Abstract
The association between isolated systolic hypertension (ISH) and incident heart failure (HF) has not been prospectively studied in a propensity-matched population of ambulatory older adults. Of the 5,795 participants in the public-use copy of the Cardiovascular Health Study (CHS) dataset, 5248 has diastolic blood pressure <90 mm Hg and were free of HF at baseline. Of these, 2000 (38%) has ISH, defined as average seated systolic blood pressure ≥ 140 mm Hg. Propensity scores for baseline ISH were calculated for each participant (based on 64 baseline covariates), and were used to match 1,260 pairs of participants with and without ISH. Matched Cox regression models were used to estimate association of ISH with incident HF during a mean follow-up of 8.7 years. Matched participants (n=2,520) had a mean (±SD) age of 74 (±6) years, 60% were women, 16% were nonwhites, 18% developed new-onset HF, and 35% died. Incident HF developed in 20% (rate, 242/10,000 person-years) and 16% (rate, 194/10,000 person-years) of participants with and without ISH respectively (matched hazard ratio {HR} when ISH was compared with no-ISH, 1.26; 95% confidence interval {CI}, 1.04–1.51; P=0.016). Pre-match unadjusted, multivariable-adjusted and propensity-adjusted HRs (95% CI) for ISH-associated incident HF were respectively 1.72 (1.51–1.97; P<0.0001), 1.35 (1.18–1.56; P<0.0001) and 1.22 (1.04–1.44; P=0.016). ISH had no association with all-cause mortality (matched HR, 1.03; 95% CI, 0.88–1.19; P=0.732). In conclusion, in a propensity-matched cohort of community-dwelling older adults who were well-balanced in 64 baseline covariates, ISH was associated with increased risk of incident HF but had no association with all-cause mortality.
Keywords: Heart failure, isolated systolic hypertension, mortality, coronary artery disease, cerebrovascular disease, propensity score
Isolated systolic hypertension (ISH) is associated with cardiovascular morbidity and mortality.1, 2 Hypertension is common in older adults and over 80% of elderly hypertensive patients have ISH.3–5 Hypertension is a major risk factor for cardiovascular morbidity including heart failure (HF).6, 7 However, the association between ISH and incident HF has not been well studied.8–10 In particular, the association between ISH and incident HF has not been prospectively studied in a propensity-matched population of community-dwelling ambulatory older adults. We used public-use copies of the Cardiovascular Health Study (CHS) datasets obtained from the National Heart, Lung, and Blood Institute (NHLBI) to test the hypothesis that baseline ISH was associated with incident HF using a propensity-matched design.
Methods
Study Design and Participants
The CHS is an NHLBI-funded, currently-ongoing, population-based, longitudinal study of 5,888 community-dwelling older adults, age ≥65 years.11 Participants were recruited in two phases from Forsyth County, North Carolina, Sacramento County, California, Washington County, Maryland, and Pittsburgh, Pennsylvania. An initial cohort (n=5,201) was recruited between 1989 and 1990. Due to under-representation of minorities, a second cohort (n=687) of African-Americans was recruited between 1992 and 1993. The objective of the CHS was to identify the onset and course of conventional and new cardiovascular disease risk factors among older adults.11
Of the 5,888 original CHS participants, data on 5,795 participants were available in the public-use copy of the datasets (93 participants did not consent to be included in the de-identified public-use copy of the data). We excluded 19 participants without data on average baseline systolic blood pressure (SBP) or diastolic blood pressure (DBP) and 278 participants with average DBP ≥90 mm Hg. We also excluded 250 participants with baseline prevalent HF. The final sample size for the current analysis consisted of 5,248 participants with ISH without diastolic hypertension and prevalent HF. From this group, we assembled a cohort of 1,260 pairs of propensity-matched participants with and without ISH for our main analyses as described later.
ISH and Other Baseline Measurements
At baseline, seated blood pressure (fifth Korotkoff sound) was measured with a random-zero sphygmomanometer, model 7076 (Hawksley and Sons Limited, Sussex, England).9 The average of two measurements of the SBP and DBP, corrected for zero values, was used. We defined ISH as SBP ≥140 mm Hg withDBP <90 mm Hg.12 Of the 5,248 participants, 2000 (38.1%) had ISH. Data on socio-demographic, clinical, sub-clinical, and laboratory variables were collected at baseline and have been previously described in detail.9, 11 Missing values for continuous variables were imputed based on values predicted by age, sex, and race.
