Abstract
Inflammation and insulin resistance associated with visceral obesity are important risk factors for the development of type 2 diabetes, atherosclerosis, and the metabolic syndrome. The 12/15-lipoxygenase (12/15-LO) enzyme has been linked to inflammatory changes in blood vessels that precede the development of atherosclerosis. The expression and role of 12/15-LO in adipocytes have not been evaluated. We found that 12/15-LO mRNA was dramatically upregulated in white epididymal adipocytes of high-fat fed mice. 12/15-LO was poorly expressed in 3T3-L1 fibroblasts and was upregulated during differentiation into adipocytes. Interestingly, the saturated fatty acid palmitate, a major component of high fat diets, augmented expression of 12/15-LO in vitro. When 3T3-L1 adipocytes were treated with the 12/15-LO products, 12-hydroxyeicosatetranoic acid (12(S)-HETE) and 12-hydroperoxyeicosatetraenoic acid (12(S)-HPETE), expression of proinflammatory cytokine genes, including tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein 1 (MCP-1), interleukin 6 (IL-6), and IL-12p40, was upregulated whereas anti-inflammatory adiponectin gene expression was downregulated. 12/15-LO products also augmented c-Jun N-terminal kinase 1 (JNK-1) phosphorylation, a known negative regulator of insulin signaling. Consistent with impaired insulin signaling, we found that insulin-stimulated 3T3-L1 adipocytes exhibited decreased IRS-1(Tyr) phosphorylation, increased IRS-1(Ser) phosphorylation, and impaired Akt phosphorylation when treated with 12/15-LO product. Taken together, our data suggest that 12/15-LO products create a proinflammatory state and impair insulin signaling in 3T3-L1 adipocytes. Because 12/15-LO expression is upregulated in visceral adipocytes by high-fat feeding in vivo and also by addition of palmitic acid in vitro, we propose that 12/15-LO plays a role in promoting inflammation and insulin resistance associated with obesity.
INTRODUCTION
Obesity is associated with inflammation and insulin resistance which in turn promote the development of type 2 diabetes and cardiovascular disease. The chronic inflammatory state in obese individuals is characterized by increased production of proinflammatory cytokines by the adipose tissue, including tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein 1 (MCP-1), interleukin 6 (IL-6), and IL-12 (1). These cytokines act locally in the adipose tissue, but also systemically as their levels in the circulation are increased and affect whole body insulin sensitivity (2–4). MCP-1 released by adipocytes contributes to macrophage infiltration into adipose tissue (5). Macrophage accumulation augments the production of proinflammatory cytokines by adipose tissue and thereby contributes to the pathophysiological consequences of obesity (6). IL-12 is associated with atherosclerosis, type 1 diabetes, and insulin resistance in rodent models (7–10). IL-12 mediates its effect by STAT4 activation, and inhibition of STAT4 activation protects nonobese diabetic mice from autoimmune diabetes (11) and reduces inflammation in the Zucker obese fatty rat (12). TNF-α and IL-6 can promote insulin resistance through several mechanisms. They increase lipolysis in adipocytes (13) and thereby increase serum fatty acid levels. In addition, they have both been linked to hypertriglyceridemia associated with adiposity (13,14). Lower levels of adiponecti are often observed in insulin-resistant patients, and intervention therapy with adiponectin reverses insulin resistance associated with both lipoatrophy and obesity (15).
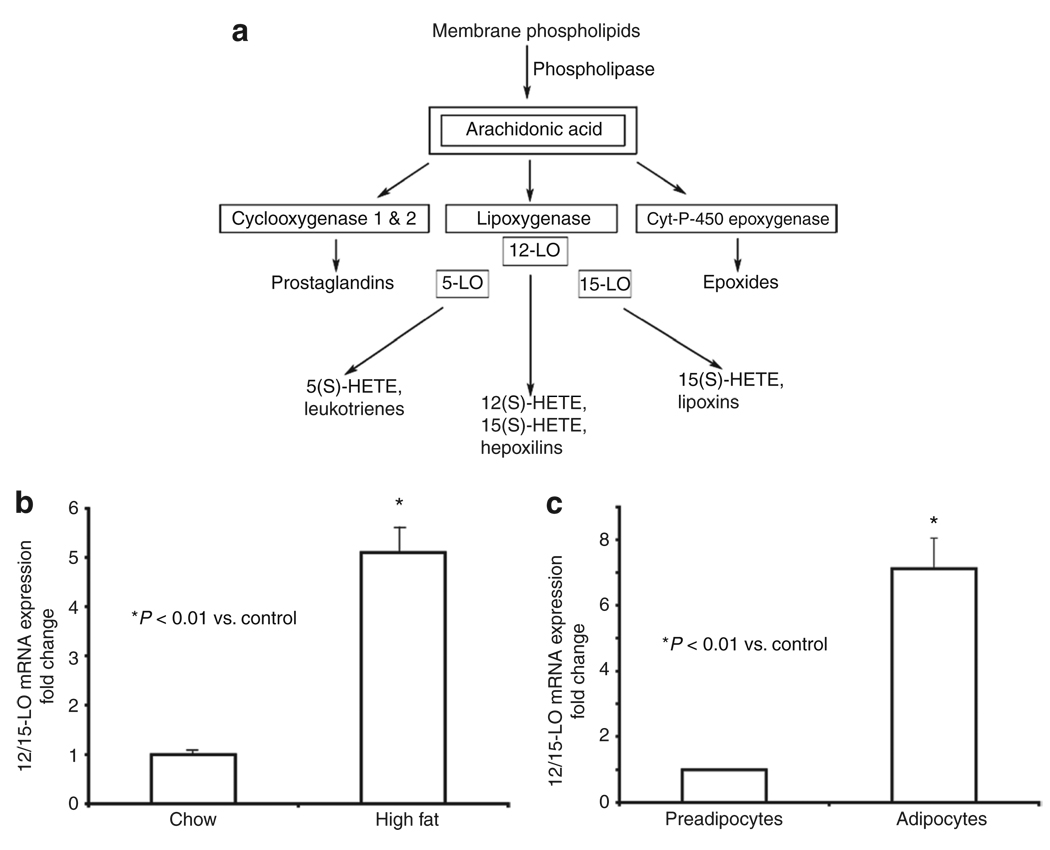
12-Lipoxygenases (12-LO), including leukocyte-12/15-LO, epidermal-12-LO, and platelet-12-LO, convert arachidonic acid by oxygenation to form the lipid inflammatory mediator 12-hydroperoxyeicosatetraenoic acid (12(S)-HPETE) which subsequently is converted to the more stable product 12-hydroxyeicosatetranoic acid (12(S)-HETE) (Figure 1a). Arachidonic acid can also be oxygenated by other enzymes leading to the generation of additional eicosanoids (Figure 1a) that, with the exception of lipoxins, all act as proinflammatory mediators.
