Abstract
Objective
Oxidized phospholipids are key factors in inflammation and associated diseases including atherosclerosis yet the initial reception mechanisms for cellular responses to the factors are poorly understood. The objective of this study was to determine if calcium-permeable channels are targets for the oxidized phospholipids, 1-palmitoyl-2-glutaroyl-phosphatidylcholine (PGPC) and 1-palmitoyl-2-oxovaleroyl-phosphatidylcholine (POVPC).
Methods and Results
Low micromolar concentrations of PGPC and POVPC evoked rises in intracellular calcium in HEK 293 cells over-expressing human Transient Receptor Potential Canonical 5 (TRPC5) but not human Transient Receptor Potential Melastatin 2 or 3. Electrophysiological experiments confirmed stimulation of TRPC5. To investigate relevance to endogenous channels we studied proliferating vascular smooth muscle cells from patients undergoing coronary artery bypass surgery. PGPC and POVPC elicited calcium entry that was inhibited by anti-TRPC5 or anti-TRPC1 antibodies or dominant-negative mutant TRPC5. Calcium-release did not occur. The effect was functionally relevant because it enhanced cell migration. Actions of PGPC and POVPC depended on Gi/o proteins but not previously identified G protein coupled receptors for oxidized phospholipids.
Conclusions
The data suggest that stimulation of calcium-permeable TRPC5-containing channels is an early event in cellular responses to oxidized phospholipids that couples to cell migration and requires an unidentified G protein coupled receptor.
Keywords: Oxidized phospholipids, Calcium channels, Transient Receptor Potential, G proteins, Vascular smooth muscle cells
INTRODUCTION
1-Palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC) is a phospholipid that is a common component of mammalian cell membranes and lipoproteins1,2. The susceptibility of PAPC and other constituent lipids to oxidation through myeloperoxidase, lipoxygenase and other enzyme activities leads to bioactive oxidation products (oxidized phospholipids)3,4. Two important products of PAPC are POVPC (1-palmitoyl-2-oxovaleroyl-phosphatidylcholine) and PGPC (1-palmitoyl-2-glutaroyl-phosphatidylcholine)1,2,5; they are two examples of oxidized phospholipids that are now recognized to constitute a diverse family of signaling lipids that accumulate during oxidative stress, apoptosis and necrosis, and are often associated with inflammatory conditions such as rheumatoid arthritis and atherosclerosis. There are also suggestions of physiological roles for these lipids that include leukocyte-endothelial interactions and pattern recognition in innate immunity6,7. Both pro- and anti-inflammatory effects of oxidized phospholipids have been detected8, suggesting context dependent actions and critical importance of the free lipid concentration. Although the importance of oxidized phospholipids is increasingly established, understanding of the initial reception and signaling mechanisms is limited. Perhaps surprisingly, given the pivotal roles of Ca2+-signaling in mammalian cells, information is lacking on the effects of oxidized phospholipids on Ca2+ channels or other Ca2+ transport mechanisms.
Mammalian transient receptor potential (TRP) proteins are ion pore-forming subunits of cationic channels with wide-ranging roles in physiology and disease9. The Canonical (C) subfamily has seven members that are generally considered to form heteromultimeric assemblies in native cells9-12. While the channels may have constitutive activity they are also stimulated by various chemical factors, potentially acting as polymodal integrators of Ca2+ movement across cell membranes10,11. TRPC5 is a commonly studied example of the TRPC subfamily11-13. It is stimulated by factors that include lanthanide ions, protons, reduced thioredoxin, and sphingosine-1-phosphate (S1P)11-14. It is also stimulated by lysophosphatidylcholine15, a major phospholipid on oxidized low-density lipoproteins. Because of the effect of lysophosphatidylcholine we investigated chemically-related phospholipids with relevance to cardiovascular disease. Here we report on effects of POVPC and PGPC on TRPC5 over-expressed in the HEK 293 cell-line and on endogenous TRPC5-containing channels in vascular smooth muscle cells (VSMCs), which are targets for the actions of oxidized phospholipids16-18.