Outcome Measures
The primary outcome for this study was definite new-onset HF during a mean follow-up of 8.7 years. The process of adjudication of HF in CHS has been well documented in the literature.6,13 Briefly, participants were asked about self-reports of a physician diagnosis of HF during semi-annual visits. The CHS Events Committee later adjudicated the diagnosis of HF through the examination of participant’s medical records for a constellation of symptoms, physical signs, and other supporting findings suggestive of HF, use of medications commonly used for HF, and follow up surveillances. Secondary outcomes were all-cause mortality, incident coronary artery disease (CAD) including acute myocardial infarction or angina pectoris, cerebrovascular disease including stroke or transient ischemic attack, and peripheral artery disease.
Propensity Score Matching
Because of significant differences in key baseline characteristics between participants with and without ISH (Table 1 and Figure 1), we used propensity score matching to assemble a population in which those with and without ISH would be well balanced in all measured baseline covariates. Propensity score is the conditional probability of having an exposure given a set of measured baseline covariates.14, 15 Propensity scores for ISH for each of the 5,248 participants were estimated using a non-parsimonious multivariable logistic regression model.16–18 In the model, ISH was used as the dependent variable, and 64 baseline characteristics displayed in Figure 1 were entered as covariates along with one significant interaction term (between age and baseline serum creatinine).
Table 1.
Baseline characteristics of participants of Cardiovascular Heart Study (CHS), by isolated systolic hypertension,* before and after propensity score matching
| n (%) or mean (±SD) | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Isolated systolic hypertension |
P value | Isolated systolic hypertension |
P value | |||
| No (n=3248) | Yes (n=2000) | No (n=1260) | Yes (n=1260) | |||
| Age, years | 72 (±5) | 75 (±6) | <0.0001 | 74 (±5) | 73 (±6) | 0.680 |
| Female | 1828 (56%) | 1234 (62%) | <0.0001 | 758 (60%) | 754 (60%) | 0.903 |
| Non-white | 428 (13%) | 359 (18%) | <0.0001 | 196 (16%) | 188 (15%) | 0.700 |
| Married | 2233 (69%) | 1272 (64%) | <0.0001 | 815 (65%) | 823 (65%) | 0.766 |
| Living alone | 381 (12%) | 275 (14%) | 0.032 | 166 (13%) | 160 (13%) | 0.764 |
| ≥College education | 1432 (44%) | 831 (42%) | 0.071 | 520 (41%) | 542 (43%) | 0.393 |
| ≥$25 thousand income | 1262 (39%) | 647 (32%) | <0.0001 | 426 (34%) | 444 (35%) | 0.476 |
| Current smoker | 438 (14%) | 208 (10%) | 0.001 | 141 (11%) | 137 (11%) | 0.849 |
| Alcohol, units/week | 2.4 (± 6.0) | 2.6 ± (6.8) | 0..395 | 2.6 (±6.8) | 2.6 (±6.6) | 0.856 |
| Body mass index, kg/m2 | 26 (±4) | 27 (±4) | <0.0001 | 27 (±4) | 27 (±4) | 0.432 |
| Self-reported fair to poor general health | 697 (22%) | 531 (27%) | <0.0001 | 307 (24%) | 312 (25%) | 0.857 |
| Myocardial infarction | 274 (8%) | 147 (7%) | 0.160 | 93 (7%) | 97 (8%) | 0.815 |
| Current angina | 559 (17%) | 342 (17%) | 0.918 | 203 (16%) | 207 (16%) | 0.872 |
| Hypertension | 953 (29%) | 1238 (62%) | <0.0001 | 604 (48%) | 597 (47%) | 0.787 |
