Abstract
RhoC protein, a known marker of metastases in aggressive breast cancers and melanoma, has also been found to be over-expressed in certain head and neck cancers, thus we investigated the correlation between RhoC expression and the metastatic behavior of head and neck squamous cell carcinoma. Selective inhibition of RhoC expression was achieved using lentiviral small hairpin RNA (shRNA) transduced and tracked with green fluorescent protein (GFP) to achieve 70-80% RhoC inhibition. Fluorescence microscopy of the RhoC knockdown stable clones showed strong green fluorescence in the majority of cells, signifying a high efficiency of transduction. Importantly, qRT-PCR showed no significant decrease in the mRNA expression levels of other members of the Ras superfamily. Cell motility and invasion were markedly diminished in RhoC depleted cell lines as compared to control transduced lines. Hematoxylin and eosin staining of lung tissue obtained from SCID mice which had been implanted with RhoC knockdown cells showed marked decrease in lung metastasis and inflammation of the blood vessels. The cultured lung tissue showed a significant decrease in cell growth in mice implanted with RhoC depleted cell lines as compared to shRNA scrambled sequence control lines. Microscopic studies of CD31 expression revealed substantial quantitative and qualitative differences in the primary tumor microvessel density as compared to parental and shRNA-scrambled controls. This study is the first of its kind to establish the involvement of RhoC specifically in head and neck metastasis. These findings suggest that RhoC warrants further investigation to delineate its robustness as a novel potentially therapeutic target.
Keywords: Tumor metastasis, lentiviral transduction, CD31, lung metastasis, RhoC
Introduction
Head and neck cancer is the sixth most common cancer world wide (1). As per the statistical report of the American Cancer Society, about 70,000 new head and neck squamous cell carcinoma will be diagnosed this year in the United States (2). In contrast to other epithelial cancers for which effective screening exists, most of the patients with head and neck cancer are diagnosed at a very late stage (stage III and IV). Despite the advancement in surgical procedures, chemotherapy, and radiation therapy, survival rates have not improved in the last several decades (3). Furthermore, it has been shown that the high rate of morbidity is due to both locoregional recurrence and distant metastases.
In the past decade, numerous studies have shown that the Rho family of GTPases, (RhoA, RhoB, RhoC, Rac1, Rac2, Rac3 and CDC42) is involved in instilling a metastatic phenotype into localized cancerous cells that are localized to the organ of origin. RhoA and RhoC are over expressed in a number of tumor types (4, 5) suggesting an oncogenic role. Among the Ras homology protein family, RhoC (molecular mass of 21 kDa) has been implicated in a wide range of cellular activities, including downstream expression of inflammatory genes and chemokines, cell proliferation, intracellular signaling, and cytoskeletal organization (6). More significantly, RhoC plays a central role in the assembling focal adhesion, by modulating the orientation of cytoskeletal fibers, resulting in cell polarity, increased cell motility and, consequently, increased invasiveness (7-9). In addition, signaling mediated by Rho proteins through Rho activating kinase (ROCK) regulate proteins that in turn regulate actin polymerization such as cofilin, profilin and formin homology (FH) proteins (10). Interestingly, high levels of RhoC and ROCK are also associated with membrane blebbing, a phenomenon that is observed in motile or invasive cells (10, 11).
RhoC over expression is now well documented in a wide range of malignant cancers suggesting an important role in changing non-invasive carcinomas into invasive forms. Interestingly, over expression of RhoC has been reported in inflammatory breast cancer and exclusively in invasive breast carcinoma (12-15). Other tumor types where over expression of RhoC has been reported are ovarian carcinoma (16), esophageal squamous cell carcinoma (17), pancreatic cancer (18), gastric cancer (17, 19) and human melanoma (11, 20). In addition, functional studies have shown that RhoC can act as a transforming oncogene when it is over expressed in human mammary epithelia converting these normally immobile cells into highly motile and invasive malignant cells (12, 21). Thus, a wide range of current studies reveal an important role of RhoC in cancer metastasis.
However, very few studies to date have investigated the role of RhoC in head and neck cancer. Studies on gene expression profiling of stage III and IV regionally metastatic head and neck squamous cell carcinoma (HNSCC) showed that there is elevated levels of RhoC when compared to stage I and II localized malignancy (22). Furthermore, in our laboratory, we have shown that there is elevated RhoC expression in tumors of patients with HNSCC when compared to normal squamous cell epithelium (21). More importantly, our study showed that increased RhoC expression is strongly associated with lymph node metastasis and could also be used to predict metastasis even in small (T1, T2) primary tumors (23). In the present study, we investigated the role of RhoC in head and neck metastasis by inhibiting its function using RNA interference (RNAi). Our in vitro findings determined that inhibiting RhoC function strongly reduced cell motility and invasion. Furthermore, we observed a remarkable reduction in tumor metastasis and microvessel density in SCID mice injected with RhoC knockdown cell lines. These findings suggest that inhibition of RhoC function in head and neck squamous cell carcinoma can diminish a tumor's aggressive behavior, thus opening new possibilities for future drug therapies targeting this pathway.