METHODS
Conditional channel expression in HEK 293 cells
HEK 293 cells stably expressing tetracycline-regulated human TRPC5 have been described10. Cells were grown in DMEM-F12 medium containing 10 % fetal calf serum (FCS), 100 units.ml−1 penicillin and 100 μg.ml−1 streptomycin. Cells were maintained at 37 °C in 95 % air and 5 % CO2 and selected with 250 μg.ml−1 zeocin and 10 μg.ml−1 blasticidin. TRP channel expression was induced by 1 μg.ml−1 tetracycline (Tet+). Non-induced cells without addition of tetracycline were controls (Tet−). Cells were replated on poly-D-lysine-coated black 96-well plates (Corning or BD Biosciences) or 13 mm glass coverslips 24 hr prior to experiments.
Vascular smooth muscle cells (VSMCs)
Freshly discarded human saphenous vein segments were obtained anonymously and with informed consent from patients undergoing open heart surgery in the General Infirmary at Leeds. Approval was granted by the Leeds Teaching Hospitals Local Research Ethics Committee. The investigation conforms to the principles outlined in the Declaration of Helsinki. Proliferating VSMCs were prepared using an explant technique19 and grown in DME medium supplemented with 10 % FCS, penicillin/streptomycin and L-glutamine at 37 °C in a 5 % CO2 incubator. Experiments were performed on cells at passage 3-4.
Intracellular Ca2+ measurement
Cells were incubated with 2 μM fluo4-AM or fura2-AM for 1 hr at 37 °C followed by 0.5 hr wash at room temperature. The loading buffer was standard bath (extracellular) solution (SBS), which contained (mM): 130 NaCl, 5 KCl, 8 D-glucose, 10 HEPES, 1.5 CaCl2, 1.2 MgCl2, titrated to pH 7.4 with NaOH. Loading buffer included 2 μM probenicid (fluo4-AM only) and 8 μM pluronic acid. Measurements were at room temperature (21±2 °C) on an inverted fluorescence microscope (see Supplemental Material) or a 96-well fluorescence plate reader (FlexStation II384, Molecular Devices). All Ca2+ data derived from the 96-well system except those of Fig 3c. With either system, changes in intracellular calcium (Ca2+i) concentration were indicated by the change (Δ) in fluo4 fluorescence (F, in arbitrary units) or the ratio of fura2 emission intensities for 340 nm and 380 nm excitation (F ratio). Fluo4 fluorescence values were divided by 104 (F*). Wells within columns of 96-well plates were loaded alternately for test and control conditions. The extracellular solution was SBS unless indicated otherwise; Ca2+-free solution was SBS without Ca2+ but with 0.4 mM EGTA.
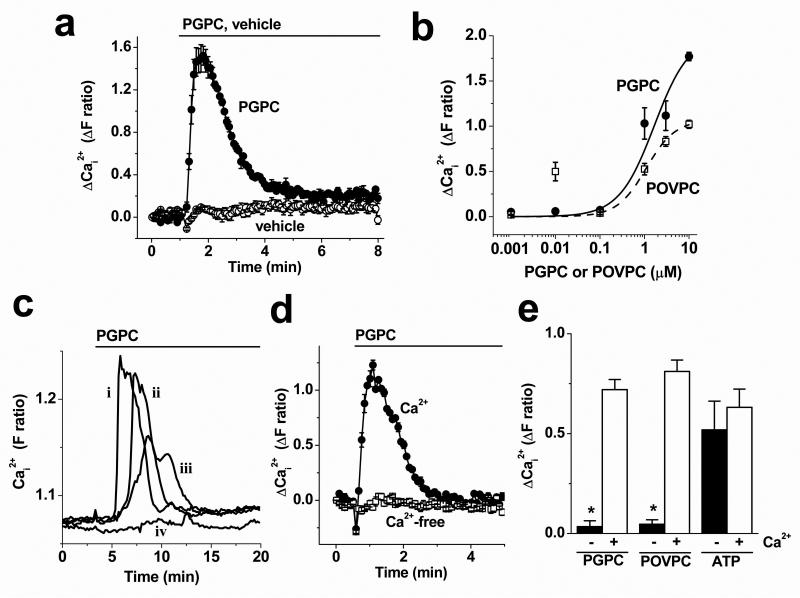
Figure 3. Responses of VSMCs to PGPC and POVPC.