Figure 1.
12/15-Lipoxygenase (12/15-LO) is upregulated in white epididymal adipocytes isolated from mice on a high-fat diet and in fully differentiated 3T3-L1 adipocytes. (a) A simplified schematic diagram depicting metabolism of arachidonic acid by cyclooxygenase 1 and 2, LO, and cytochrome P-450 epoxygenase enzymes to bioactive eicosanoids. All eicosanoids depicted, except the lipoxins, act as proinflammatory lipid mediators. Certain LOs can convert other fatty acid substrates, like linoleic acid, to produce additional products. 12/15-LO mRNA expression in epididymal adipocytes isolated from mice fed for 8 weeks with the (b) chow or high-fat diet and (c) in preadipocytes and differentiated 3T3-L1 adipocytes was determined by real-time RT-PCR using gene-specific primers and SYBR Green I. The data were normalized to total actin and fold differences were calculated using the 2−ΔΔCT method. Data represent means ± s.e.m. for adipocytes from six to eight mice (b) and three independent experiments (c). *Values are statistically significantly different (P < 0.01) when compared to chow-fed mice (b) or undifferentiated 3T3-L1 cells (c).
Leukocyte-12/15-LO (Alox 15), from now on referred to as 12/15-LO, has been implicated to play a key role in the progression of type 1 diabetes and atherosclerosis (16–20). Preliminary studies indicate 12/15-LO expression is elevated in adipocytes isolated from insulin-resistant obese Zucker rats when compared to lean rats (21). Furthermore, 12/15-LO deficiency in macrophages leads to defective IL-12 synthesis (22). These data suggest a correlation between 12/15-LO activation and an increased inflammatory response. To examine whether 12/15-LO products have a role in increased production of proinflammatory cytokines by adipocytes (free from macrophage contamination), we tested the direct effect of 12/15-LO products on 3T3-L1 adipocytes. We show that 12/15-LO products not only upregulate proinflammatory cytokines, but also impair insulin signaling as well. This is the first report demonstrating a direct effect of 12/15-LO products on the production of proinflammatory cytokines and impairment of insulin signaling in 3T3-L1 adipocytes.
METHODS AND PROCEDURES
Reagents
3T3-L1 cells were kindly provided by the late Dr John C. Lawrence Jr (University of Virginia, Charlottesville, VA). Dulbecco’s modified Eagle’s medium (DMEM), penicillin/streptomycin, and trypsin were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum was purchased from Zen-Bio (Research Triangle Park, NC). Dexamethasone, isobutylmethylxanthine, protease inhibitors and palmitic acid were purchased from Sigma-Aldrich (St Louis, MO). 12(S)-HETE, 12(S)-HPETE, and 12(R)-HETE were from BIOMOL (Plymouth Meeting, PA). The PCR oligonucleotides were purchased from Operon Biotechnologies (Huntsville, AL). Antibodies were obtained as following: Akt, phospho-Akt (Ser473), IRS-1, phospho-IRS-1 (Ser307), and phospho-SAPK/JNK (Thr183/Tyr185) (Cell Signaling Technology, Danvers, MA); phospho-IRS-1 (Tyr896) (Invitrogen).
Animal study
Male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were placed on a chow or a high-fat “western-type” diet beginning at 6–8 weeks of age. The chow and western diet foods were purchased from Harlan Teklad (Madison, WI); the western diet consisted of 42% of calories from fat, 15.3% of calories from protein, and 42.7% of calories from carbohydrate, primarily sucrose (TD#88137). All mice were placed on the diet for 8 weeks. Mice were housed in a pathogen-free facility at the Centers for Comparative Medicine at the University of Virginia and Eastern Virginia Medical School. All experiments were performed in accordance with an animal study protocol approved by the Institutional Animal Care and Use Committees of University of Virginia and Eastern Virginia Medical School.