Results
RhoC mRNA expression is greatly reduced in knockdown clones from head and neck squamous cell carcinoma cell lines
To understand the role of RhoC expression on head and neck metastasis, we constructed cellular reagents in which RhoC expression was down regulated by small hairpin RNA (shRNA) in UM-SCC-11A and -1 cell lines. These cells exhibit strong invasive phenotype and have been shown in our previous studies that RhoC is constitutively active in these lines (23).
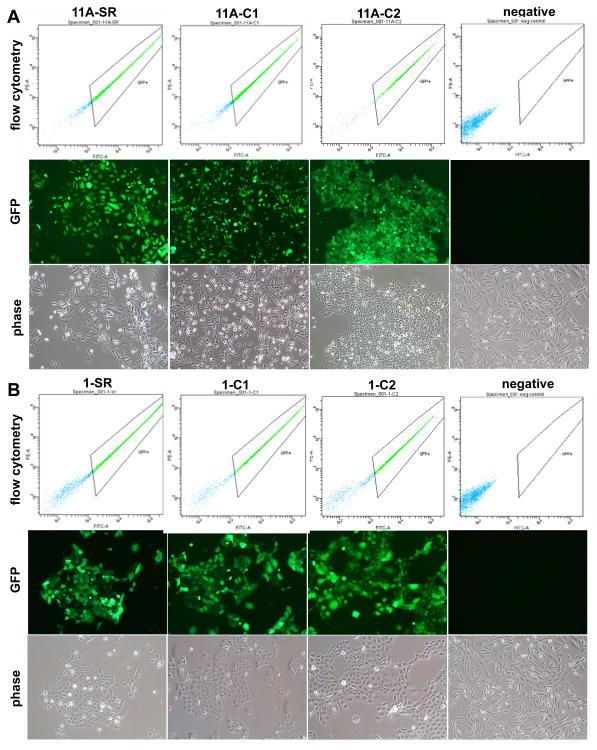
The inhibition of RhoC expression was achieved using RNA interference (RNAi) and lentiviral transfection and transduction technology. After lentiviral infection, positive (stable) clones were selected using puromycin antibiotic. Fluorescence microscopy of the stable clones showed a strong green fluorescence in the majority of the cells, signifying a high efficiency of transfection (Fig.1).
FIGURE 1.
Lentivirus infected cells showing GFP expression levels. Panel (A) is representation of UM-SCC-11A cell line transfected with: shRNA-scrambled sequence control (SR), RhoC Knockdown clone -1 (C1), RhoC knockdown clone -2 (C2), and uninfected cells as the control (negative). The upper panel shows the histograms obtained by flow cytometry, while the middle and lower panels represent GFP labeled cells in fluorescent and bright lights respectively. Panel (B) is a representation of UM-SCC-1. All other notations are the same as described above. As shown by the GFP expression levels, a high number of cells (80-90%) were successfully infected with recombinant lentivirus.
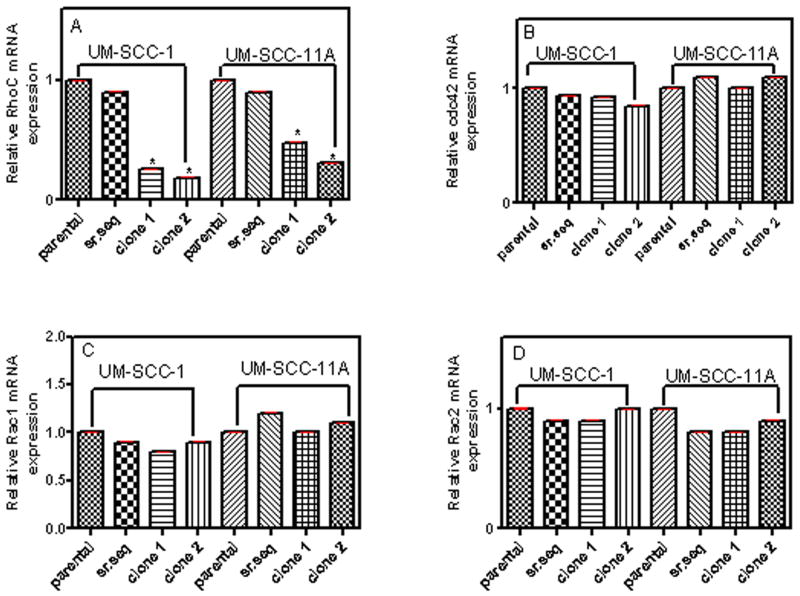
We then tested the effectiveness of shRNA in depleting RhoC mRNA expression in the lentiviral infected cell lines using real time quantitative PCR (qPCR). Because only a small number of specific gene sequences are capable of activating the RNA degradation pathway, we used two different RhoC knockdown clones (namely C1 and C2 along with a parental and shRNA-scrambled sequence infected control) to ensure the effectiveness of depleting levels of RhoC. The results show greatly reduced expression levels of RhoC gene in the C1 and C2 RhoC knockdown clones, while normal RhoC expression was observed in clones with shRNA-scrambled sequence (Fig.2). The relative RhoC mRNA expression in parental, shRNA-scrambled control and RhoC knockdown clones 1 and 2 were evaluated by quantitative RT-PCR and the CT values thus obtained were normalized using two housekeeping genes as described in Material and Methods. As shown in figure 2(A) RhoC mRNA expression decreased about 75% and 80% in RhoC knockdown clone 1 and clone 2 of UM-SCC- 1 respectively. A similar decrease of 40% and 70% in RhoC mRNA levels was observed in RhoC knockdown clone 1 and clone 2 of UM-SCC-11A respectively. However, the control shRNA-scrambled sequence in either of the cell lines did not show any significant reduction in RhoC mRNA expression level (Fig. 2A). To confirm that only RhoC expression was being inhibited, the mRNA levels of other Rho family members, Cdc42, Rac1 and Rac2 were also analyzed by quantitative RT-PCR. As shown in figures 2B, C, and D, the expression levels of Cdc42, Rac1, and Rac2 are very similar to the parental lines, thus confirming that our shRNA process is highly specific to RhoC only. These studies provided a clear insight about the “switching off” of the RhoC machinery by decreasing total levels of RhoC mRNA expression, and therefore, further detailed studies on its functional roles are defensible. One of the most basic clinical questions that arise at this point is how inhibition of the RhoC transcript affects metastasis in head and neck cancer. To address this question, we investigated two characteristic behaviors of metastatic cells, invasion and motility in the transduced cell lines.