Data are from intracellular Ca2+ measurements. (a) Typical response to bath-applied 3 μM PGPC compared with vehicle control. (b) Concentration-response data for PGPC and POVPC (n/N=4/12 for each point). Mid-points of the fitted Hill equations were at 1.5 μM (PGPC) and 1.09 μM (POVPC). (c) Example single VSMC responses to 3 μM PGPC observed using the microscope system (4 cells, labeled i-iv). (d) Responses to 3 μM PGPC in the presence and absence of extracellular Ca2+ (N=6 for each). (e) As for (d) but mean data from independent experiments for 3 μM PGPC, 3 μM POVPC and 100 μM ATP (n/N=3/18 for each).
Membrane current recording
Recordings were made under voltage-clamp using the whole-cell configuration of the patch-clamp technique at room temperature. Signals were amplified and sampled using an Axopatch 200B amplifier and pCLAMP software (Molecular Devices). The extracellular solution was SBS and the patch pipette solution contained (mM): 135 CsCl, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2ATP and 0.1 Na2GTP; titrated to pH 7.2 with NaOH and filtered using a 0.2-μm filter. The voltage paradigm was a 200-ms ramp protocol (−100 to +100 mV) applied every 10 s from a holding potential of 0 mV. Data were filtered at 2 kHz and digitally sampled at 4 kHz.
Linear wound assay
After transfection, cells were serum-starved for 16 hr, wounded, washed and photographed, and then treated with vehicle or PGPC before being photographed and analysed. For more details see the Supplemental Material.
Reagents
Unless indicated otherwise, reagents were from Sigma UK. Stocks of chemicals were reconstituted in an appropriate vehicle: fura2-AM and fluo4-AM (Invitrogen) were dissolved at 1 mM in dimethylsulphoxide (DMSO); lysophosphatidylcholine (LPC) was dissolved at 50 mM in methanol; 1-palmitoyl-2-glutaroyl-phosphatidylcholine (PGPC), 1-palmitoyl-2-oxovaleroyl-phosphatidylcholine (POVPC) (Cayman Chemicals), 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC, Hycult Biotechnology) and oleic acid were dissolved at 16.4-16.84 mM, and sphingosine-1-phosphate (S1P) at 10 mM in ethanol. Oxidized PAPC (OxPAPC, Hycult Biotechnology) had unknown molecular mass but was assumed to have the same mass as PAPC and was similarly dissolved in ethanol. 9-Nitro oleate (Cayman Chemicals) was dissolved at 1.5267 mM in ethanol. N-Oleoylethanolamide and oleamide were dissolved at 50 mM in DMSO. Stocks of prostaglandin-E2 (PGE2) and (±)-15-deoxy-16R-hydroxy-17-cyclobutyl PGE1 ((R)-butaprost) at 10 mM and 3-(4-[2-chlorophenyl]-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-α]diazepine-2-yl)-1-propanone (WEB2086, Tocris Bioscience) at 100 mM were prepared in ethanol. Vascular endothelial growth factor (VEGF) was dissolved in water. The stock of pertussis toxin (PTX) was 0.2 mg.ml−1 in glycerol; denatured PTX was generated by boiling by PTX for 10 min. 2-Aminoethoxydiphenylborate (2-APB) was dissolved at 75 mM in DMSO. Pluronic acid F-127 (Invitrogen) was stored at 10 % w/v in DMSO at room temperature. Probenecid was freshly prepared in SBS. Final working concentrations of solvents were: 0.01 % methanol, ≤0.05 % ethanol, and ≤0.06 % DMSO. Functional anti-TRPC5 (T5E3) and anti-TRPC1 (T1E3) antibodies have been described12,14,20. For control experiments, pre-adsorption with antigenic peptide (10 μM) occurred overnight at 4 °C prior to use. T1E3 was used at 1:500 dilution and T5E3 at 1:100. Cells were exposed to antibody during loading with Ca2+ indicator and for the 0.5 hr wash period. Subsequently cells were washed with fresh SBS to remove excess antibody. For the DN-TRPC5 construct and vector, see the Supplemental Material.
Data analysis and presentation
Mean data are presented as mean ± standard error of the mean (SEM), where the n represents the number of independent experiments and the N represents the number of wells of a 96-well plate used in a single experiment. For patch-clamp experiments, n was the number of recordings from individual cells. Student t-tests were used for comparisons between pairs of data. Data from linear wound assays were analyzed by ANOVA followed by a Newman Keuls multiple comparison test. P values <0.05 were taken as indicating significant difference. Data were analyzed and presented using Origin software (Microcal Inc.).