Isolation of white epididymal adipocytes
Isolation of white epididymal adipocytes was based on a protocol previously described (11). Briefly, mice were killed by CO2 asphyxiation. Epididymal fat pads were removed and the tissues were finely minced and digested with collagenase in Krebs Ringer Hepes (KRH)–bovine serum albumin (BSA) buffer for 60 min at 37°C in a shaking water bath. Once digestion was complete, samples were passed through a nylon mesh (400 µm). The cells were washed by adding KRH–BSA 10 times the volume of the cell suspension. Adipocytes were allowed to float. Floating cells were collected and washed with 10 times the volume of KRH–BSA for two more times. After the final wash, the adipocytes were suspended in five times the volume of KRH–BSA and centrifuged at 200g for 1 min at room temperature. Two-milliliter Trizol (Invitrogen) was added per 1 ml of cell suspension and lysates prepared by passing the cells through an 18-gauge needle. Cell lysates were stored at −80°C until processing for RNA extraction.
3T3-L1 cell culture and differentiation
3T3-L1 preadipocytes were grown to confluence at 37 °C in 100-mm culture dishes in DMEM, containing 10% fetal bovine serum in an incubator equilibrated with 9% CO2. At 2 days postconfluence (day 0), differentiation was induced with isobutylmethylxanthine (0.5 mmol/l), dexamethasone (0.25 µmol/l), and insulin (1 µg/ml) in DMEM containing 10% fetal bovine serum as described in ref. 11. After 2 days, the isobutylmethylxanthine and dexamethasone were removed and insulin was maintained for two additional days. On day 4, and thereafter, medium was replaced with DMEM (without insulin supplementation) plus 10% fetal bovine serum every 2 days. Cells were used for experiments on day 8 of differentiation. Before each experiment, cells were kept in DMEM containing 1% serum overnight. Cells were then washed with DMEM containing 0.2% BSA and incubated in the same medium for 2 h before treatment with 12(S)-HETE, 12(S)-HPETE, or ethanol (EtOH) (solvent control) for an additional 2 or 4 h. Palmitic acid treatment was performed in DMEM containing 0.25% serum. The palmitic acid–BSA complex (3:1) used for the treatments was prepared as described in ref. 23.
RNA extraction and real-time PCR
RNA was prepared using the Ribo-Pure Kit (Ambion, Foster City, CA) for 3T3-L1 cells and Trizol (Invitrogen) for mouse white adipocytes. cDNA was made from 5 µg of total RNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen) in 20 µl reaction volume using random hexamers (Invitrogen). For quantitative measurement of PCR products, a double-stranded DNA dye, SYBR Green I (Molecular Probes, Carlsbad, CA), was used with Jump StartTaq Polymerase (Sigma-Aldrich). A volume of 3 µl of the cDNA reaction (fivefold diluted) was used as template for PCR in a reaction volume of 25 µl for PCR (24). Thermal cycling was performed using the iCycler (Bio-Rad, Hercules, CA). Cycling parameters for amplification of cytokines and adiponectin were 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 30 s for 40 cycles (25). For the amplification of 12/15-LO, the cycling conditions were 95 °C for 30 s, 62 °C for 30 s, and 72 °C for 30 s followed by 81 °C for 15 s. The 12/15-LO PCR product was subcloned into the T/A cloning vector TOPO (Invitrogen), and three to four clones from each PCR were sequenced to confirm the identity of the amplified fragment. Primer sequences are presented in Table 1. All reactions were performed in triplicate and the data were normalized to a housekeeping gene, GAPDH or actin, and evaluated using the 2−ΔΔCT method (24). Expression levels are presented as fold induction/downregulation of transcripts of respective genes relative to control.
Table 1.
Primer sequences used in real-time RT-PCR amplification of cDNA prepared from white epididymal adipocytes of chow vs. high-fat fed mice as well as preadipocytes vs. differentiated 3T3-L1 adipocytes
| Mouse genes | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| Leukocyte type 12/15-LO | ctctcaaggcctgttcagga | gtccattgtccccagaacct |
| IL-6 | gaggataccactcccaacagacc | aagtgcatcatcgttgttcataca |
| IL-12p40 | ggaagcacggcagcagaata | aacttgagggagaagtaggaatgg |
| MCP-1 | cttctgggcctgctgttca | ccagcctactcattgggatca |
| TNF-α | catcttctcaaaattcgactcacaa | tgggagtagacaaggtacaaccc |
| Adiponectin | gttgcaagctctcctgttcc | atccaacctgcacaagttcc |
| GAPDH | tcaccaccatggagaaggc | gctaagcagttggtggtgca |
| Actin | aggtcatcactattggcaacga | ccctccatgatggaattgaatgtagtt |
12/15-LO, 12/15-lipoxygenase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL-6, interleukin 6; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor-α.
Cytokine release measurements using enzyme-linked immunosorbent assay
Cytokine protein concentrations, IL-6 and IL-12p40, in 3T3-L1 adipocyte supernatants were measured following the manufacturer’s instructions by enzyme-linked immunosorbent assay using a kit from R&D System (Minneapolis, MN) and a Procarta Assay (Panomics, Fremont, CA). The treatment was done in the same way as described under “3T3-L1 cell culture and differentiation” except that 3T3-L1 adipocytes were supplemented with 12/15-LO products every 8 h and supernatants were collected after 48 h of incubation with the 12/15-LO products.
