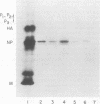
Abstract
Monoclonal antibodies were used to study antigenic variation in the nucleoprotein of influenza A viruses. We found that the nucleoprotein molecule of the WSN/33 strain possesses at least five different determinants. Viruses of other influenza A virus subtypes showed antigenic variation in these nucleoprotein determinants, although changes in only one determinant were detected in H0N1 and animal strains. The nucleoprotein of human strains isolated from 1933 through 1979 could be divided into six groups, based on their reactivities with monoclonal antibodies; these groups did not correlate with any particular hemagglutinin or neuraminidase subtype. Our results indicate that antigenic variation in the nucleoproteins of influenza A viruses proceeds independently of changes in the viral surface antigens and suggest that point mutations and genetic reassortment may account for nucleoprotein variability.
Full text
PDF

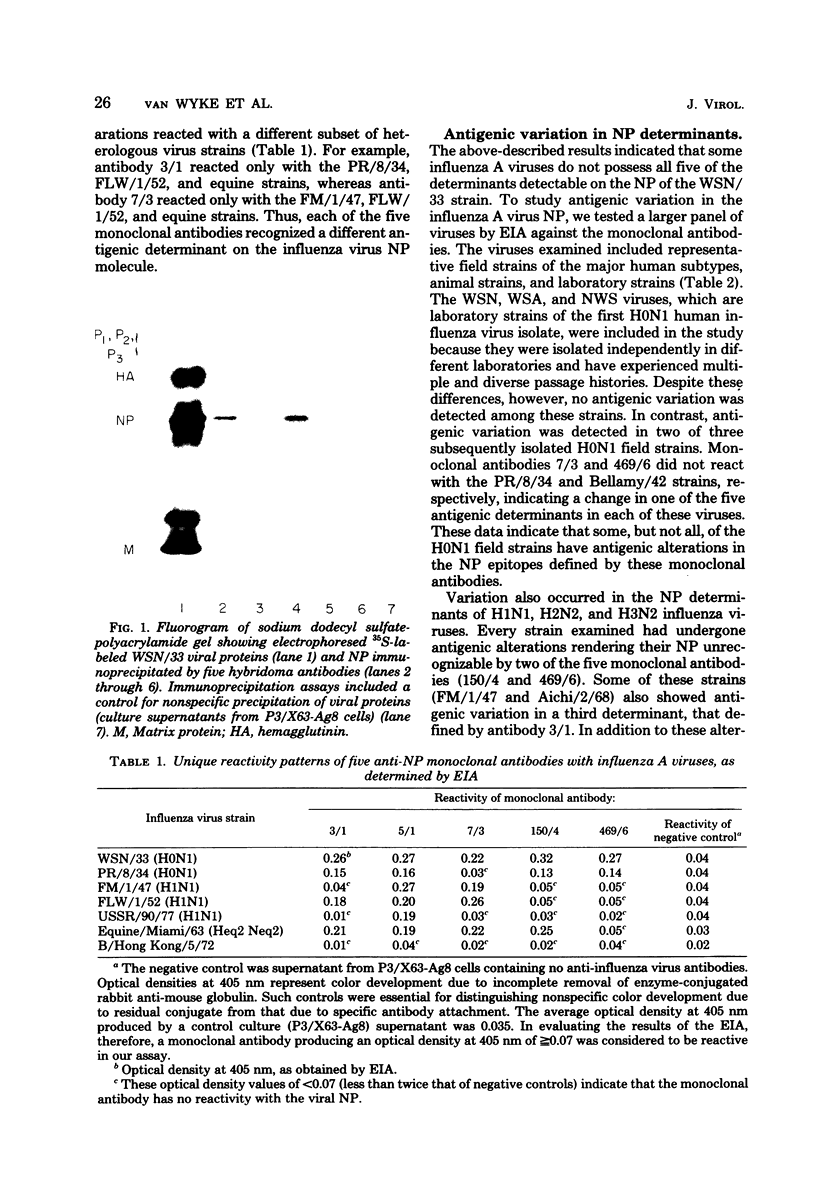
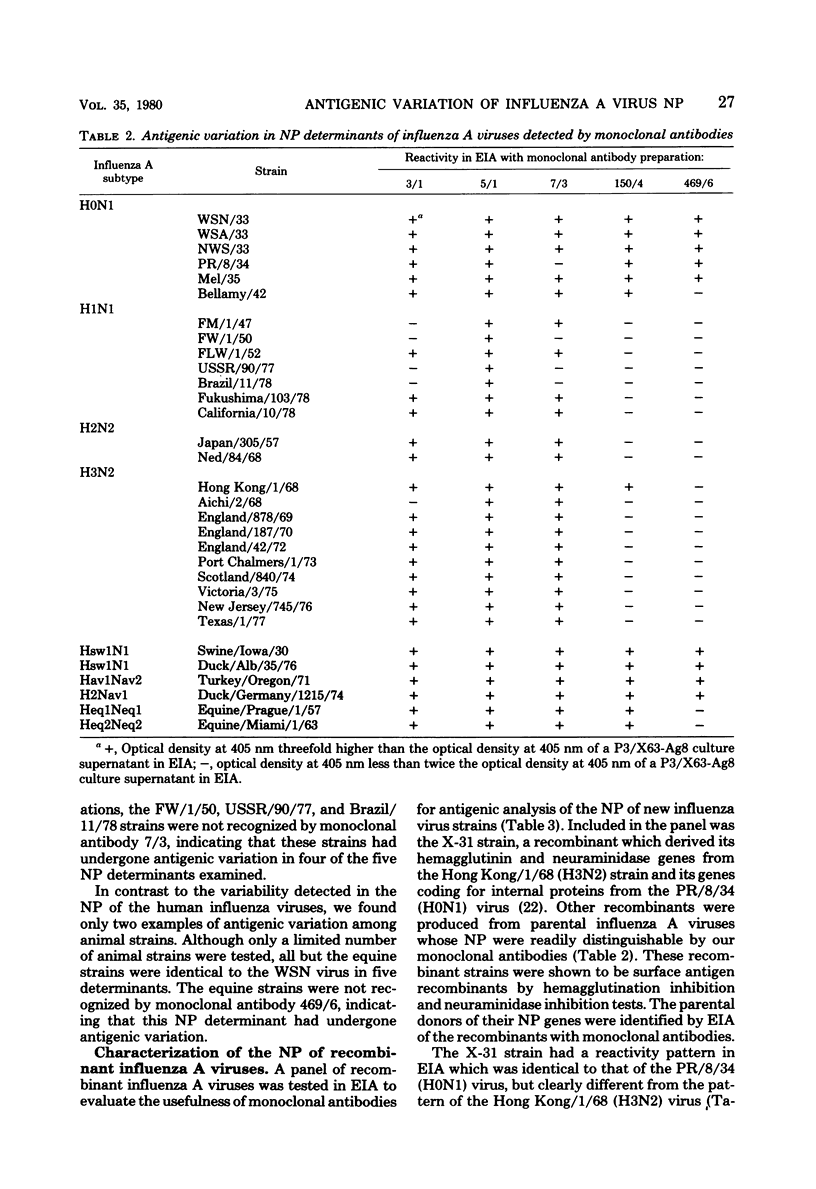
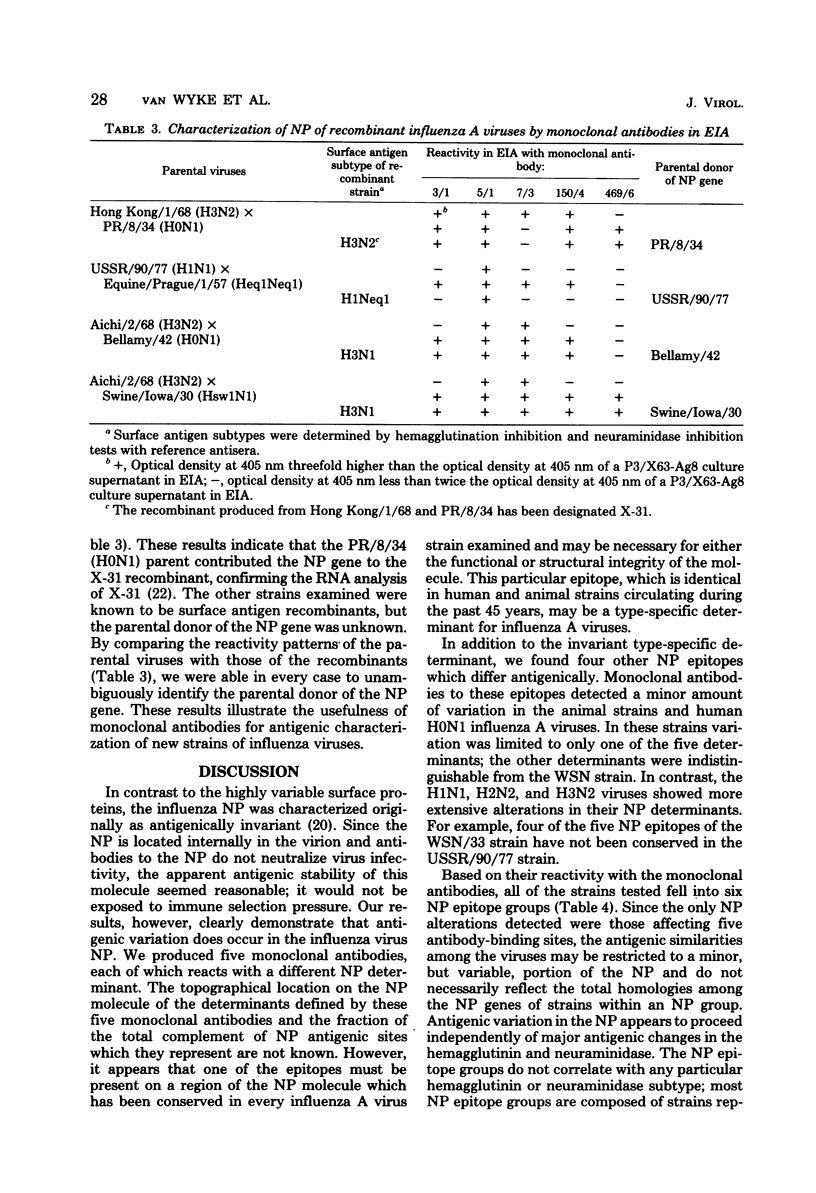

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond J. W., McGeoch D., Barry R. D. Method for assigning temperature-sensitive mutations of influenza viruses to individual segments of the genome. Virology. 1977 Aug;81(1):62–73. doi: 10.1016/0042-6822(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT F. M., ROTT R., SCHAEFER W. Physical and biological properties of influenza virus components obtained after ether treatment. J Exp Med. 1960 Nov 1;112:765–782. doi: 10.1084/jem.112.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C., Yolken R. H., Krokan H., Hsu I. C. Ultrasensitive enzymatic radioimmunoassay: application to detection of cholera toxin and rotavirus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5336–5339. doi: 10.1073/pnas.76.10.