RESULTS
Ca2+ signals in cells over-expressing TRPC5
Stimulation of Ca2+-entry in HEK 293 cells conditionally expressing human TRPC5 was investigated in 96-well Ca2+-indicator assays. In half of each plate, cells were induced to express TRPC5 by tetracycline (Tet+), while in the other half cells were not exposed to tetracycline (Tet−) to provide controls for TRPC5-independent effects. Cells were exposed to the test substance while maintaining the vehicle constant. Small effects occurred on solution application (Fig 1a, b) but much larger rises in Ca2+ were observed on exposure of TRPC5 (Tet+) cells to 10 μM oxidized PAPC, 3 μM PGPC or 3 μM POVPC (Fig 1a-d). There were no stimulatory effects of PAPC or the vehicle (Fig 1a, b). There were also no effects of PGPC or POVPC in control (Tet−) cells (Fig 1a-e). Therefore, PGPC and POVPC did not have TRPC5-independent effects, such as evoking Ca2+-release. In TRPC5 Tet+ cells, effects of PGPC occurred at >0.1 μM (Fig 1e). Effects of POVPC mostly occurred at similar concentrations to those of PGPC except some experiments showed additional effects at 10 nM (Fig 1f). The data suggest that PGPC and POVPC are relatively potent stimulators of TRPC5.
Figure 1. Ca2+-entry stimulated by PGPC and POVPC in TRPC5-expressing HEK 293 cells.
Ca2+ measurement data, where black symbols indicating responses to OxPAPC, PGPC or POVPC in TRPC5-expressing (Tet+) cells and white symbols indicating PAPC, vehicle controls or responses in Tet− cells. The ordinate scale is the change (Δ) in fluo4 fluorescence divided by 104 (F*) in arbitrary units. (a) Responses to bath-applied 10 μM OxPAPC or 10 μM PAPC (N=6 each). (b) Responses to bath-applied 3 μM PGPC or vehicle (N=6 each). (c, d) Mean data for PGPC, POVPC and vehicle responses in Tet+ and Tet− cells (n/N=3/18 for each point). (e, f) Concentration-response data for PGPC and POVPC in Tet+ and Tet− cells (n/N=3/9 for each point). Curves are Hill equations fitted to Tet+ cell data with mid-points at 2.24 μM (e) and 1.52 μM (f).
Ionic current in cells over-expressing TRPC5
An alternative method for measuring TRPC channel activity is whole-cell patch-clamp recording. Large ionic currents were evoked in TRPC5 (Tet+) cells by PGPC (Fig 2a, b). Currents were blocked by the non-specific TRPC5 inhibitor 2-APB (Fig 2a) and they reversed polarity near 0 mV, consistent with non-selective cationic permeability of the underlying channels (Fig 2c). Importantly, the evoked current-voltage relationships (I-Vs) had double-rectifying characteristics expected of TRPC5 (Fig 2c)14,15,21. POVPC evoked similar responses (Fig 2d). Effects of PGPC and POVPC appeared to occur in two phases – early and late (e.g. Fig 2a, c, d), as has previously been observed for receptor-activation of TRPC521. The data are consistent with PGPC and POVPC stimulating TRPC5 and suggest activation via a receptor-coupling mechanism.
Figure 2. Ionic current stimulated by PGPC or POVPC in TRPC5-expressing HEK 293 cells.
Whole-cell patch-clamp recordings from Tet+ cells under voltage-clamp. (a) Time-series plot showing the effect of bath-applied 3 μM PGPC in the continuous presence of the ethanol vehicle, and then inhibition by 75 μM 2-APB. (b) Mean time-matched data comparing current responses to the vehicle (ethanol) and 3 μM PGPC at −80 mV and +80 mV (n=6 each). (c) For the experiment of (a), current-voltage relationships (I-Vs) for currents induced by PGPC at the early and late stages of the response. (d) Response to bath-applied 3 μM POVPC shown as I-Vs for currents induced by POVPC.
Lack of effect on other TRP channels in the same expression system
As further controls for the expression system and to investigate specificity, PGPC and POVPC were tested against other TRP channels expressed via the same conditional system as TRPC5. No stimulation of Ca2+-entry was observed in response to PGPC or POVPC in TRPM2- or TRPM3-expressing cells (Supplemental Fig I).