FIGURE 2.
Quantitative RT-PCR of cell lines UMSCC-1 and -11A showing the relative mRNA expression levels of (A) RhoC, (B) Cdc42, (C) Rac1 and (D) Rac2 in parental (control), shRNA-scrambled sequence control and RhoC knockdown clones -1 and -2 after selection and establishment of positive clones. Results were analyzed using 2-ΔΔCT methods. A significant decrease in mRNA levels of RhoC knockdown clones were obtained while the expression of Cdc42, Rac1 and Rac2 were remained unchanged (p<0.05).
RhoC knockdown clones show decrease in cell invasion and motility
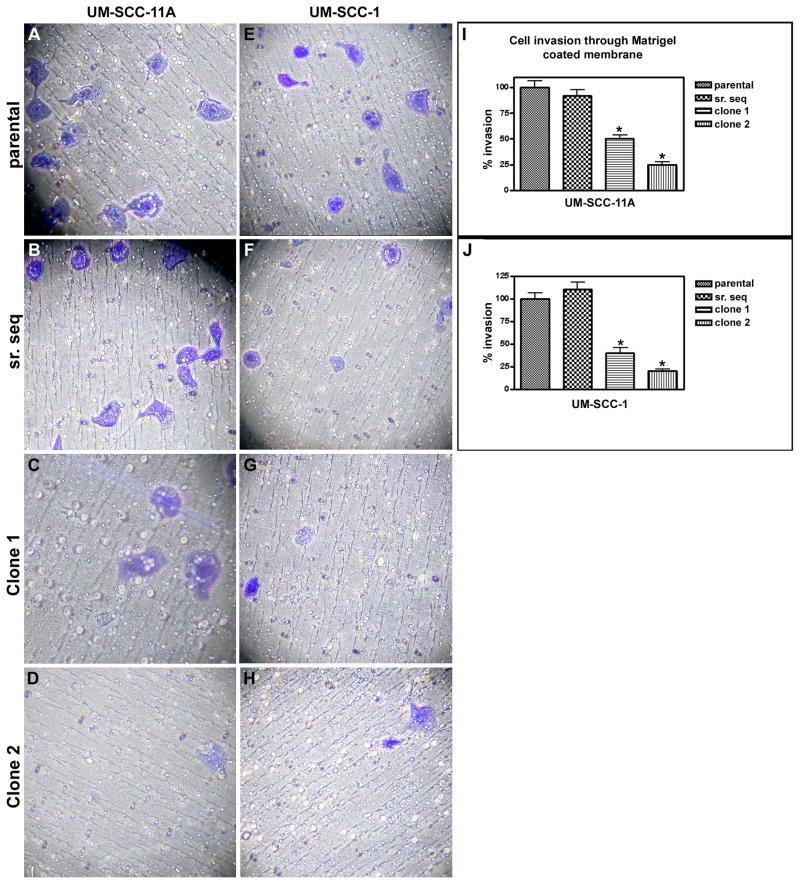
In the invasion assays, RhoC-depleted clones of UMSCC-11A and -1 were remarkably less invasive and motile compared to the parental or shRNA-scrambled controls (Fig. 3). Notably, cell invasion was decreased by 50% and 75% in RhoC knockdown clone 1 and 2 respectively, in the transduced UM-SCC-11A cell line (Fig. 3 I). A similar decrease of 60% and 80% in clones 1 and 2 respectively was observed in UM-SCC-1 lines (Fig.3 J) when compared to their parental or shRNA-scrambled controls (n=3; p< 0.003).
FIGURE 3.
Cell invasion assay of UM-SCC-11A and -1 lines transfected with RhoC sh-RNA. (A&E) Parental cell lines; (B&F) shRNA-scrambled controls; (C&G) RhoC knockdown clones -1; (D&H) RhoC knockdown clones -2 of UM-SCC-11A and 1 respectively (magnification, ×40 & ×100). Columns (I & J), rates of invasion, bars, 95% CI P< 0.05.