| Diabetes | 443 (14%) | 373 (19%) | <0.0001 | 211 (17%) | 211 (17%) | 1.000 |
| Atrial fibrillation by ECG | 67 (2%) | 40 (2%) | 0.876 | 25 (2%) | 31 (3%) | 0.504 |
| LVH by ECG | 70 (2%) | 146 (7%) | <0.0001 | 51 (4%) | 60 (5%) | 0.417 |
| Transient ischemic attack | 149 (5%) | 136 (7%) | 0.001 | 70 (6%) | 68 (5%) | 0.928 |
| Stroke | 104 (3%) | 96 (5%) | 0.003 | 43 (3%) | 46 (4%) | 0.830 |
| Claudication | 58 (2%) | 58 (3%) | 0.008 | 27 (2%) | 31 (3%) | 0.689 |
| Chronic obstructive pulmonary disease | 400 (12%) | 256 (13%) | 0.606 | 156 (12%) | 155 (12%) | 1.000 |
| Chronic kidney disease | 619 (19%) | 464 (23%) | <0.0001 | 264 (21%) | 267 (21%) | 0.922 |
| Cancer | 483 (15%) | 272 (14%) | 0.203 | 178 (14%) | 183 (15%) | 0.822 |
| Medications | ||||||
| ACE inhibitors | 157 (5%) | 179 (9%) | <0.0001 | 106 (8%) | 97 (8%) | 0.550 |
| Beta blockers | 380 (12%) | 278 (14%) | 0.019 | 165 (13%) | 172 (14%) | 0.729 |
| Calcium channel blockers | 335 (10%) | 316 (16%) | <0.0001 | 156 (12%) | 151 (12%) | 0.806 |
| Aspirin | 111 (3%) | 72 (4%) | 0.726 | 47 (4%) | 44 (4%) | 0.832 |
| Statins | 73 (2%) | 45 (2%) | 0.995 | 29 (2%) | 27 (2%) | 0.894 |
| Loop diuretics | 149 (5%) | 104 (5%) | 0.314 | 58 (5%) | 61 (5%) | 0.853 |
| Thiazide diuretics | 328 (10%) | 261 (13%) | 0.001 | 157 (13%) | 161 (13%) | 0.859 |
| Potassium-sparing diuretics | 22 (1%) | 20 (1%) | 0.203 | 14 (1%) | 12 (1%) | 0.845 |
| NSAIDs | 378 (12%) | 284 (14%) | 0.007 | 160 (13%) | 168 (13%) | 0.679 |
| Pulse rate, beats/min | 68 (±11) | 68 (±12) | 0.811 | 68 (±10) | 68 (±12) | 0.739 |
| Diastolic blood pressure, mm Hg | 67 (±9) | 74 (±10) | <0.0001 | 71 (±8) | 71 (±10) | 0.836 |
| Serum creatinine, mg/dL | 0.95 (±0.39) | 0.96 (±0.37) | 0.164 | 0.94 (±0.32) | 0.95 (±0.38) | 0.481 |
| Serum uric acid, mg/dL | 5.63 (±1.49) | 5.66 (±1.52) | 0.484 | 5.67 (±1.51) | 5.67 (±1.53) | 0.973 |
| Plasma glucose, mg/dL | 109 (±36.5) | 112 (±36.4) | 0.001 | 111 (±37.1) | 112 (±36.3) | 0.657 |
| Serum insulin, mcU/mL | 16 (±21) | 18 (±29) | 0.030 | 17 (±21) | 17 (±23) | 0.997 |
| Serum potassium, mEq/L | 4.19 (±0.36) | 4.13 (±0.39) | <0.0001 | 4.16 (±0.38) | 4.16 (±0.39) | 0.751 |
| Total cholesterol, mg/dL | 210 (±39) | 214 (±39) | 0.001 | 213 (±39) | 213 (±39) | 0.949 |
| Triglyceride, mg/dL | 138 (±74) | 143 (±81) | 0.025 | 139 (±72) | 143 (±77) | 0.157 |
| Albumin, g/dL | 3.99 (±0.29) | 4.00 (±0.29) | 0.179 | 4.00 (±0.29) | 4.00 (±0.29) | 0.862 |
| Fibrinogen, mg/dL | 318 (±71) | 319 (±75) | 0.382 | 318 (±72) | 318 (±75) | 0.896 |
| C-reactive protein, mg/dL | 4.4 (±7.6) | 4.9 (±8.5) | 0.030 | 4.7 (±7.9) | 4.9 (±8.4) | 0.585 |
| Hemoglobin, g/dL | 14.1 (±1.4) | 14.0 (±1.4) | 0.012 | 14.0 (±1.3) | 14.0 (±1.5) | 0.914 |
| White blood cell, 103/μL | 6.23 (±2.07) | 6.34 (±2.14) | 0.066 | 6.25 (±1.84) | 6.30 (±1.78) | 0.481 |
| Platelet, 103/μL | 243 (±66) | 246 (±69) | 0.051 | 245 (±64) | 247 (±69) | 0.403 |
Isolated systolic hypertension is defined as average systolic blood pressure ≥140 and diastolic blood pressure <90 mm Hg; ACE=angiotensin converting enzyme; ECG=electrocardiography; LVH=left ventricular hypertrophy; MI=myocardial infarction; NSAID=non-steroidal anti-inflammatory drug
Figure 1.