Ca2+ signals in VSMCs endogenously expressing TRPC5
Endogenous expression of TRPC5 usually occurs concomitant with expression of other TRP channels, leading to heteromultimeric channels. To investigate TRPC5 in such a context we used human saphenous vein VSMCs that express TRPC5 along with other TRP proteins including TRPC112,20. PGPC and POVPC elicited Ca2+ responses in cells from about half of the donors (Fig 3a, Supplemental Fig II). Effects occurred at concentrations >0.1 μM (Fig 3b) but, as in HEK 293 cells over-expressing TRPC5, there were anomalous responses to 10 nM POVPC (Fig 3b).
TRPC-dependent Ca2+-entry
Responses to PGPC and POVPC in VSMCs were often transient, resembling the time-course of Ca2+ events caused by Ca2+-release (Fig 3a). Similar data were obtained using two different Ca2+ indicator dyes, fura2 (Fig 3a) and fluo4 (data not shown), or when recording signals from individuals cells using microscope-based imaging (Fig 3c). Unlike Ca2+-release events, however, responses to PGPC and POVPC were absent when Ca2+ was omitted from the extracellular medium (Fig 3d, e). ATP-evoked Ca2+-release was unaffected by the absence of extracelullar Ca2+ (Fig 3e). The data suggest that PGPC and POVPC evoke predominantly Ca2+ entry in VSMCs.
To investigate if TRPC5-containing channels were involved in PGPC- and POVPC-evoked Ca2+ entry in VSMCs we first used anti-TRPC5 and anti-TRPC1 antibodies that target extracellular loops and acutely inhibit channel function12,14,20. Either antibody inhibited responses to PGPC (Fig 4a-c) and POVPC (Fig 4d, e) in paired experiments using antibodies preadsorbed to antigenic peptides as controls. As an independent test of the contribution of TRPC5-containing channels we used an ion-pore mutant of TRPC5 (DN-TRPC5) that fails to pass current and acts as a dominant negative, presumably because it damages ion permeation by entering in the heteromultimeric complex12. Transfection of this mutant into VSMCs inhibited PGPC-evoked Ca2+-entry (Fig 4f; P<0.05, n=8). The data suggest that endogenous channels containing TRPC5 and TRPC1 contributed to Ca2+ entry evoked by PGPC and POVPC.
Figure 4. Contribution of endogenous TRPC5 and TRPC1.
Data are from intracellular Ca2+ measurements from VSMCs. (a, b) Responses to 3 μM PGPC after pretreatment with anti-TRPC antibody alone or anti-TRPC antibody preadsorbed to its antigenic peptide (+pep.) (N=5 each): T5E3 anti-TRPC5 antibody (a); T1E3 anti-TRPC1 antibody (b). (c) As for (a, b) but mean data for independent experiments (n/N=3/15). (d, e) As for (a, c) but using POVPC instead of PGPC (n/N=3/15). (f) Example from 8 independent paired experiments of the response to 3 μM PGPC after transfection with dominant negative mutant TRPC5 (DN-TRPC5) or vector control (N=16 for each).
Positive impact on cell migration
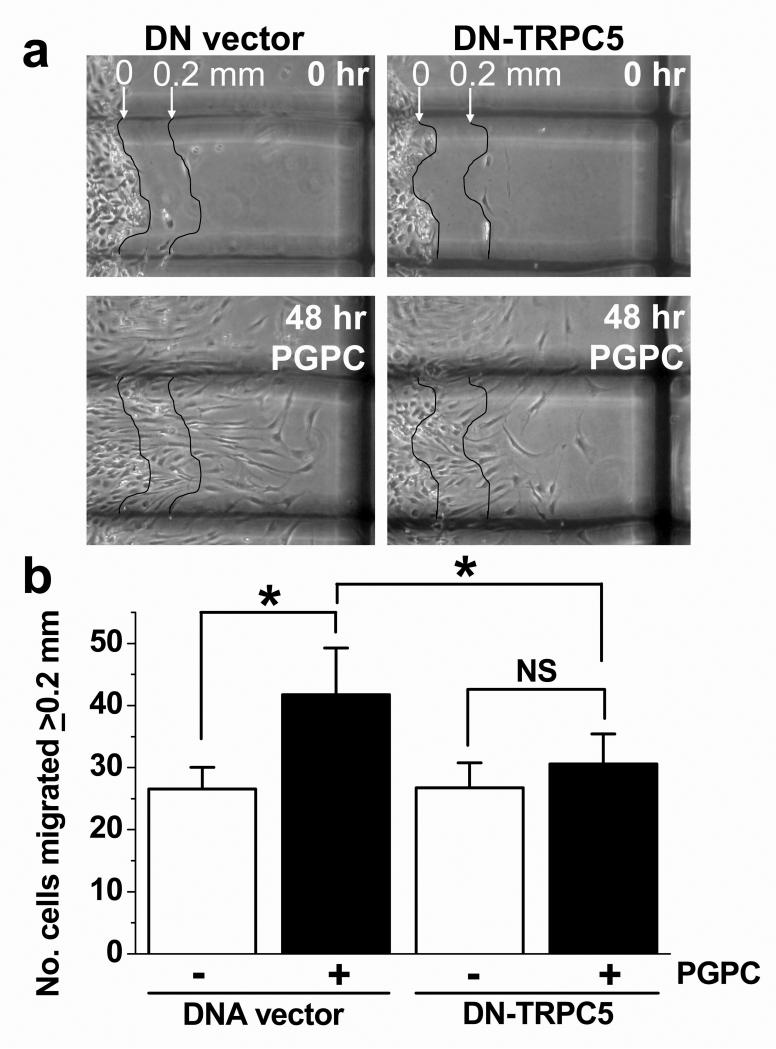