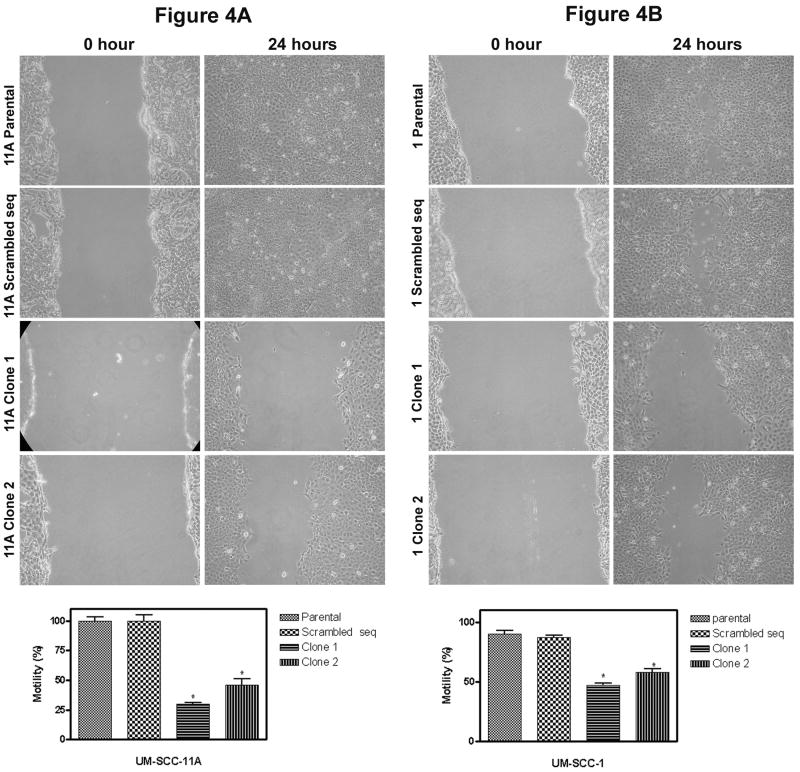
We hypothesized that RhoC plays an important role in cell motility in head and neck squamous cell carcinoma. We therefore investigated the effect of RhoC on the cell motility using the scratch model. A noticeable decrease in cell motility was observed in RhoC knockdown clones as compared to the parental or sh-RNA scrambled sequence control lines (Fig. 4A and 4B), (n=3; p< 0.005). These in vitro assays provide evidence for the first time that RhoC plays an important role in cell invasion and motility and suggest that RhoC is important for metastasis in head and neck cancer.
FIGURE 4.
The effect of RhoC knockdown on cell motility. Panels A and B show the slow movement of RhoC knockdown cells (after 24 hours) as compared to its parental or sh-RNA scrambled control in UM-SCC-11A and 1 respectively (magnification, × 40). Bar graphs show the percent motility (p<0.05) with initial reference point as zero hour.
RhoC plays an important role in lung metastasis and microvessel density formation
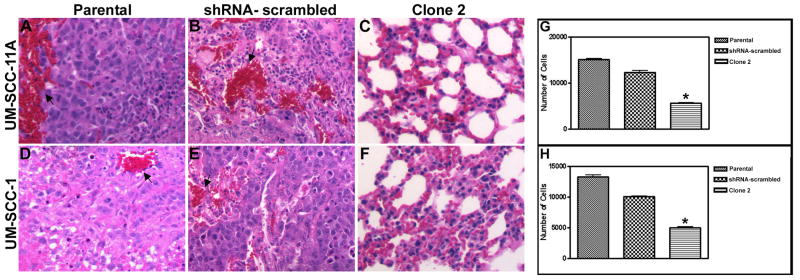
Besides localized tumor growth, lung metastasis is common and frequent occurrence in head and neck cancer patients (24). Keeping in view this aspect we designed an in vivo study where we could analyze the effect of RhoC inhibition on lung metastasis and primary tumor vascularity. This was achieved by injecting transduced cell lines through tail veins of SCID mice and analyzing them for lung metastasis. Since both clones gave similar results in our cell motility and invasion assays we selected RhoC knockdown clone 2 for our subsequent in vivo studies and all results discussed hereafter are based on this clone. The xenograft mice were sacrificed two weeks after implantation and their lungs were analyzed for metastasis using H&E stain. As shown in figure 5A and B, in the mice injected with UM-SCC-11A parental or shRNA-scrambled control, a colossal protuberance of metastatic tissue and inflamed blood vessels were observed in the lung region (marked by arrows). A similar set of results were obtained for the UM-SCC-1 parental and sh-RNA scrambled sequence control (Fig. D and F). In contrast, mice injected with RhoC knockdown clone have very small metastatic tissue with barely visible patches of inflamed blood vessels in UM-SCC-11A and -1 respectively (Fig. 5 C and F).
FIGURE 5.
The effect of RhoC knockdown on lung metastasis in SCID mice injected through tail vein with UM-SCC-11A AND -1 cell lines transfected with RhoC shRNA. The lung sections were stained with hematoxylin and eosin (H&E) dye to show degree of metastasis. (A&D) Parental; (B&E) shRNA-scrambled controls; (C&F) RhoC knockdown clone 2. Arrows (black) indicate the inflamed blood vessels present only in parental and scrambled-sequence controls (magnification, ×100). (G&H) numbers of cells obtained by culturing the lungs for UMS-CC-11A and -1 respectively showing a marked reduction in RhoC knockdown clone 2 (p<0.05).