Love plots for absolute standardized differences for covariates between participants with and without isolated systolic hypertension, before and after propensity score matching (ACE=angiotensin-converting enzyme; COPD=chronic obstructive pulmonary disease; NSAID=non-steroidal anti-inflammatory drug)
Propensity score models are sample-specific adjusters and are not intended to be used for out-of-sample prediction or estimation of coefficients. Therefore, the assessment of the model’s effectiveness is not measured by fitness or discrimination but rather by the quality of the covariate balance achieved after matching. Using a greedy matching protocol, described elsewhere in detail, we were able to match 1,260 (63% of the 2,000) CHS participants with ISH with 1,260 participants without ISH who had similar propensity scores.16–18 We estimated absolute standardized differences for all 64 covariates between participants with and without ISH to assess pre-match imbalance and post-match balance and displayed them as a Love plot.16–20 Absolute standardized differences directly quantify balance in the means (or proportions) of covariates across the groups, and are expressed as percentages of pooled standard deviations. An absolute standardized difference of 0% on a covariate indicates no between-group imbalance for that covariate and values <10% indicate inconsequential imbalance.
Statistical Analysis
For descriptive analyses Pearson Chi square, Wilcoxon rank-sum tests, McNemar’s tests and paired sample t-tests were used as appropriate for pre- and post-match between-group comparisons. To estimate the association between ISH and outcomes, we used Kaplan Meier and matched Cox proportional hazard analyses. Proportional hazards assumptions were checked using log-minus-log scale survival plots. We then repeated our analysis in the matched cohort using systolic blood pressure as a continuous variable and as dummy variables. For the dummy variable analysis, SBP was grouped in 10 mm Hg categories as follows: 140–149 mm Hg, 150–159 mmHg, 160–169 mm Hg, and ≥170mm Hg and SBP <140 mm Hg was used as the reference category. To determine if the association between ISH and incident HF was homogeneous across various subgroups of matched patients, we conducted subgroup analyses and formally tested for interactions using Cox regression models. Finally, we examined the association of ISH and incident HF in the full pre-match cohort of 5,248 participants using three different approaches: (1) unadjusted, (2) multivariable-adjusted, using all covariates used in the propensity score model, and (3) propensity score adjusted. All statistical tests were two-tailed with 95% confidence levels and p-values <0.05 were considered significant. SPSS for Windows (Version 15) was used for all data analysis.21
Sensitivity Analyses
Even though our matched cohort was well balanced in 64 measured baseline covariates between participants with and without ISH, bias due to imbalances in unmeasured covariates is possible. As such, we conducted a formal sensitivity analysis to quantify the degree of a hidden bias that would need to be present to invalidate our main conclusions.22
Results
Baseline Characteristics
Overall, matched participants had a mean age (±SD) age of 73.5 (±5.5) years, 60% were women, and 15% were non-whites. Pre-match imbalances and post-match balances between participants with and without ISH are displayed in Table 1 and Figure 1. In general, before matching, compared with participants without ISH, those with ISH were more likely to be older, women, nonwhites, have higher mean body mass index, chronic kidney disease (CKD), diabetes, stroke, and left ventricular hypertrophy (LVH). After matching, absolute standardized differences for all measured covariates were <10% (most <5%), suggesting substantial significant covariate balance across the two groups (Figure 1).
Association of ISH with New-Onset HF
Overall, 452 (18%) matched participants developed new-onset HF during 20,729 person-years of follow up. Kaplan-Meier survival curves for incident HF are displayed in Figure 2. New-onset HF occurred in 20% (rate, 242/10,000 person-years) and 16% (rate, 194/10,000 person-years) participants with and without ISH respectively (matched hazard ratio {HR} when ISH was compared with no-ISH, 1.26; 95% confidence interval {CI}, 1.04–1.51; P=0.016; Table 2). When average SBP was used as a continuous variable, every one mm Hg increase in SBP was associated with an increased risk of new-onset HF (HR, 1.01; 95% CI, 1.01–1.02; P<0.0001). When compared with SBP <140 mm Hg, HRs (95% CIs) for incident HF for SBP categories 140–149 mm Hg, 150–159 mm Hg, 160–169 mm Hg and ≥170 mm Hg were respectively 1.01 (0.80–1.28; P=0.942), 1.31 (1.01–1.71; P=0.044), 1.88 (1.34–2.63; P<0.0001), and 1.89 (1.27–2.80; P=0.002). Among the 5,248 pre-match participants, 890 (17%) developed HF. Incident HF occurred in 22% and 14% of participants with and without ISH respectively (unadjusted HR, 1.72; 95% CI, 1.51–1.97; P<0.0001; Table 2). Multivariable- and propensity-adjusted associations of ISH and incident HF before matching are displayed in Table 2.