Western blotting
Differentiated 3T3-L1 adipocytes grown in six-well plates were harvested in 250 µl/well sodium dodecyl sulfate gel loading buffer containing trypsin inhibitor (10 µg/ml), leupeptin (5 µg/ml), aprotinin (5 µg/ml), phenylmethanesulphonylfluoride (1 mmol/l), sodium orthovanadate (1 mmol/l), benzamidine (1 mmol/l), sodium fluoride (50 mmol/l), and dithiothreitol (25 mmol/l). The lysates were sheared by passing through a 23-gauge needle. Protein determination was done by the Lowry method (DC Protein Assay; Bio-Rad) and as described in ref. 26. Of the total protein, 50 µg was loaded in each lane of a 7.5% sodium dodecyl sulfate–polyacrylamide gel, transferred to polyvinylidene fluoride (Millipore, Bedford, MA) membrane using a semidry apparatus (Bio-Rad), immunoblotted with phospho-IRS-1, total IRS-1, phospho-Akt, and total-Akt antibodies. Detection was performed with secondary horseradish peroxidase–conjugated antibodies and ECL plus (GE Healthcare, Piscataway, NJ) according to manufacturer’s instructions. Western blot quantitation was performed using NIH ImageJ software and band intensity values are represented as fold change of phosphorylated band intensity normalized to control.
Data analysis
Data are presented as the means ± s.e.m. Student’s t-test was used to establish statistically significant differences between samples. Data were considered statistically significantly different at P < 0.05.
RESULTS
12/15-LO is expressed in white mouse adipocytes and 3T3-L1 adipocytes
Obesity is associated with inflammation of adipose tissue. To evaluate whether 12/15-LO could play a role in the inflammatory response of the adipose tissue, we first tested whether high-fat diet feeding of mice alters the expression of 12/15-LO. As shown in Figure 1b, 12/15-LO mRNA expression was upregulated 5.2-fold in white epididymal adipocytes after 8 weeks of high-fat feeding of mice when compared to chow-fed mice. As it is difficult to evaluate a direct role of 12/15-LO and its products in adipose tissue inflammation in vivo, we used the well-established 3T3-L1 adipocytes in culture. We first established 12/15-LO expression in these cells. When comparing undifferentiated preadipocytes and fully differentiated adipocytes, we found that 12/15-LO mRNA expression was upregulated sevenfold in fully differentiated adipocytes (Figure 1c). On the other hand, we saw no discernible expression or upregulation of the other forms of 12-LO mRNA, epidermal-, or platelet-12-LO, in 3T3-LI adipocytes (data not shown), suggesting that 12/15-LO products are primarily generated by leukocyte-12/15-LO in fat cells.
12/15-LO products modify the expression and production of proinflammatory cytokines and adiponectin in 3T3-L1 adipocytes
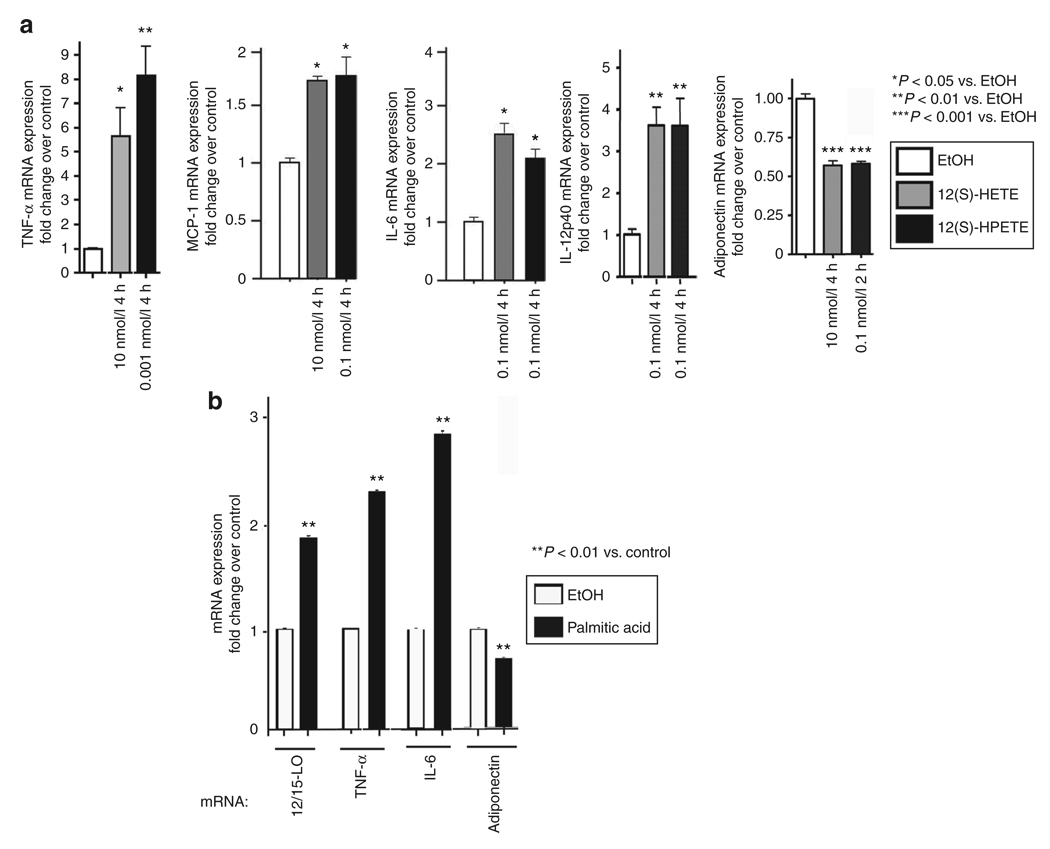