In addition, the remaining dissected lung tissues were cultured for observation of cell growth by the metastatic tumors. The bar graph shows the number of cancer cells grown in digested lung of mice which includes parental, shRNA-scrambled control and RhoC knockdown clones. Interestingly, there is a 67% and 58% decrease in cell number in RhoC knockdown clones of UM-SCC-11A and -1 respectively, when compared to their parental lines (Fig.5 G and H). These results strongly suggest that inhibition of RhoC expression greatly reduces metastasis in vivo.
Furthermore, to test the angiogenic role of RhoC, parental and RhoC knockdown cells were implanted in the flank region of the SCID mice. Microvessel density of the localized solid primary tumor which grows into a sizable volume after 12 weeks of implantation in the flank region was analysed using CD31 antibody. Microscopic analysis of the CD31 stained tumor revealed a remarkable difference in microvessels formation in mice implanted with RhoC knockdown clones when compared to the corresponding either parental or shRNA-scrambled control. In the control groups, well developed microvessels were observed in the tumors, which was in strong contrast to the poorly developed microvessels in mice implanted with RhoC knockdown clone (Fig. 6). Our results are in coherence of the published work about the essential role of RhoC in angiogenesis (25, 26). A similar pattern of microvessels development was observed in UM-SCC-11A parental, shRNA-scrambled, and RhoC knockdown clone (data not shown). These results suggest that RhoC is required for proper formation of the vascular network in a developing tumor.
FIGURE 6.
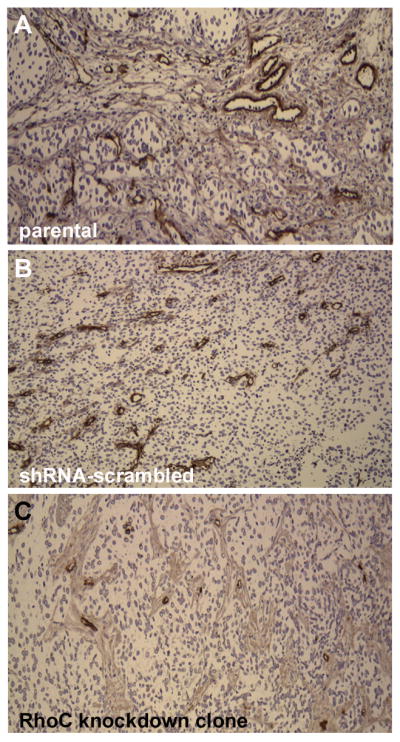
Assessment of microvessl density in UM-SCC-1 lines using immunostaining with CD31 antibody. (A&B) Parental and shRNA-scrambled sequence with well developed microvessels; (C) RhoC knockdown clone 2 with much smaller and poorly developed microvessels (magnification, ×40).
Discussion
Tumor metastasis is well correlated with the over expression of certain oncogenes. The over expression of the Rho gene family has been reported in many malignant forms of cancer (27), including pancreatic cancer (18), gastric cancer (17, 19) and human melanoma (11, 20). However, there have been very few studies on whether over expression of RhoC is involved in head and neck tumor metastasis. Previous studies in our laboratories have shown that RhoC is actively expressed in several well established University of Michigan Squamous Cell Carcinoma cell lines (UM-SCC). Among the cell lines tested, the UM-SCC- 11A and -1 lines exhibited considerably high levels of RhoC protein (23). In particular, the active form of RhoC (RhoC GTPase) was observed to be constitutively expressed in the UM-SCC lines. Therefore, for our current study, we selected two UM-SCC lines (UMSCC-11A and UMSCC-1) to evaluate the role of RhoC in head and neck squamous cell carcinoma metastasis. Our first and foremost aim was to inhibit RhoC expression in the two selected cell lines and analyze its function in vitro. Our expectation was that the motility and invasion would be greatly reduced in RhoC depleted cell lines as compared to parental lines. In this study we have demonstrated a successful inhibition of RhoC gene expression and, subsequently, its function using shRNA techniques (Fig.2). Furthermore, our data show that cell invasiveness and motility which are characteristics of aggressive head and neck cancer cell lines were diminished when RhoC expression was inhibited (Figs.3 and 4). Therefore, these results suggest that RhoC over expression drives cell invasion and motility in HNSCC. It is reported that one of the major functions of Rho-family of proteins is to control cytoskeletal organization (28). Cytoskeletal proteins are involved pre-dominantly in cell motility. Therefore RhoC may control metastasis by modulating cell motility (29). In order to facilitate the movement of cells, they need to turn over both cell-extra cellular matrix and cell to cell adhesions which includes both adherence junctions and tight junctions (30, 31). It has also been reported that RhoC plays a predominant role over RhoA in the weakening of adherence junctions, which is an important step towards transforming cells into an invasive phenotype (6). These studies therefore, raise the question as to what effect RhoC inhibition would create in vivo. Our in vivo results showed that both inflamed blood vessels of lungs and a large volume of lung metastases were present in animals which were administrated by tail vein injection of either parental or shRNA-scrambled sequence (control) cell lines. In contrast, the lungs of mice implanted or injected with RhoC knockdown lines were free from any pathological findings, specifically very minimal lung metastases and very low level of inflammation in lung tissues and blood vessels (Fig.5). Furthermore, the level of angiogenesis in the localized tumors was assessed using CD31 antibody and these results showed a remarkable difference both in quality as well quantity of the microvessels in the tumors. The mice implanted with RhoC knockdown lines showed markedly fewer and less poorly developed microvessels as compared to the far greater in number and clearly defined vessels in parental or shRNA-control cell lines (Fig. 6).