TRPC channel activity has been linked to cell migration12. Therefore, the effect of PGPC on VSMC migration was investigated in linear wound assays with and without the presence of DN-TRPC5 (Fig 5a). PGPC was found to enhance cell movement into the wounded area (Fig 5b; Supplemental Fig III). Transfection of VSMCs from 8 patients with DN-TRPC5 did not affect baseline cell movement but prevented the stimulatory effect of PGPC (Fig 5b). The data suggest that PGPC promotes VSMC migration via a mechanism that depends on ion permeation through TRPC channels.
Figure 5. VSMC migration evoked by PGPC and depending on TRPC channel function.
(a) Representative images showing VSMCs at time zero (0 hr) and 48 hr after scratch wound in the presence of 3 μM PGPC. The images show a paired comparison of cells from the same patient transfected with DNA vector or vector expressing DN-TRPC5. In each image, VSMCs before migration are on the left of the thin line that delineated the origin (0 mm); the parallel thin line to the right defines the 0.2 mm distance required for cells to migrate before counting. The thick dark shadow is the 1 mm grid. (b) For experiments of the type shown in (a), mean data for VSMCs of 8 patients after transfection with DNA vector or vector containing the dominant negative (DN) TRPC5 insert. VSMCs were treated with vehicle (ethanol) or 3 μM PGPC and the numbers of migrated VSMCs were measured.
Dependence on Gi/o signaling
Lipid factors may act directly on TRPC channels or through intermediate signaling pathways. Sphingosine-1-phosphate, for example, acts via a G protein-coupled receptor where as lysophosphatidylcholine acts relatively directly12,15. In the case of sphingosine-1-phosphate, receptor coupling occurs via a Gi or Go protein as indicated by sensitivity to pertussis toxin12 which ADP-ribosylates and inactivates Gi/o proteins.
Pertussis toxin strongly inhibited effects of PGPC and POVPC on TRPC5 (Fig 6a, b). Inhibition of the PGPC effect tended to be less than inhibition of the POVPC effect, as shown by comparison of the summary data (Fig 6a, b). In confirmation of previous results15, pertussis toxin lacked effect on TRPC5 stimulation by lysophosphatidylcholine (Fig 6c). The data suggest that PGPC and POVPC acted on TRPC5 through a Gi/o protein signaling pathway and that lysophosphatidylcholine acted via a different mechanism.
Figure 6. Role of Gi/o-signaling but not PAF receptors.
Data are from intracellular Ca2+ measurements from TRPC5-expressing HEK 293 cells (a-c) and VSMCs (d-f). Cells were pretreated with 1 μg ml−1 pertussis toxin (PTX) or denatured (denat.) PTX for 4 hr at 37 °C. (a-c) Responses were evoked by 3 μM PGPC (a, n/N=3/9), 5 μM POVPC (b, n/N=3/9) or 5 μM lysophosphatidylcholine (c, n/N=3/18). (d) Representative paired experiment showing responses to 3 μM PGPC after treatment with PTX or denatured PTX (N=5 each). (e) Responses were evoked by 3 μM PGPC or 5 μM POVPC (n/N=4/20 each). (f) Responses to 3 μM PGPC in cells pre-incubated and maintained in the presence of 100 μM WEB2086 or its vehicle (N=6 each and representative of 3 independent experiments).