We next examined whether addition of 12/15-LO products directly to 3T3-L1 adipocytes alters mRNA expression levels of proinflammatory cytokines. Cells were serum-starved and treated with physiological concentrations of the active 12/15-LO products, 12(S)-HETE at 0.1 and 10 nmol/l, or 12(S)-HPETE at 1 pmol/l and 0.1 nmol/l for 2 or 4 h. Early time points were chosen to assess direct effects of 12/15-LO products on 3T3-L1 adipocytes and 2 h is the earliest time point at which a proinflammatory response was observed (25). Results for maximal effects are shown in Figure 2a. All the cytokines evaluated, TNF-α, MCP-1, IL-6, and IL-12p40, were increased in response to 12(S)-HETE and 12(S)-HPETE. Maximal stimulation of TNF-α mRNA expression, 5.5- and 7.8-fold, respectively, was obtained with 12(S)-HETE at 10 nmol/l and 12(S)-HPETE at 1 pmol/l after 4 h (Figure 2a). MCP-1 mRNA expression was induced 1.6–1.7-fold in 4 h by both 12(S)-HETE at 10 nmol/l and 12(S)-HPETE at 0.1 nmol/l (Figure 2a). For IL-6 mRNA, the maximal fold stimulation (2–2.5) was detected after 4 h with 12(S)-HETE and 12(S)-HPETE at 0.1 nmol/l (Figure 2a). Clear increases in mRNA expression (3.5-fold) were also observed for IL-12p40 after 4-h treatment with both 12(S)-HETE and 12(S)-HPETE at 0.1 nmol/l (Figure 2a). In contrast, when treating cells with the inactive 12-LO analogue, 12(R)-HETE, we did not see noticeable changes in gene expression (data not shown). Addition of 12/15-LO products to undifferentiated 3T3-L1 cells resulted in no significant change in the expression of the genes analyzed (data not shown).
Figure 2.
Addition of 12/15-lipoxygenase (12/15-LO) products and activation of endogenous 12/15-LO by palmitic acid upregulate proinflammatory cytokine and downregulate anti-inflammatory adiponectin mRNA expression. 3T3-L1 adipocytes were treated with 12-hydroxyeicosatetranoic acid (12(S)-HETE) and 12-hydroperoxyeicosatetraenoic acid (12(S)-HPETE) at (a) concentrations and for times indicated, and (b) with 0.25 mmol/l palmitic acid for 4 h. The mRNA measurements were done by real-time RT-PCR using gene-specific primers and SYBR Green I. Ethanol (EtOH) was used as a control. The data were normalized to total actin. The fold changes in expression were calculated relative to ethanol control. All data represent the means ± s.e.m. from three independent experiments, and asterisks indicate that values are statistically significantly different (P < 0.001–0.05) when compared to ethanol control. Shown are effects of 12(S)-HETE and 12(S)-HPETE on mRNA expression of tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein 1 (MCP-1), interleukin 6 (IL-6), IL-12p40, and adiponectin (a), as well as palmitic acid on mRNA expression of 12/15-LO, TNF-α, IL-6, and adiponectin (b).
Adiponectin is a key anti-inflammatory adipokine. When testing the effect of the 12/15-LO products on adiponectin mRNA in 3T3-L1 adipocytes, we found that both 12(S)-HETE at 10 nmol/l and 12(S)-HPETE at 0.1 nmol/l significantly decreased adiponectin gene expression by 40% in 4 and 2 h, respectively (Figure 2a). These results indicate that 12/15-LO products have differential effects on proinflammatory cytokine and anti-inflammatory cytokine adiponectin expression in adipocytes.
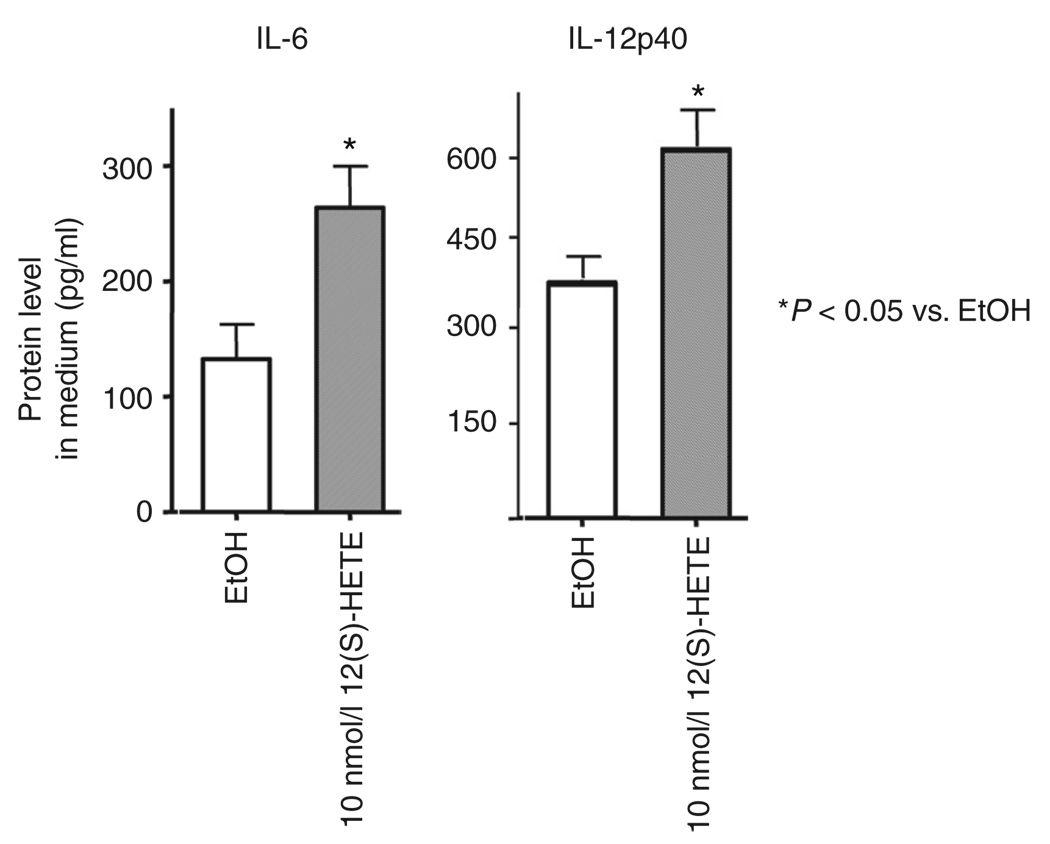
To test whether the changes in inflammatory cytokines observed at the mRNA level could also be observed at the protein level, we evaluated cytokine protein concentration in media of 3T3-L1 adipocytes treated with 12(S)-HETE. As shown in Figure 3, IL-6 and IL-12p40 increased 2- and 1.7-fold, respectively, after 48 h of incubation with 10 nmol/l 12(S)-HETE. Similar results were observed with MCP-1 protein (data not shown).