The implications of the findings in this study provide a fertile area of research in head and neck squamous cell carcinoma. For instance, recent work has shown that matrix metalloproteinases, which are well known mediators of invasive tumor behavior, have been identified as a specific and critical player for the formation of lung metastasis (32, 33). Li et al, 2006, reported that the oncogene AF1Q which is responsible for primary breast tumor growth and pulmonary metastasis are at least, in part, regulates other MMPs and RhoC expression (34). The remodeling of the actin cytoskeleton is a critical and important step in the formation of pulmonary metastasis due to changes in cell shape, polarity, cell interactions and eventual migration of the cancer cells. Interestingly, studies by Nelson et al 2008 (35) have shown that the expression of MMP3 gene that induces epithelial-mesenchymal transition in mammary epithelial cells is brought about by change in cell shape through Rac1 (also a member of the Rho family) mediated changes in cytoskeletal structure. Clearly, future studies elucidating the specific interactions between the MMP 2, 3 and 9 (major MMP proteins in head and neck squamous cell carcinoma) and RhoC are indicated and may prove to be one of the signaling pathways for RhoC mediated function.
In conclusion, the findings presented in this study illustrate that both in in vivo and in vitro conditions RhoC plays an important role in head and neck cancer progression and metastasis. With additional investigations and ongoing development of RhoC specific inhibitors, this may prove to be an important therapeutic target in this patient population.
Materials and Methods
Cell culture and generation of stable RhoC knockdown clones
University of Michigan squamous cell carcinoma cell lines (UM-SCC)-11A and (UM-SCC)-1 are a well established cell lines derived from a 65-year-old patient with a T2 N2a of the epiglottis and 46-year-old patient with T2N0 of the false vocal cord (36, 37). These cell lines were grown at 37°C in a humidified atmosphere with 95% air-5% CO2. The cultures were maintained in Dulbecco Modified Eagle Medium (DMEM), (Gibco/BRL; Gaithersburg, MD) containing 10% heat-inactivated fetal bovine serum (Hyclone; Logan, UT) and supplemented with 50μg/ml of penicillin G, and 50μg/ml of streptomycin sulphate.
RhoC knockdown and scrambled sequence constructs with green fluorescence protein (GFP) tag and puromycin resistance sites were synthesized by the vector core facility of the University of Michigan (www.med.umich.edu/vcore). The sequences used for RhoC constructs are available in open biosystems (www.openbiosystems.com) and are: oligo ID V2LHS_69446 and V2LHS_69410, accession number NM_001042678. The sequences of the constructs are 69446 = 5′-ATACTGTCTTTGAGAACTATAT (Sense)[for RhoC knockdown clone 1] and 69410=5′- CACCAGCACTTTATACACTTC (Sense) [for RhoC knockdown clone2]. The sequence of shRNA miR non-silencing (scrambled)controlis:ATCTCGCTTGGGCGAGAGTAAGTGCTGTTGACAGTAAGCGATCTCGCTTGGGCGAGAGTAAGTAGTGAAGCCACAGATGTACTTACTCTCGCCCAGCGAGAGTGCCTACTGCCTCGGA. This control sequence does not match any known mammalian genes (the sequence had at least 3 or more mismatches against any gene which was determined via nucleotide alignment/BLAST of target 22mer sequence). This is the non-silencing shRNAmir hairpin sequence found in the pSM2, pSMP, pGIPZ, pTRIPZ and pLemiR non-silencing controls.
293FT cells (Invitrogen, Carlsbad, CA) were infected with 250mM CaCl2 solution containing RhoC shRNA construct, 25μM chloroquine and viral particles (namely Gag, Pol, and Env) and grown overnight. Media was changed after 12 hours to remove chloroquine and fresh DMEM-10% FBS was added to the growing 293FT cells to produce the virus. The supernatants from the infected cells were collected and 1ml of this solution was added to growing UM-SCC-11A and -1 lines. Cells were incubated at 37°C and the GFP expression was monitored after 48 hours of infection. Positive (stable) clones were selected using Puromycin antibiotic (1.6 μg / ml and 2.0 μg / ml for UM-SCC-11A and -1 respectively). These were then analyzed using fluorescence microscopy which showed a strong green fluorescence in the majority of the cells, signifying a high efficiency of infection (Fig. 1A and 1B). Furthermore, flow cytometry analyses showed that that the numbers of non-infected cells were significantly low (Fig. 1A and 1B).
Flow cytometry analyses
About 70-80% confluent lentivirus infected cells were harvested using trypsin-EDTA solution and re-suspended in phosphate buffer saline containing 3% FBS, 0.5mm EDTA and 60U/ml DNase. Flow cytometry analysis was performed using a BD FACS Aria IIU flow cytometer equipped with a 488nm, 15mW, air-cooled Argon laser. (Analytical Cytometry Laboratory, Ohio State University Comprehensive Cancer Centre). GFP positive cells were sorted out and grow for subsequent experiments.