Figure 2.

Kaplan-Meier plots for incident heart failure by isolated systolic hypertension (ISH) in Cardiovascular Health Study (HR=hazard ratio; CI=confidence interval)
Table 2.
Association of isolated systolic hypertension with incident heart failure
| Outcomes | Rate, per 10,000 person-years follow-up (% events) |
Rate difference* (per 10,000 person-years) | Hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Isolated systolic hypertension | |||||
| No | Yes | ||||
| Before matching (n=5248) | n=3248 | n=2000 | |||
| Unadjusted | 164 (14) | 277 (22) | + 113 | 1.72 (1.51–1.97) | <0.0001 |
| Multivariable-adjusted | – | – | – | 1.35 (1.18–1.56) | <0.0001 |
| Propensity-adjusted | – | – | – | 1.22 (1.04–1.44) | 0.016 |
| After matching (n=2520) | n=1260 | n=1260 | |||
| Incident heart failure | 194 (16) | 242 (20) | + 48 | 1.26 (1.04–1.51) | 0.016 |
Absolute rate differences were calculated by subtracting the rates of new-onset heart failure in the no isolated systolic hypertension group from those in the isolated systolic hypertension group (before values were rounded).
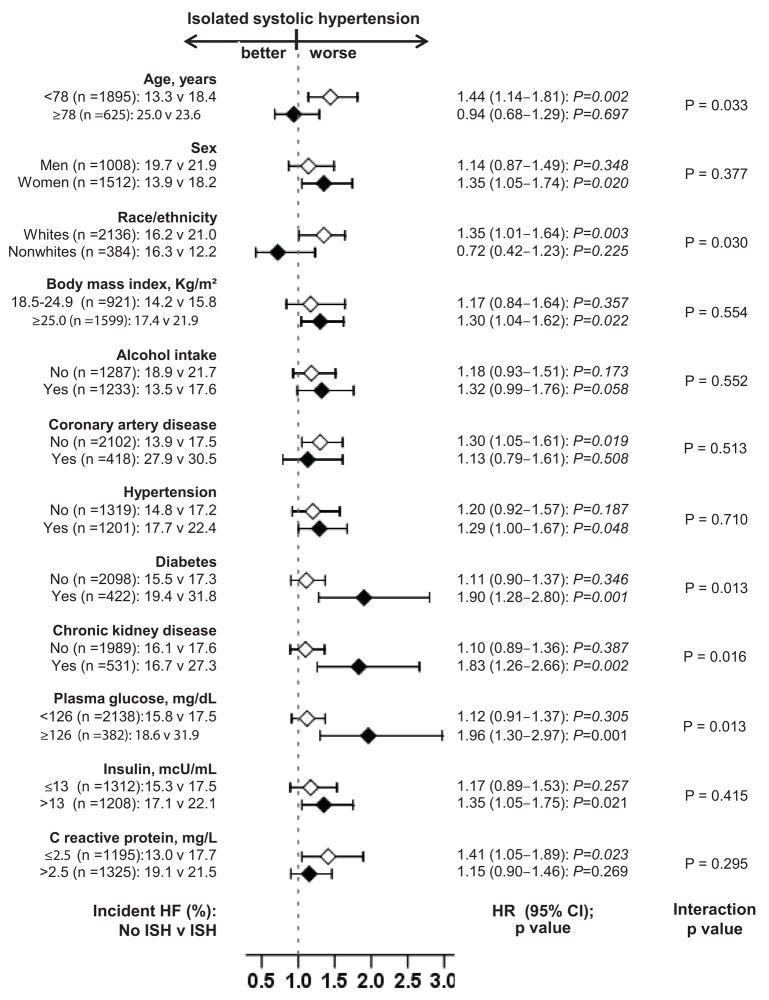
Subgroup Analyses
The association of ISH and incident HF was homogenous across a wide spectrum of participants except for those by diabetes mellitus and CKD (Figure 3). The association between ISH and incident HF was significantly more pronounced in participants with a history of diabetes mellitus (p for interaction=0.013), high mean baseline serum glucose (p for interaction=0.013), and CKD (p for interaction=0.016; Figure 3).