Figure 3.
12-Hydroxyeicosatetranoic acid (12(S)-HETE) increases cytokine protein secretion. 3T3-LI adipocytes were treated with 10 nmol/l 12(S)-HETE every 8 h for 48 h, and interleukin 6 (IL-6) and IL-12p40 concentrations in the culture medium were measured by enzyme-linked immunosorbent assay. All data represent the means ± s.e.m. and asterisks indicate that values are statistically significantly different (P < 0.05) when compared to ethanol control (EtOH).
Palmitic acid upregulates 12/15-LO and modifies expression of proinflammatory cytokines and adiponectin in 3T3-L1 adipocytes
We tested whether addition of palmitic acid to 3T3-L1 adipocytes altered 12/15-LO expression. 3T3-L1 adipocytes were treated with 0.125, 0.25, and 0.5 mmol/l palmitic acid. Concentration of 0.25 mmol/l palmitic acid consistently produced maximal gene expression changes; at higher concentrations gene induction decreased (Figure 2b; data not shown). As shown in Figure 2b, addition of palmitic acid (0.25 mmol/l) for 4 h upregulated 12/15-LO expression by 1.9-fold. We also looked at simultaneous gene expression changes of two proinflammatory cytokines, TNF-α and IL-6, and the anti-inflammatory adiponectin. As shown in Figure 2b, both TNF-α and IL-6 expression were upregulated 2.4- and 2.9-fold, respectively, with 0.25 mmol/l palmitic acid. This is similar to previously reported observations (27). Furthermore, adiponectin expression was reduced by 0.7-fold with 0.25 mmol/l palmitic acid.
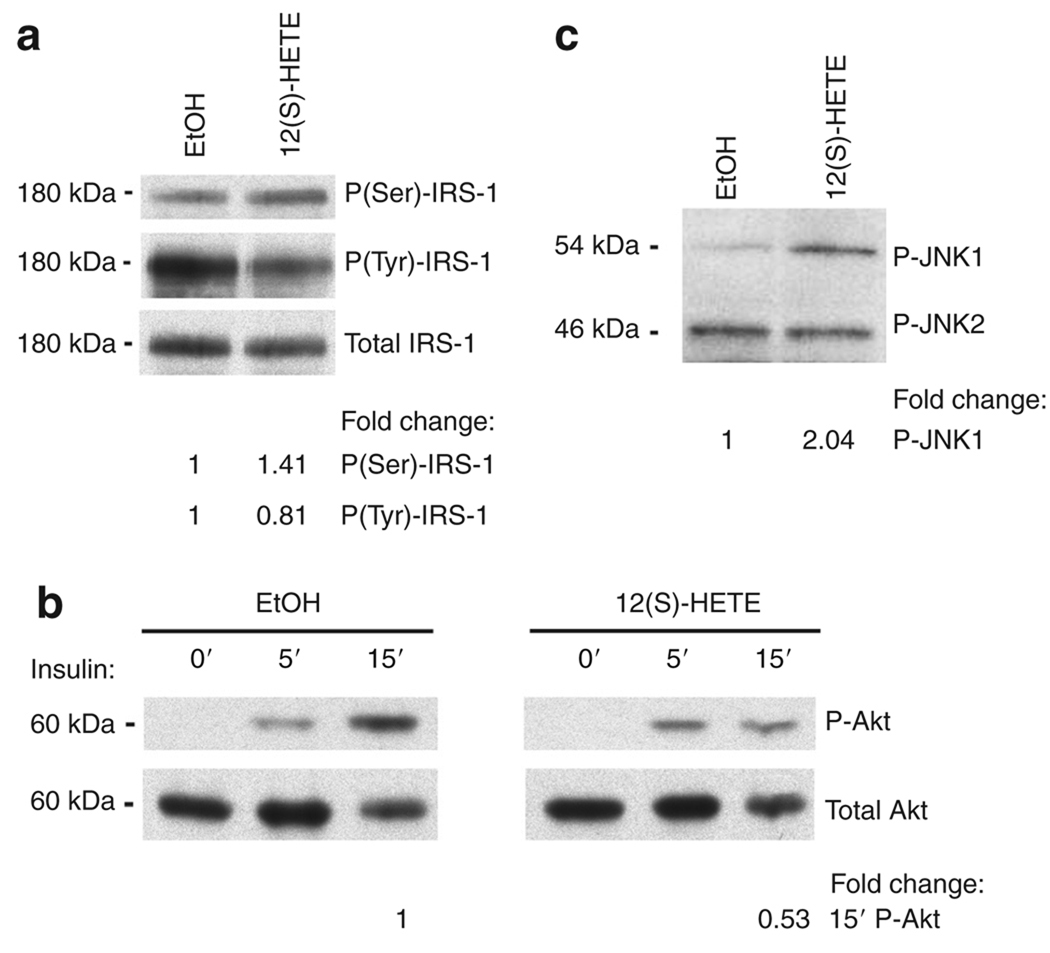
12(S)-HETE impairs insulin signaling
Given that obesity is associated with inflammation and insulin resistance (28), we examined whether 12(S)-HETE also impaired insulin signaling in 3T3-L1 adipocytes. Insulin signaling was studied in differentiated 3T3-L1 adipocytes after incubation with 10 nmol/l 12(S)-HETE for 4 h. We observed that IRS-1(Ser) phosphorylation increased (1.41-fold) and IRS-1(Tyr) phosphorylation decreased (0.81-fold) in 12(S)-HETE-treated vs. control cells stimulated with insulin for 10 min (Figure 4a). These changes were followed by impairment of downstream Akt phosphorylation (0.53-fold) after 15 min of insulin stimulation (Figure 4b). Simultaneously, we found that 12(S)-HETE treatment increased c-Jun N-terminal kinase 1 (JNK-1) phosphorylation (2.04-fold) (Figure 4c). Increased JNK-1 phosphorylation has been shown to be associated with impaired insulin signaling (29). 12(S) HETE treatment could thus through activation of JNK-1 lead to the observed increased IRS-1(Ser) phosphorylation and consequent impaired IRS-1(Tyr) and Akt phosphorylation. Taken together, these suggest that 12(S)-HETE reduces insulin signal transduction in 3T3-LI adipocytes.
Figure 4.