Quantitative reverse transcriptase polymerase chain reactions (qRT-PCR)
Total RNA was isolated according to the standard procedure using TRIzol reagent (Invitrogen, Carlsbad, CA). Quantitative reverse transcriptase polymerase chain reactions (qRT-PCR) were conducted by Taqman probe system, from Applied Bio Systems (Foster City, CA) by using the following products cd42: Hs03044122_g1, Rac1: Hs01025984_m1, Rac2: Hs01032884_m1 and RhoC: Hs00733980_m1. Beta actin and G3PDH were used as the data normalizers. Relative changes in gene expressions were calculated using 2-ΔΔCT method (38).
Cell invasion and motility assay
Invasion assay
Cell invasion assays were performed using BD BioCoat Matrigel Invasion Chamber which was obtained from BD Biosciences, Bedford, MA USA. The procedure was followed according to manufacturer instructions. Briefly, about 2.5 ×105 cells in 2 ml of serum free DMEM were added at the top of the insert and 1ml of media was added in the bottom wells of each insert. Fetal bovine serum albumin (FBS) was added in the media of lower chamber (final concentration of FBS was 10%, v/v) which acts as a chemo attractant. Cells were incubated for 22 hours in a humidified cell culture incubator, at 37°C, 5%CO2 atmosphere. Next, the non-invading cells at the top of the insert were scraped out with the help of cotton –tipped swab. The invading cells which were attached to the under side of the membrane were fixed in 100% methanol and stained with 1% Toluidine prepared in 100% methanol. After repeated washing of the membrane with distilled water, stained cells were allowed to air dry at room temperature before it was visualized under microscope. A parallel experiment with control insert (without Matrigel) was also run. Matrigel invaded cells were counted microscopically at 40 × and 100 × magnifications.
Motility assay
Cell motility assays were performed in 100 mm Petri dishes. At about 80% confluence of the cells, cells were washed with PBS and a fine scratch in the form of groove was made by the help of sterile pipette tip and immediately photographed. We designate this time as the zero hour. Next cells were supplemented with DMEM containing 10% FBS and allowed to grow. A migration of cells from the edge of the groove towards the centre was monitored microscopically at 40× magnifications after 24 hours to asses the extent of scratched area covered. The width of the scratch was measured at zero hour and after 24 hours to calculate the percentage of the gape covered by the cells in 24 hours time.
Animal xenograft
Athymic severe combined immune deficient (SCID) mice were obtained from the Jackson laboratory, Bar Harbor, ME- USA; 6 weeks old mice were housed in cages of 5 animals in each. Five animals per treatments were selected to receive parental, shRNA-scrambled sequence control and RhoC knockdown clone, resulting 15 animals per cell lines per set of experiments. About 5 ×106 UM-SCC-11A and -1 cells were suspended in 100 μl serum free DMEM and injected thorough the tail vein and or in the flank region into the mice using 0.5 inch, 27-gauge needle. Animals were monitored every other day for their general health and activities. At the end of second weeks the animals were euthanized using a CO2 chamber. The lungs were dissected and half of the lungs were fixed in buffered formalin for 6 hours thereafter transferred to 70 % methanol and then processed to form paraffin embedded tissue blocks Hematoxylin and eosin (H and E) staining were done. Remaining half of the lungs was digested in collagenase for culturing the cells. At the end of week 12, tumors in flank region was fully grown. The animals were euthanized and tumors were dissected and fixed in the same way as described above for CD31 staining.
Lung metastases
Slides of five- micrometer sections of lungs were prepared and stained with H & E stained. Five random fields in a blind fashion way were examined microscopically at 100 × magnification to detect metastases.
Microvessel density
Microvessel density in all primary tumors was assessed using anti mouse CD31 antibody (PharMingen, San Diego, CA) at a dilution of 1:250. Five random low power fields (40 × magnification) were selected to visualize the microvessels. The mean was reported in a blind fashion for each tumor.
Statistical analysis
Statistical analyses were performed using sigma graph pad prism 4 software. The mean was reported with Standard deviation (±SD). Differences were considered to be statistically significant when p values were less than 0.05.
Acknowledgments
The authors thank Dr. N. S. Mahfooz for critical reading of the manuscript, Dr. Thomas Carey for providing the cell lines for use in this work and the NCI SPORE program for grant support.
