors (6, 32), and interneurons in the dentate gyrus exhibit immunoreactivity for α1 and δ subunits along the cell body surface and proximal dendrites (33). Consistent with these observations, we found that δ mRNA expression increased across postnatal development of the monkey dorsolateral pre-frontal cortex, paralleling the previously reported increase in α1 mRNA in the same animals (34), whereas levels of α4 mRNA decreased across postnatal development. These findings support the idea that δ-containing GABAA receptors in the adult dorsolateral prefrontal cortex preferentially coassemble with α1 subunits. Furthermore, unlike in the rodent hippocampus (33), GABAA receptors containing both δ and α1 subunits are likely to be found principally in pyramidal cells in the primate neocortex. For example, a previous study of postmortem human motor cortex (18) revealed that δ subunit mRNA clustered mainly over pyramidal cells. Consistent with this finding, our analysis of δ mRNA silver grains indicated that in the human dorsolateral prefrontal cortex, δ mRNA expression was also clustered primarily over putative pyramidal cells, characterized by their faint Nissl staining and large nuclei (see Figure S1 in the online data supplement). Therefore, the δ subunit mRNA appears to be predominantly expressed by cortical pyramidal cells, although we cannot exclude a lower level of expression in GABA-containing interneurons. This interpretation is further supported by the absence of δ mRNA expression in layer 1 of the dorsolateral prefrontal cortex (Figure 1), which contains GABA-containing interneurons but not pyramidal cells.
Thus, lower levels of δ mRNA in schizophrenia might reflect a reduced complement of α1βxδ GABAA receptors in dorsolateral prefrontal cortex pyramidal neurons. Consistent with this interpretation, the differences in α1 (20) and δ mRNA levels were significantly correlated as measured by in situ hybridization (r=0.74, p<0.0001) in the subjects used in this study and as measured by microarray (r=0.81, p<0.001) in a previous study involving a subset of the subjects studied here (10). However, further studies are needed to determine whether the lower levels of α1 and δ subunit mRNAs in the dorsolateral prefrontal cortex of subjects with schizophrenia are paralleled by lower levels of their cognate proteins and assembled GABAA receptors.
The lower mRNA levels of δ subunit in schizophrenia might be a consequence of lower levels of α1 subunit, given that δ mRNA levels are significantly reduced in the medial frontal cortex of α1 knockout mice. However, it should be noted that δ mRNA levels were unchanged in other brain regions of α1 knockout mice (35). Thus, it is unclear whether the potential effect of lower α1 subunits on the mRNA expression levels of δ is limited to certain cortical regions. Although it has been suggested that the disruption of excitatory signaling through NMDA receptors could produce the alterations in presynaptic markers of GABA neurotransmission seen in schizophrenia (24), δ mRNA levels were not altered in three animal models with two forms of reduced excitatory drive in the medial frontal cortex: 1) reduced number of NMDA receptors and 2) reduced presynaptic excitatory inputs from the thalamus or hippocampus. Thus, these findings do not support the hypothesis that a chronic reduction in the activity of excitatory inputs represents a pathogenetic mechanism resulting in lower expression of δ subunit mRNA in subjects with schizophrenia.
Alternatively, lower levels of δ-containing GABAA receptors (and potentially of tonic inhibition) in schizophrenia might represent a compensatory response to presynaptic reductions in GABA neurotransmission. Release of GABA provides inhibitory control over postsynaptic cells via both synaptic and extrasynaptic GABAA receptors. An important functional role of synaptic receptors, which mediate phasic inhibition, is the generation of rhythmic activities in neuronal networks (6). The resulting synchronized activity gives rise to network oscillations that are thought to contribute to cognitive processes, such as working memory. For instance, the synchronized firing of neuronal networks at 30–80 Hz, known as gamma band oscillations, is induced and sustained in the human dorsolateral pre-frontal cortex during working memory tasks (36, 37). Thus, lower levels of prefrontal GAD67 mRNA, leading to a deficit in GABA synthesis and impaired phasic inhibition, have been proposed to be a substrate for reduced frontal lobe gamma band power and working memory deficits in schizophrenia (38, 39). Although the role of extrasynaptic receptors and tonic inhibition in the generation or maintenance of oscillations is less clear, a decrease in tonic inhibition could represent a compensatory response since mutant mice with a complete loss of tonic inhibition in the hippocampus exhibit an increase in the power of gamma band oscillations (40).