In VSMCs, pertussis toxin partially inhibited responses to PGPC (Fig 6d, e) and almost abolished responses to POVPC (Fig 6e). The data suggest that PGPC and POVPC couple to endogenous TRPC channels substantially through a Gi/o signaling pathway and presumably because they bind and activate a membrane receptor.
Investigation of the receptor type
Previous studies have suggested that oxidized phospholipids are ligands at prostaglandin E (PGE) and platelet-activating factor (PAF) G protein-coupled receptors3,8,22,23. However, neither PGE2 (n=3, data not shown) nor the synthetic PGE2 receptor agonist (R)-butaprost stimulated TRPC5 (Supplementary Figure IV) and a high concentration of the PAF receptor antagonist WEB2086 failed to inhibit PGPC-evoked responses in VSMCs (Fig 6f). VEGF receptors are suggested to be involved in oxidized phospholipid effects24, but we confirmed that the VSMCs and Tet+ HEK 293 cells show no Ca2+ responses to 100 ng.mL−1 VEGF; contrasting with endothelial cells from the saphenous vein (data not shown). The data indicate that previously suggested oxidized phosholipid receptors are not involved in mediating TRPC channel stimulation.
To obtain more information on the character of the receptor we tested 3 μM 9-nitro-oleate, 3 μM oleic acid, 10 μM oleoylethanolamide and 10 μM oleamide in Ca2+ measurements assays of TRPC5 Tet+ cells, using 3 μM PGPC as a positive control. None of these additional lipid factors evoked TRPC5 activity (3 independent experiments for each lipid, data not shown).
DISCUSSION
The study reveals that bioactive oxidation products of a common membrane phospholipid have acute stimulatory effects on Ca2+-entry via TRPC1/5 channels and a G protein signaling mechanism, without causing Ca2+-release. The mechanism was shown to be relevant to proliferating VSMCs.
Dominant negative TRPC5 mutant had no effect on basal migration of VSMCs but prevented migration evoked by PGPC. The result adds to an expanding picture of positive roles of TRPC1/5 in remodeling12,23,25,26. The effect is consistent with a pro-atherogenic effect of the phospholipids and may be important in physiological events of formation, remodeling and response-to-injury of blood vessels. PGPC and POVPC were found to be potent stimulators of the channels, acting at concentrations considerably lower than those used in many studies or detected in animals fed atherogenic diets2-5,27.
PGPC and POVPC generated relatively sustained Ca2+ signals in the TRPC5 Tet+ cells but mostly transient signals in VSMCs. The difference may have arisen because of the more complex channel composition in the VSMCs. TRPC1 was involved in the VSMC signal and is susceptible to Ca2+-induced inactivation28; it may, therefore confer the decay in the PGPC and POVPC responses. A previous study described opposite effects of PGPC and POVPC on endothelial cells6. Our data show equivalent effects of the oxidized phospholipids on VSMCs except for two features: greater inhibition of POVPC responses by pertussis toxin, and anomalous responses to 10 nM POVPC. The 10 nM POVPC effect occurred in HEK 293 cells induced to express TRPC5 but not non-induced cells, suggesting dependence on TRPC5. A separate intriguing observation was made with lysophosphatidylcholine, which is chemically closely related to PGPC and POVPC and functionally active in similar contexts29. Unlike PGPC and POVPC, lysophosphatidylcholine stimulated TRPC5 independently of G protein signaling (Fig 6)15. Chemical features in PGPC and POVPC presumably prevented these lipids from mimicking the effect of lysophosphatidylcholine.
The receptors mediating the TRPC effects of PGPC and POVPC were not identified. PGE2 and PAF receptors are unlikely to be involved. PGE2 and (R)-butaprost were ineffective and POVPC was previously found to lack effect at PGE2 receptors22. The PAF antagonist WEB2086 failed to block the action of PGPC and a previous study found that WEB2086 inhibited effects of POVPC but not PGPC23. A candidate receptor for the PGPC and POVPC effects in our experiments was an S1P receptor because S1P also acts via a Gi/o pathway12. However, PGPC and POVPC could not have been agonists at S1P receptors because, unlike S1P, they did not cause Ca2+-release. Although oxidized phospholipids bind to scavenger receptors like CD36, these receptors are not thought to couple to G protein signaling.