12-Hydroxyeicosatetranoic acid (12(S)-HETE) impairs insulin signaling. 3T3-LI adipocytes were treated with ethanol (EtOH) or 10 nmol/l 12(S)-HETE for 4 h. Cells were either stimulated with (a) 10 nmol/l insulin for 10 min, (b) the indicated time periods, (c) or unstimulated. Total cell lysates were immunoblotted with phospho-IRS-1 and total IRS-1 (a), phospho-Akt and total Akt (b), or phospho-JNK (c) antibodies. Data are representative of two to three independent experiments. P = phospho. The fold changes in phosphorylation were calculated relative to control.
DISCUSSION
Visceral obesity is associated with inflammation of visceral adipose tissue that is marked by macrophage infiltration and increased production of proinflammatory cytokines (30). The proinflammatory cytokines in turn cause insulin resistance at the local (adipocyte) and systemic level. The 12/15-LO products, 12(S)-HETE and 12(S)-HPETE, have been recognized as potent inflammatory compounds (31). Our studies focus on the leukocyte form of 12-LO, one of the three forms of mammalian 12-LO which produces 12(S)-HETE and 15(S)-HETE at a ratio of 3:1. 12(S)-products can be generated from other forms of 12-LO, e.g., platelet- and epidermal-12-LO. However, we observed very low mRNA expression of platelet-12-LO and epidermal-12-LO by quantitative PCR in 3T3-L1 adipocytes (data not shown). This suggests that the majority of 12(S)-products in differentiated 3T3-L1 adipocytes is derived from 12/15-LO activity. 12/15-LO can react with other saturated fatty acids and generate other metabolites, such as 15(S)-HETE/15(S)-HPETE, HODEs (hydroxyoctadeca-10E, 12Z-dienoic acid), lipoxins (32), and hepoxilins (33) (Figure 1a). Furthermore, there are other pathways of ara-chidonic acid metabolism, such as the cyclooxygenase and cytochrome P-450 pathways. It is not clear whether these pathways are functional in adipocytes. Current studies in our lab are investigating the contribution of these products to the proinflammatory response and impaired insulin signaling.
We had previously reported that 12/15-LO products, when added to cultured macrophages, cause inflammation by upregulating the expression of TNF-α and MCP-1 (25). In this study, we show that the addition of 12/15-LO products directly to 3T3-LI adipocytes significantly upregulates the expression of key proinflammatory genes, TNF-α, MCP-1, IL-6, and IL-12p40, and downregulates an important anti-inflammatory gene, adiponectin. The upregulation of these proinflammatory cytokines and downregulation of adiponectin are associated with visceral adiposity, insulin resistance, atherosclerosis, and diabetes (7–15). As we started investigating the signal transduction mechanisms by which 12/15-LO products could mediate the increase in inflammatory gene expression and reduction of adiponectin expression, we found that 12(S)-HETE increases the activation of JNK-1 in 3T3-L1 adipocytes suggesting that signaling through mitogen-activated protein kinase pathways may be involved. JNK-1 has been clearly associated with reduced insulin signaling by increasing serine phosphorylation of IRS-1 (29). In the current study, 12(S)-HETE-mediated induction of JNK-1 may be one mechanism for reduced insulin action in 3T3-LI adipocytes.
