Abstract
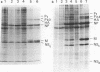
Virus-specific protein and RNA syntheses have been analyzed in chicken embryo fibroblast cells infected with two group IV temperature-sensitive (ts) mutants of influenza A (fowl plague) virus in which the ts lesion maps in RNA segment 8 (J. W. Almond, D. McGeoch, and R. D. Barry, Virology 92:416-427, 1979), known to code to code for two nonstructural proteins, NS1 and NS2. Both mutants induced the synthesis of similar amounts of all the early virus-specific proteins (P1, P2, P3, NP, and NS1) at temperatures that were either permissive (34 degrees C) or nonpermissive (40.5 degrees C) for replication. However, the synthesis of M protein, which normally accumulates late in infection, was greatly reduced in ts mutant-infected cells at 40.5 degrees C compared to 34 degrees C. The NS2 protein was not detected at either temperature in cells infected with one mutant (mN3), and was detected only at the permissive temperature in cells infected with mutant ts47. There was no overall reduction in polyadenylated (A+) complementary RNA, which functions as mRNA, in cells infected with these mutants at 40.5 degrees C compared to 34 degrees C, nor was there any evidence of selective accumulation of this type of RNA within the nucleus at the nonpermissive temperature. No significant differences in ts mutant virion RNA transcriptase activity were detected by assays in vitro at 31 and 40.5 degrees C compared to wild-type virus. Virus-specific non-polyadenylated (A-) complementary RNA, which is believed to act as the template for new virion RNA production, accumulated normally in cells at both 34 and 40.5 degrees C, but at 40.5 degrees C accumulation of new virion RNA was reduced by greater than 90% when compared to accumulation at 34 degrees C.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond J. W., McGeoch D., Barry R. D. Method for assigning temperature-sensitive mutations of influenza viruses to individual segments of the genome. Virology. 1977 Aug;81(1):62–73. doi: 10.1016/0042-6822(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Almond J. W., McGeoch D., Barry R. D. Temperature-sensitive mutants of fowl plague virus: isolation and genetic characterization. Virology. 1979 Jan 30;92(2):416–427. doi: 10.1016/0042-6822(79)90146-6. [DOI] [PubMed] [Google Scholar]
- Barrett T., Wolstenholme A. J., Mahy B. W. Transcription and replication of influenza virus RNA. Virology. 1979 Oct 15;98(1):211–225. doi: 10.1016/0042-6822(79)90539-7. [DOI] [PubMed] [Google Scholar]
- Barry R. D., Mahy B. W. The influenza virus genome and its replication. Br Med Bull. 1979 Jan;35(1):39–46. doi: 10.1093/oxfordjournals.bmb.a071540. [DOI] [PubMed] [Google Scholar]
- Borland R., Mahy B. W. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in cells infected with influenza virus. J Virol. 1968 Jan;2(1):33–39. doi: 10.1128/jvi.2.1.33-39.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Gandhi S. S., Bell H. B., Burke D. C. Abortive infection of L cells by fowl plague virus: comparison of RNA and protein synthesis in infected chick and L cells. J Gen Virol. 1971 Dec;13(3):424–432. doi: 10.1099/0022-1317-13-3-423. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J. Influenza virus transcription. Br Med Bull. 1979 Jan;35(1):47–50. doi: 10.1093/oxfordjournals.bmb.a071541. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Barrett T., Brown C. M., Almond J. W. The smallest genome RNA segment of influenza virus contains two genes that may overlap. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3790–3794. doi: 10.1073/pnas.76.8.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Mahy B. W. Polypeptides specified by the influenza virus genome. 3. Control of synthesis in infected cells. Virology. 1979 May;95(1):154–164. doi: 10.1016/0042-6822(79)90410-0. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., McGeoch D. J., Mahy B. W. Polypeptides specified by the influenza virus genoma. 2. Assignement of protein coding functions to individual genome segments by in vitro translation. Virology. 1977 May 15;78(2):522–536. doi: 10.1016/0042-6822(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Etkind P. R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973 Nov;56(1):334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Soeiro R. Studies on the intranuclear localization of influenza virus-specific proteins. Virology. 1975 Apr;64(2):378–387. doi: 10.1016/0042-6822(75)90114-2. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4908–4912. doi: 10.1073/pnas.76.10.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. S. Isolation of temperature-sensitive mutants and the construction of a preliminary genetic map for influenza virus. J Gen Virol. 1970 Jan;6(1):63–75. doi: 10.1099/0022-1317-6-1-63. [DOI] [PubMed] [Google Scholar]