The data suggest that TRPC channels are components of an initial reception mechanism enabling cellular responses to biologically-active oxidized phospholipids. The mechanism is indicated to involve binding to a previously unrecognized G protein-coupled receptor that couples to the channels but not Ca2+-release. An identified functional consequence is the promotion of VSMC migration, consistent with prior suggestion that oxidized phospholipids play key roles in the proliferating phenotype of VSMCs16.
Supplementary Material
ACKNOWLEDGEMENTS
The work was supported by the Wellcome Trust and the British Heart Foundation.
Footnotes
CONFLICT OF INTEREST None declared.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 2.Ashraf MZ, Kar NS, Podrez EA. Oxidized phospholipids: biomarker for cardiovascular diseases. Int J Biochem Cell Biol. 2009;41:1241–1244. doi: 10.1016/j.biocel.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berliner JA, Gharavi NM. Endothelial cell regulation by phospholipid oxidation products. Free Radic Biol Med. 2008;45:119–123. doi: 10.1016/j.freeradbiomed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50(Suppl):S207–212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochkov VN. Inflammatory profile of oxidized phospholipids. Thromb Haemost. 2007;97:348–354. [PubMed] [Google Scholar]
- 9.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 10.Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, Beech DJ. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beech DJ. Canonical transient receptor potential 5. Handb Exp Pharmacol. 2007:109–123. doi: 10.1007/978-3-540-34891-7_6. [DOI] [PubMed] [Google Scholar]
- 12.Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- 14.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, Benham CD, Bateson AN, Muraki K, Beech DJ. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- 16.Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 17.Fruhwirth GO, Hermetter A. Mediation of apoptosis by oxidized phospholipids. Subcell Biochem. 2008;49:351–367. doi: 10.1007/978-1-4020-8831-5_13. [DOI] [PubMed] [Google Scholar]
- 18.Cherepanova OA, Pidkovka NA, Sarmento OF, Yoshida T, Gan Q, Adiguzel E, Bendeck MP, Berliner J, Leitinger N, Owens GK. Oxidized phospholipids induce type VIII collagen expression and vascular smooth muscle cell migration. Circ Res. 2009;104:609–618. doi: 10.1161/CIRCRESAHA.108.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter KE, Naik J, Turner NA, Dickinson T, Thompson MM, London NJ. Simvastatin inhibits human saphenous vein neointima formation via inhibition of smooth muscle cell proliferation and migration. J Vasc Surg. 2002;36:150–157. doi: 10.1067/mva.2002.122029. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res. 2008;103:e97–104. doi: 10.1161/CIRCRESAHA.108.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obukhov AG, Nowycky MC. TRPC5 activation kinetics are modulated by the scaffolding protein ezrin/radixin/moesin-binding phosphoprotein-50 (EBP50) J Cell Physiol. 2004;201:227–235. doi: 10.1002/jcp.20057. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98:642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 23.Subbanagounder G, Leitinger N, Shih PT, Faull KF, Berliner JA. Evidence that phospholipid oxidation products and/or platelet-activating factor play an important role in early atherogenesis: in vitro and In vivo inhibition by WEB 2086. Circ Res. 1999;85:311–318. doi: 10.1161/01.res.85.4.311. [DOI] [PubMed] [Google Scholar]
- 24.Zimman A, Mouillesseaux KP, Le T, Gharavi NM, Ryvkin A, Graeber TG, Chen TT, Watson AD, Berliner JA. Vascular endothelial growth factor receptor 2 plays a role in the activation of aortic endothelial cells by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2007;27:332–338. doi: 10.1161/01.ATV.0000252842.57585.df. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, Iijima T, Ito H. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis. 2007;195:287–296. doi: 10.1016/j.atherosclerosis.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 27.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson AD, Faull KF, Fogelman AM, Berliner JA. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol. 2000;20:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 28.Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS. Calmodulin regulates Ca2+-dependent feedback inhibition of store-operated Ca2+ influx by interaction with a site in the C terminus of TrpC1. Mol Cell. 2002;9:739–750. doi: 10.1016/s1097-2765(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 29.Berliner JA, Subbanagounder G, Leitinger N, Watson AD, Vora D. Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc Med. 2001;11:142–147. doi: 10.1016/s1050-1738(01)00098-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.