Furthermore, insulin-stimulated activation of Akt is necessary for normal glucose metabolism in muscle and fat cells (34). Consistent with the data presented in this study, we have found that in vivo 12/15-LO deletion improves glucose metabolism in mice fed a high-fat diet (35). As we also show in this study, 12/15-LO mRNA expression is elevated in white epididymal adipocytes of high-fat fed mice, consistent with a possible role of 12/15-LO in obesity-related inflammation. In the current study, we have now provided mechanistic evidence suggesting that fatty acids such as palmitic acid can increase 12/15-LO expression. Support for the in vivo role of 12/15-LO is provided by our earlier study showing that mice deficient for 12/15-LO are protected against a proinflammatory response and consequent metabolic defects induced by a high-fat diet (35).
In conclusion, we provide evidence for two important responses to the treatment of 3T3-L1 adipocytes with 12/15-LO products: the induction of an inflammatory response and impairment of insulin signaling. We are aware that this study has been performed in murine adipocytes and mice and thus has to be confirmed in humans. Further mechanisms of how 12/15-LO products mediate these responses are currently being investigated. Intervention with anti-inflammatory agents may help to dissect the sequence of events. Because 12/15-LO mRNA expression is upregulated in white epididymal adipocytes isolated from mice on a high-fat diet, blockade of the 12/15-LO pathway may provide a new therapeutic approach to reduce inflammation and improve insulin sensitivity, thereby reducing the complications associated with adiposity. Further studies will aim at understanding how 12/15-LO induction affects glucose metabolism and insulin sensitivity in 3T3-LI adipocytes. Identification of immediate upstream factors of the 12/15-LO gene regulated, for example, by saturated fatty acids, such as palmitate, will be useful in determining early intervention targets in blocking 12/15-LO-mediated effects as observed in this study.
ACKNOWLEDGMENTS
We express our thanks to George E. Vandenhoff for performing the enzyme-linked immunosorbent assay. This work was supported by the National Institutes of Health Grants P01 HL55798 and ROI DK55240.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabie N, Delfs MW, Westrich JR, et al. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Invest. 2003;111:671–680. doi: 10.1172/JCI16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adorini L. Interleukin 12 and autoimmune diabetes. Nat Genet. 2001;27:131–132. doi: 10.1038/84732. [DOI] [PubMed] [Google Scholar]
- 9.Wegner M, Winiarska H, Bobkiewicz-Kozlowska T, Dworacka M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine. 2008;42:312–316. doi: 10.1016/j.cyto.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Wen Y, Gu J, Li SL, et al. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology. 2006;147:2518–2525. doi: 10.1210/en.2005-0519. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Chen M, Ellett JD, et al. Autoimmune diabetes is blocked in Stat4-deficient mice. J Autoimmun. 2004;22:191–200. doi: 10.1016/j.jaut.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Pei H, Gu J, Thimmalapura PR, Mison A, Nadler JL. Activation of the 12-lipoxygenase and signal transducer and activator of transcription pathway during neointima formation in a model of the metabolic syndrome. Am J Physiol Endocrinol Metab. 2006;290:E92–E102. doi: 10.1152/ajpendo.00133.2005. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51:2929–2935. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]
- 14.Nonogaki K, Fuller GM, Fuentes NL, et al. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology. 1995;136:2143–2149. doi: 10.1210/endo.136.5.7720663. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Chen M, Carter JD, et al. Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem Biophys Res Commun. 2006;344:1017–1022. doi: 10.1016/j.bbrc.2006.03.177. [DOI] [PubMed] [Google Scholar]
- 17.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 18.Reilly KB, Srinivasan S, Hatley ME, et al. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem. 2004;279:9440–9450. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48:486–495. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- 20.McDuffie M, Maybee NA, Keller SR, et al. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57:199–208. doi: 10.2337/db07-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadler JL, Pei H, Bevard M, Bruce A. Reduced macrophage infiltration in visceral adipose tissue of 12-lipoxygenase knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:E48. [Google Scholar]
- 22.Zhao L, Cuff CA, Moss E, et al. Selective interleukin-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atherosclerosis in a mouse model of familial hypercholesterolemia. J Biol Chem. 2002;277:35350–35356. doi: 10.1074/jbc.M205738200. [DOI] [PubMed] [Google Scholar]
- 23.Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001;50:315–321. doi: 10.2337/diabetes.50.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarti SK, James JC, Mirmira RG. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem. 2002;277:13286–13293. doi: 10.1074/jbc.M111857200. [DOI] [PubMed] [Google Scholar]
- 25.Wen Y, Gu J, Chakrabarti SK, et al. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology. 2008;148:1313–1322. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- 26.Peterson GL, et al. A Simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 27.Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- 28.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto H, Obici S, Accili D, Rossetti L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest. 2005;115:1314–1322. doi: 10.1172/JCI23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huo Y, Zhao L, Hyman MC, et al. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 32.O’Meara SJ, Rodgers K, Godson C. Lipoxins: update and impact of endogenous pre-resolution lipid mediators. Rev Physiol Biochem Pharmacol. 2007;160:47–69. doi: 10.1007/112_2006_0606. [DOI] [PubMed] [Google Scholar]
- 33.Nigam S, Zafiriou MP, Deva R, Ciccoli R, Roux-Van der Merwe R. Structure, biochemistry and biology of hepoxilins: an update. FEBS J. 2007;274:3503–3512. doi: 10.1111/j.1742-4658.2007.05910.x. [DOI] [PubMed] [Google Scholar]
- 34.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Nunemaker CS, Chen M, Pei H, et al. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol Endocrinol Metab. 2008;295:E1065–E1075. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]