Supplementary Material
Acknowledgments
Dr. Lewis receives research support from the BMS Foundation, Merck, and Pfizer and has served as a consultant to Bristol-Myers Squibb, Lilly, Merck, Neurogen, Pfizer, Hoffman-La Roche, Sepracor, and Wyeth. All other authors report no competing interests.
Supported by NIH grants MH079732 (to Dr. Maldonado-Avilés), MH043784 (to Dr. Lewis), MH084053 (to Dr. Lewis), and MH057683 (to Dr. O’Donnell). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIMH or NIH. The authors thank Drs. Marc Caron and Gonzalo Torres for their help and advice and the members of the Clinical Services and Diagnostics Core of the Conte Center for the Neuroscience of Mental Disorders (MH084053) for their assistance in diagnostic assessments.
References
- 1.Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- 2.Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- 3.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of pre-frontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 5.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 6.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 7.Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- 8.Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J Neurophysiol. 2007;97:2293–2300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- 9.Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 12.Jensen O, Kaiser J, Lachauz JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia: regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- 14.Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorph-Petersen K-A, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 17.Huntsman MM, Wood TM, Jones EG. Laminar patterns of expression of GABAA receptor subunit mRNAs in monkey sensory motor cortex. J Comp Neurol. 1995;362:565–582. doi: 10.1002/cne.903620410. [DOI] [PubMed] [Google Scholar]
- 18.Petri S, Krampfl K, Hashemi F, Grothe C, Hori A, Dengler R, Bufler J. Distribution of GABAA receptor mRNA in the motor cortex of ALS patients. J Neuropathol Exp Neurol. 2003;62:1041–1051. doi: 10.1093/jnen/62.10.1041. [DOI] [PubMed] [Google Scholar]
- 19.Petryshen TL, Middleton FA, Tahl AR, Rockwell GN, Purcell S, Aldinger KA, Kirby A, Morley CP, McGann L, Gentile KL, Waggoner SG, Medeiros HM, Carvalho C, Macedo A, Albus M, Maier W, Trixler M, Eichhammer P, Schwab SG, Wildenauer DB, Azevedo MH, Pato MT, Pato CN, Daly MJ, Sklar P. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia. Mol Psychiatry. 2005;10:1074–1088. doi: 10.1038/sj.mp.4001739. [DOI] [PubMed] [Google Scholar]
- 20.Beneyto M, Hashimoto T, Bazmi H, Arion D, Lewis DA. Altered expression of cortical GABA-A receptor α1 subunit in schizophrenia. Biol Psychiatry. 2008;63(suppl 7):298S. [Google Scholar]
- 21.Gault LM, Siegel RE. NMDA receptor stimulation selectively initiates GABA(A) receptor delta subunit mRNA expression in cultured rat cerebellar granule neurons. J Neurochem. 1998;70:1907–1915. doi: 10.1046/j.1471-4159.1998.70051907.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Choi HS, Lee SY, Ohio S. Changes of GABA(A) receptor binding and subunit mRNA level in rat brain by infusion of sub-toxic dose of MK-801. Brain Res. 2000;880:28–37. doi: 10.1016/s0006-8993(00)02687-1. [DOI] [PubMed] [Google Scholar]
- 23.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 24.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 26.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain, I: telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertens S, Benke D, Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by alpha 5- and delta-subunit-specific immunopurification. J Biol Chem. 1993;268:5965–5973. [PubMed] [Google Scholar]
- 30.Baude A, Bleasdale C, Dalezios Y, Somogyi P, Klausberger T. Immunoreactivity for the GABAA receptor alpha1 subunit, somatostatin, and connexin36 distinguishes axoaxonic, basket, and bistratified interneurons of the rat hippocampus. Cereb Cortex. 2007;17:2094–2107. doi: 10.1093/cercor/bhl117. [DOI] [PubMed] [Google Scholar]
- 31.Sun C, Sieghart W, Kapur J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor α1 and α2 subunit expression in primate prefrontal cortex. Biol Psychiatry. doi: 10.1016/j.biopsych.2009.01.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponomarev I, Maiya R, Harnett MT, Schafer GL, Ryabinin AE, Blednov YA, Morikawa H, Boehm SL, Homanics GE, Berman AE, Lodowski KH, Bergeson SE, Harris RA. Transcriptional signatures of cellular plasticity in mice lacking the alpha1 subunit of GABAA receptors. J Neurosci. 2006;26:5673–5683. doi: 10.1523/JNEUROSCI.0860-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 38.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 39.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.