Figure 3.
Hazard ratio and 95% confidence interval (CI) for incident heart failure (HF) associated with isolated systolic hypertension (ISH) in subgroups of patients in Cardiovascular Heart Study
Association of ISH with Other Outcomes
All-cause mortality occurred in 879 (35%) participants, which was similar (35% each) in those with and without ISH (matched HR, 1.03; 95% CI, 0.88–1.19; P=0.732; Table 3). New-onset CAD occurred in 17% participants (9% due to AMI and 15% due to angina pectoris). CAD occurred in 19% and 15% of participants with and without ISH respectively (matched HR, 1.34; 95% CI, 1.08–1.66; P=0.008; Table 3). ISH was associated with new cerebrovascular disease events (matched HR, 1.33, 95% CI 1.05–1.67; P=0.017) but had no significant association with peripheral artery disease (matched HR, 0.84, 95% CI 0.50–1.41; P=0.508; Table 3).
Table 3.
Isolated systolic hypertension and other outcomes in matched participants
| Outcomes | Rate, per 10,000 person-years follow-up (% events) |
Rate difference* (per 10,000 person-years) | Hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Isolated systolic hypertension | |||||
| No (n =1260) | Yes (n =1260) | ||||
| All-cause mortality | 401 (35) | 398 (35) | −3 | 1.03 (0.88–1.19) | 0.732 |
| Coronary artery disease | 181 (15) | 240 (19) | + 59 | 1.34 (1.08–1.66) | 0.008 |
| Acute myocardial infarction | 95 (8) | 125 (10) | + 30 | 1.33 (0.99–1.77) | 0.057 |
| Angina pectoris | 154 (13) | 209 (17) | + 55 | 1.38 (1.09–1.74) | 0.006 |
| Cerebrovascular disease | 150 (13) | 199 (16) | + 49 | 1.33 (1.05–1.67) | 0.017 |
| Stroke | 125 (11) | 164 (14) | + 39 | 1.32 (1.03–1.70) | 0.028 |
| Transient ischemic attack | 28 (3) | 39 (4) | + 11 | 1.67 (0.97–2.86) | 0.064 |
| Peripheral arterial disease | 37 (3) | 28 (2) | −9 | 0.84 (0.50–1.41) | 0.508 |
Absolute rate differences were calculated by subtracting the rates of new events in the no isolated systolic hypertension group from those in the isolated systolic hypertension group (before values were rounded).
Results of Sensitivity Analysis
In the absence of hidden confounder, a sign-score test for matched data with censoring provides strong evidence (p=0.0244) that ISH increased risk of incident HF. A hidden binary covariate that is a near-perfect predictor of incident HF would need to increase the odds of ISH by only 3.17% to explain away this association.
Discussion
The results of the current analysis demonstrate that in a propensity-matched population of community-dwelling older adults, baseline ISH was associated with increased risk of incident HF. Baseline ISH was also associated with increased risk of CAD and cerebrovascular disease, but had no association with peripheral artery disease or all-cause mortality. To the best of our knowledge, this is the first report of a significant association between ISH and incident HF in a propensity-matched population of community-dwelling older adults. These findings indicate an intrinsic association between ISH and incident HF, as participants with and without ISH were well balanced in 64 measured baseline characteristics including traditional and non-traditional risk factors.
There are three possible explanations for these findings. First, a direct effect of ISH mediated via an elevated SBP; second, an effect of a low DBP; and third, confounding by comorbidities associated with ISH. The ISH is the result of a complex interaction between age-related changes in vascular distensibility and left ventricular function.23 Compared to older adults with essential hypertension, those with ISH are more likely to have myocardial and vascular remodeling, LVH, and atherosclerosis, which may predispose them to increased cardiovascular morbidity and mortality.24 A decrease in DBP is known to cause coronary under-perfusion and may increase risk of AMI.25 However, in our matched cohort, the mean average DBP was relatively normal and was well balanced between the groups (Table 1) suggesting that the increased pulse pressure was solely due to an increase in SBP rather than a decrease in DBP. Low DBP has been shown to have a substantially stronger association with AMI than stroke.26 However, in our matched population, ISH had a significant association only with stroke and not with AMI suggesting a lesser role of DBP. Our pre-match participants with ISH were older with high prevalence of risk factors for HF. However, our post-match cohort was well balanced in all measured covariates including known risk factors for HF such as age, LVH, history of hypertension, diabetes mellitus, coronary artery disease, and CKD. This suggests that our finding of an association between ISH and incident HF may not be explained by baseline imbalances in any of the 64 covariates used in our study.