- Mahy B. W., Barrett T., Briedis D. J., Brownson J. M., Wolstenholme A. J. Influence of the host cell on influenza virus replication. Philos Trans R Soc Lond B Biol Sci. 1980 Feb 25;288(1029):349–357. doi: 10.1098/rstb.1980.0011. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Taylor J. M., Broni B., Krug R. M. Nuclear accumulation of influenza viral RNA transcripts and the effects of cycloheximide, actinomycin D, and alpha-amanitin. J Virol. 1979 Feb;29(2):744–752. doi: 10.1128/jvi.29.2.744-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markushin S. G., Ghendon Y. Z. Genetic classification and biological properties of temperature-sensitive mutants of fowl plague virus. Acta Virol. 1973 Sep;17(5):369–376. [PubMed] [Google Scholar]
- McGeoch D., Kitron N. Influenza virion RNA-dependent RNA polymerase: stimulation by guanosine and related compounds. J Virol. 1975 Apr;15(4):686–695. doi: 10.1128/jvi.15.4.686-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Dimmock N. J. Selective inhibition of influenza virus protein synthesis by inhibitors of DNA function. Virology. 1977 May 15;78(2):393–406. doi: 10.1016/0042-6822(77)90116-7. [DOI] [PubMed] [Google Scholar]
- Morrongiello M. P., Dales S. Characterization of cytoplasmic inclusions formed during influenza/WSN virus infection of chick embryo fibroblast cells. Intervirology. 1977;8(5):281–293. doi: 10.1159/000148903. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. P1 and P3 proteins of influenza virus are required for complementary RNA synthesis. J Virol. 1977 Mar;21(3):1187–1195. doi: 10.1128/jvi.21.3.1187-1195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Bowles A. L. Isolation and characterization of temperature-sensitive mutants of fowl plague virus. Virology. 1975 Oct;67(2):576–587. doi: 10.1016/0042-6822(75)90457-2. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Harms E., Rohde W., Orlich M., Rott R. Correlation between RNA fragments of fowl plague virus and their corresponding gene functions. Virology. 1976 Oct 15;74(2):332–344. doi: 10.1016/0042-6822(76)90340-8. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Compans R. W. Isolation and characterization of cytoplasmic inclusions from influenza A virus-infected cells. J Virol. 1978 Feb;25(2):608–615. doi: 10.1128/jvi.25.2.608-615.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Early polypeptide synthesis in influenza virus-infected cells. Virology. 1973 Nov;56(1):394–399. doi: 10.1016/0042-6822(73)90320-6. [DOI] [PubMed] [Google Scholar]
- Sugiura A., Tobita K., Kilbourne E. D. Isolation and preliminary characterization of temperature-sensitive mutants of influenza virus. J Virol. 1972 Oct;10(4):639–647. doi: 10.1128/jvi.10.4.639-647.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Ueda M., Tobita K., Enomoto C. Further isolation and characterization of temperature-sensitive mutants of influenza virus. Virology. 1975 Jun;65(2):363–373. doi: 10.1016/0042-6822(75)90042-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Hampson A. W., Layton J. E., White D. O. The polypeptides of influenza virus. IV. An analysis of nuclear accumulation. Virology. 1970 Nov;42(3):744–752. doi: 10.1016/0042-6822(70)90320-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Litwin S., Herring L., Broni B., Krug R. M. Use of specific radioactive probes to study transcription and replication of the influenza virus genome. J Virol. 1977 Feb;21(2):530–540. doi: 10.1128/jvi.21.2.530-540.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M. Temperature-sensitive mutants of influenza virus. Isolation and preliminary characterization. Arch Gesamte Virusforsch. 1972;39(4):360–368. doi: 10.1007/BF01241015. [DOI] [PubMed] [Google Scholar]