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Gerhard W., Webster R. G., Frankel M. E., Air G. M. Antigenic drift in type A influenza virus: peptide mapping and antigenic analysis of A/PR/8/34 (HON1) variants selected with monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1425–1429. doi: 10.1073/pnas.76.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Selection of antigenic mutants of influenza viruses. Isolation and peptide mapping of their hemagglutination proteins. Virology. 1968 Feb;34(2):193–202. doi: 10.1016/0042-6822(68)90230-4. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Oxford J. S., McGeoch D. J., Schild G. C., Beare A. S. Analysis of virion RNA segments and polypeptides of influenza A virus recombinants of defined virulence. Nature. 1978 Jun 29;273(5665):778–779. doi: 10.1038/273778a0. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. Mapping of the influenza virus genome. II. Identification of the P1, P2, and P3 genes. Virology. 1977 Jan;76(1):114–121. doi: 10.1016/0042-6822(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Palese P. Influenza B virus genome: assignment of viral polypeptides to RNA segments. J Virol. 1979 Jan;29(1):361–373. doi: 10.1128/jvi.29.1.361-373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A., Brosi B. J., Buys J. Reliability of the enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of Trichinella spiralis infections in conventionally raised pigs. J Immunol Methods. 1976;10(1):67–83. doi: 10.1016/0022-1759(76)90008-9. [DOI] [PubMed] [Google Scholar]