Grant and financial support: National Cancer Institute Head and Neck Cancer SPORE grants (TN Teknos and SD Merajver), NIH CA 77612 (SDM), Breast Cancer Research Foundation (SDM), Burroughs Wellcome Fund (SDM)
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Society AC. Cancer Facts and Figures. 2009;2009 [Google Scholar]
- 3.Tran N, Rose BR. role of human papilomavirus in the etiology of head and neck cancer head and neck. 2007;29:64–70. doi: 10.1002/hed.20460. [DOI] [PubMed] [Google Scholar]
- 4.Kleer CG, van Golen KL, Zhang Y, Wu ZF, Rubin MA, Merajver SD. Characterization of RhoC expression in benign and malignant breast disease: a potential new marker for small breast carcinomas with metastatic ability. Am J Pathol. 2002;160(2):579–84. doi: 10.1016/S0002-9440(10)64877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27(6):602–13. doi: 10.1002/bies.20238. [DOI] [PubMed] [Google Scholar]
- 6.Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4(6):408–15. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 7.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 8.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16(10):5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepage M, Dow WC, Melchior M, et al. Noninvasive detection of matrix metalloproteinase activity in vivo using a novel magnetic resonance imaging contrast agent with a solubility switch. Mol Imaging. 2007;6(6):393–403. [PubMed] [Google Scholar]
- 10.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26(4):273–87. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 11.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406(6795):532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 12.van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60(20):5832–8. [PubMed] [Google Scholar]
- 13.van Golen KL, Davies S, Wu ZF, et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5(9):2511–9. [PubMed] [Google Scholar]
- 14.Kleer CG, Zhang Y, Pan Q, et al. WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer. Breast Cancer Res. 2004;6(1):R110–5. doi: 10.1186/bcr755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo AC, Kleer CG, Banerjee M, et al. Molecular epidemiologic features of inflammatory breast cancer: a comparison between Egyptian and US patients. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horiuchi A, Imai T, Wang C, et al. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83(6):861–70. doi: 10.1097/01.lab.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 17.Faried A, Faried LS, Kimura H, et al. RhoA and RhoC proteins promote both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo. Eur J Cancer. 2006;42(10):1455–65. doi: 10.1016/j.ejca.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Kusama T, Mukai M, Endo H, et al. Inactivation of Rho GTPases by p190 RhoGAP reduces human pancreatic cancer cell invasion and metastasis. Cancer Sci. 2006;97(9):848–53. doi: 10.1111/j.1349-7006.2006.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Q, Bao LW, Teknos TN, Merajver SD. Targeted disruption of protein kinase C epsilon reduces cell invasion and motility through inactivation of RhoA and RhoC GTPases in head and neck squamous cell carcinoma. Cancer Res. 2006;66(19):9379–84. doi: 10.1158/0008-5472.CAN-06-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruth MC, Xu Y, Maxwell IH, Ahn NG, Norris DA, Shellman YG. RhoC promotes human melanoma invasion in a PI3K/Akt-dependent pathway. J Invest Dermatol. 2006;126(4):862–8. doi: 10.1038/sj.jid.5700211. [DOI] [PubMed] [Google Scholar]
- 21.van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD. RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia. 2000;2(5):418–25. doi: 10.1038/sj.neo.7900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmalbach CE, Chepeha DB, Giordano TJ, et al. Molecular profiling and the identification of genes associated with metastatic oral cavity/pharynx squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(3):295–302. doi: 10.1001/archotol.130.3.295. [DOI] [PubMed] [Google Scholar]
- 23.Kleer CG, Teknos TN, Islam M, et al. RhoC GTPase expression as a potential marker of lymph node metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2006;12(15):4485–90. doi: 10.1158/1078-0432.CCR-06-0376. [DOI] [PubMed] [Google Scholar]
- 24.Hsu YB, Chu PY, Liu JC, et al. Role of chest computed tomography in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134(10):1050–4. doi: 10.1001/archotol.134.10.1050. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Wu F, Fang F, Tao Y, Yang L. RhoC is essential for angiogenesis induced by hepatocellular carcinoma cells via regulation of endothelial cell organization. Cancer Sci. 2008;99(10):2012–8. doi: 10.1111/j.1349-7006.2008.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merajver SD, Usmani SZ. Multifaceted role of Rho proteins in angiogenesis. J Mammary Gland Biol Neoplasia. 2005;10(4):291–8. doi: 10.1007/s10911-006-9002-8. [DOI] [PubMed] [Google Scholar]
- 27.Bos JL. The ras gene family and human carcinogenesis. Mutat Res. 1988;195(3):255–71. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 28.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11(18):2295–322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 29.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 30.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 31.Aspenstrom P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377(Pt 2):327–37. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2(4):289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 33.Ikoma T, Takahashi T, Nagano S, et al. A definitive role of RhoC in metastasis of orthotopic lung cancer in mice. Clin Cancer Res. 2004;10(3):1192–200. doi: 10.1158/1078-0432.ccr-03-0275. [DOI] [PubMed] [Google Scholar]
- 34.Li DQ, Hou YF, Wu J, et al. Gene expression profile analysis of an isogenic tumour metastasis model reveals a functional role for oncogene AF1Q in breast cancer metastasis. Eur J Cancer. 2006;42(18):3274–86. doi: 10.1016/j.ejca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Nelson CM, Khauv D, Bissell MJ, Radisky DC. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem. 2008;105(1):25–33. doi: 10.1002/jcb.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey TE, Kimmel KA, Schwartz DR, Richter DE, Baker SR, Krause CJ. Antibodies to human squamous cell carcinoma. Otolaryngol Head Neck Surg. 1983;91(5):482–91. doi: 10.1177/019459988309100503. [DOI] [PubMed] [Google Scholar]
- 37.Krause CJ, Carey TE, Ott RW, Hurbis C, McClatchey KD, Regezi JA. Human squamous cell carcinoma. Establishment and characterization of new permanent cell lines. Arch Otolaryngol. 1981;107(11):703–10. doi: 10.1001/archotol.1981.00790470051012. [DOI] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]