The findings of a significantly stronger association of ISH and incident HF in participants with baseline diabetes mellitus, higher serum glucose, and CKD, despite small numbers of participants in these subgroups, deserve further discussion. Participants without diabetes and CKD respectively represented 83% and 79% of all matched participants. Yet, ISH had no significant association with HF in these participants. These findings suggest that an increased SBP may interact with increased serum glucose levels and an impaired kidney function to augment its deleterious effect on the cardiovascular system. Hypertension is common in patients with diabetes and CKD, which are also risk factors for HF.6 Our findings highlight the importance of blood pressure control in these patients.
There are several potential explanations for the apparent lack of an association of ISH with all-cause mortality, despite its significant associations with prognostically important major cardiovascular morbidities. HF is a strong predictor of mortality. However, as HF developed at different time points during follow up, the mean follow-up between incident HF and mortality was likely short. Further, an additional 48 cases of HF (in those with ISH) over 10,000 person-years of follow-up may have been too small and did not have the power to affect mortality. Finally, it is unlikely that the natural history of HF associated with ISH would vary from those not associated with ISH, especially in a population that is well balanced in 64 baseline covariates. We have recently demonstrated that hypertension is not intrinsically associated with mortality in patients with chronic HF.27
Most studies of association of ISH with cardiovascular morbidity and mortality have used an older definition of ISH (SBP ≥160 mm Hg) and did not use incident HF as an outcome.8, 9 In the original Framingham Heart Study (n=2767), compared with normal blood pressure, borderline ISH (SBP 140–159 mm Hg and DBP <90 mm Hg) was associated with incident HF.10 However, this association was only adjusted for age, sex, body mass index, smoking, serum cholesterol, and glucose tolerance. Our study is distinguished from those studies by its large sample size, long follow-up, and adjustment for 64 baseline covariates. Our use of propensity-matched design is a particular strength of our study, which not only balanced all measured baseline covariates but also allowed a visually pleasant display of that balance (Table 1 and Figure 1).
According to the 2005 ACC/AHA Chronic HF Guidelines, presence of hypertension constitutes Stage A HF, which denotes increased risk for HF in the absence of structural heart disease or HF symptoms.28 The presence of ventricular remodeling and LVH without HF symptoms indicates Stage B HF, while HF symptoms mark the development of Stage C or clinical HF. Over 80% of the about 5 million Stage C HF patients in the US are older adults, and the incidence of HF increases exponentially with age.29 Essential hypertension has the highest population-attributable risk for HF and is highly prevalent among older adults.6 Essential hypertension is often preceded by ISH denoting a potential pre-Stage A in the development of HF.10 Lowering of blood pressure in early hypertension has been shown to reduce ventricular remodeling and incident HF.1, 2, 30, 31 Finding from our study highlight the importance of SBP and its reduction, a Healthy People 2010 goal,32 especially among those with diabetes and CKD.
Our study has several limitations. While propensity score technique can account for imbalances in all measured covariates, it may or may not balance unmeasured covariates. However, for such an unmeasured covariate to become a confounder, in addition to being a near-perfect predictor of outcomes (incident HF), it must also be associated with exposure (baseline ISH) and should not be strongly correlated with any of the 64 covariates used in our propensity score model. Participants without ISH at baseline may have developed ISH during follow-up and vice-versa. This regression dilution may have underestimated the true associations observed in our study.33
Perspectives
This analysis suggests that, in a propensity-matched population of community-dwelling older adults who were well balanced in 64 measured baseline demographic, clinical, subclinical, and biochemical covariates including history of hypertension and diastolic blood pressure, the presence of baseline isolated systolic hypertension had a significant independent association with the development of new-onset heart failure, coronary artery disease, and cerebrovascular disease, but had no association with mortality. Elevated systolic blood pressure in older adults with isolated systolic hypertension should be properly controlled to reduce cardiovascular morbidity including heart failure.