- Schild G. C. Evidence for a new type-specific structural antigen of the influenza virus particle. J Gen Virol. 1972 Apr;15(1):99–103. doi: 10.1099/0022-1317-15-1-99. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Oxford J. S., Newman R. W. Evidence for antigenic variation in influenza A nucleoprotein. Virology. 1979 Mar;93(2):569–573. doi: 10.1016/0042-6822(79)90259-9. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Pereira H. G. Characterization of the ribonucleoprotein and neuraminidase of influenza A viruses by immunodiffusion. J Gen Virol. 1969 Apr;4(3):355–363. doi: 10.1099/0022-1317-4-3-355. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Becht H., Rott R. Inhibition of influenza RNA polymerase by specific antiserum. Virology. 1971 Jan;43(1):137–143. doi: 10.1016/0042-6822(71)90231-5. [DOI] [PubMed] [Google Scholar]
- Webster R. G. Antigenic hybrids of influenza A viruses with surface antigens to order. Virology. 1970 Nov;42(3):633–642. doi: 10.1016/0042-6822(70)90309-0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Kendal A. P., Gerhard W. Analysis of antigenic drift in recently isolated influenza A (H1N1) viruses using monoclonal antibody preparations. Virology. 1979 Jul 15;96(1):258–264. doi: 10.1016/0042-6822(79)90189-2. [DOI] [PubMed] [Google Scholar]
- Young J. F., Desselberger U., Palese P. Evolution of human influenza A viruses in nature: sequential mutations in the genomes of new H1N1. Cell. 1979 Sep;18(1):73–83. doi: 10.1016/0092-8674(79)90355-6. [DOI] [PubMed] [Google Scholar]
- Young J. F., Palese P. Evolution of human influenza A viruses in nature: recombination contributes to genetic variation of H1N1 strains. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6547–6551. doi: 10.1073/pnas.76.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]