Acknowledgments
“The Cardiovascular Health Study (CHS) was conducted and supported by the NHLBI in collaboration with the CHS Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the CHS Study or the NHLBI.”
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through a grant from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama. “The Cardiovascular Health Study (CHS) was conducted and supported by the NHLBI in collaboration with the CHS Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the CHS Study or the NHLBI.
Footnotes
Author Contributions
Dr. Ahmed conceived the study hypothesis and design, and performed statistical analyses in collaboration with Drs. Ekundayo, Aban and Love. Drs. Ahmed and Ekundayo prepared the first draft of the manuscript. All authors interpreted the data, participated in critical revision of the manuscript for important intellectual content, and approved the final version of the article. Drs. Ahmed and Ekundayo had full access to all data.
Conflict of Interest/Disclosure Statement
None.
References
- 1.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 2.Staessen JA, Gasowski J, Wang JG, Thijs L, Den Hond E, Boissel JP, Coope J, Ekbom T, Gueyffier F, Liu L, Kerlikowske K, Pocock S, Fagard RH. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet. 2000;355:865–872. doi: 10.1016/s0140-6736(99)07330-4. [DOI] [PubMed] [Google Scholar]
- 3.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 4.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 5.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 6.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med. 1972;287:781–787. doi: 10.1056/NEJM197210192871601. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell CJ, Ridker PM, Glynn RJ, Berger K, Ajani U, Manson JE, Hennekens CH. Hypertension and borderline isolated systolic hypertension increase risks of cardiovascular disease and mortality in male physicians. Circulation. 1997;95:1132–1137. doi: 10.1161/01.cir.95.5.1132. [DOI] [PubMed] [Google Scholar]
- 9.Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O’Leary DH, Bild DE, Robbins J, Fried LP, Reid C. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA. 1992;268:1287–1291. [PubMed] [Google Scholar]
- 10.Sagie A, Larson MG, Levy D. The natural history of borderline isolated systolic hypertension. N Engl J Med. 1993;329:1912–1917. doi: 10.1056/NEJM199312233292602. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.The Joint National Committee on Prevention D, and Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 13.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 15.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 16.Ahmed A, Aban IB, Vaccarino V, Lloyd-Jones DM, Goff DC, Jr, Zhao J, Love TE, Ritchie C, Ovalle F, Gambassi G, Dell’Italia LJ. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–1590. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 21.SPSS for Windows, Rel. 15. Chicago, IL: SPSS Inc; 2007. [computer program]. Version. [Google Scholar]
- 22.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. Vol. 1. New York: Springer-Verlag; 2002. pp. 105–170. [Google Scholar]
- 23.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 24.Pini R, Cavallini MC, Bencini F, Silvestrini G, Tonon E, De Alfieri W, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ. Cardiovascular remodeling is greater in isolated systolic hypertension than in diastolic hypertension in older adults: the Insufficienza Cardiaca negli Anziani Residenti (ICARE) a Dicomano Study. J Am Coll Cardiol. 2002;40:1283–1289. doi: 10.1016/s0735-1097(02)02159-9. [DOI] [PubMed] [Google Scholar]
- 25.Smulyan H, Safar ME. The diastolic blood pressure in systolic hypertension. Ann Intern Med. 2000;132:233–237. doi: 10.7326/0003-4819-132-3-200002010-00010. [DOI] [PubMed] [Google Scholar]
- 26.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 27.Filippatos GS, Adamopoulos C, Sui X, Love TE, Pullicino PM, Lubsen J, Bakris G, Anker SD, Howard G, Kremastinos DT, Ahmed A. A propensity-matched study of hypertension and increased stroke-related hospitalization in chronic heart failure. Am J Cardiol. 2008;101:1772–1776. doi: 10.1016/j.amjcard.2008.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 29.Kannel WB. Vital epidemiologic clues in heart failure. J Clin Epidemiol. 2000;53:229–235. doi: 10.1016/s0895-4356(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed A, Perry GJ, Husain A. VALIDD should not invalidate angiotensin-receptor blockers. Lancet. 2007;369:2053–2054. doi: 10.1016/S0140-6736(07)60955-6. [DOI] [PubMed] [Google Scholar]
- 31.Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 32.Fine LJ, Cutler JA. Hypertension and the treating physician: understanding and reducing therapeutic inertia. Hypertension. 2006;47:319–320. doi: 10.1161/01.HYP.0000200692.23410.c9. [DOI] [PubMed] [Google Scholar]
- 33.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]