Abstract
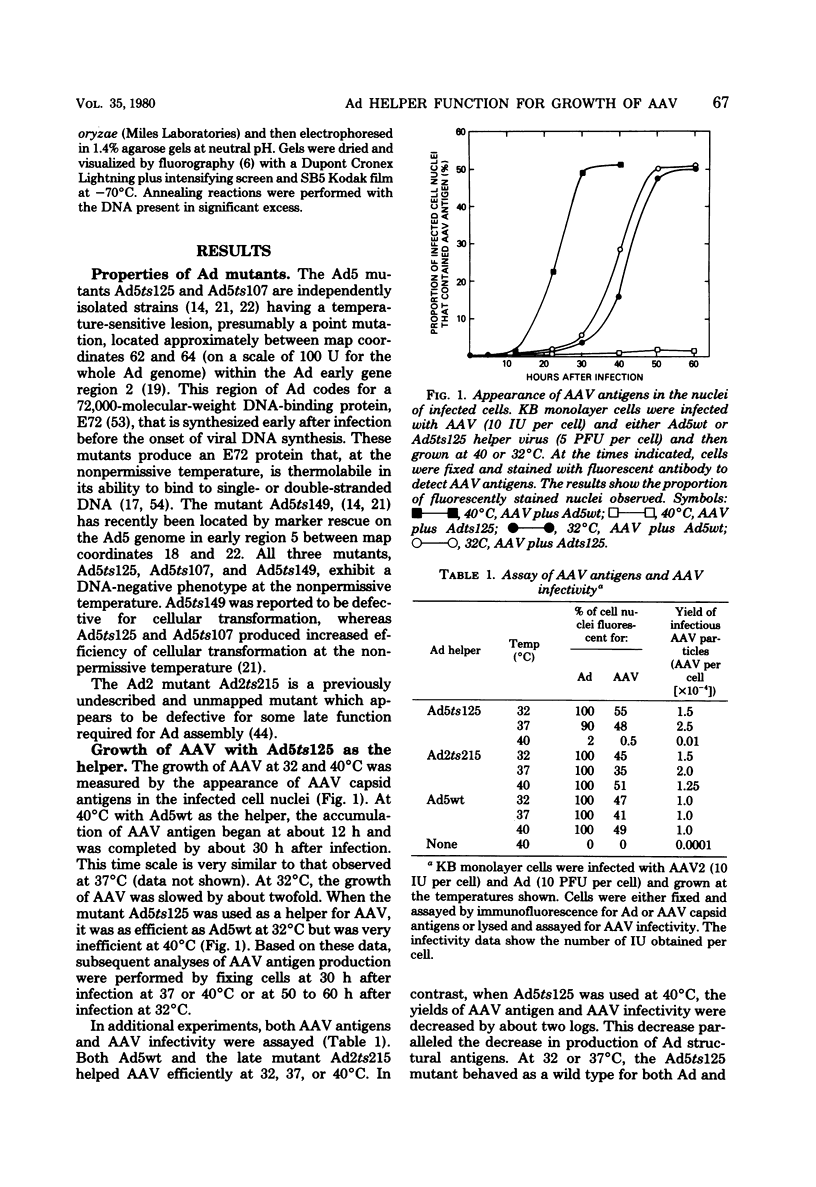
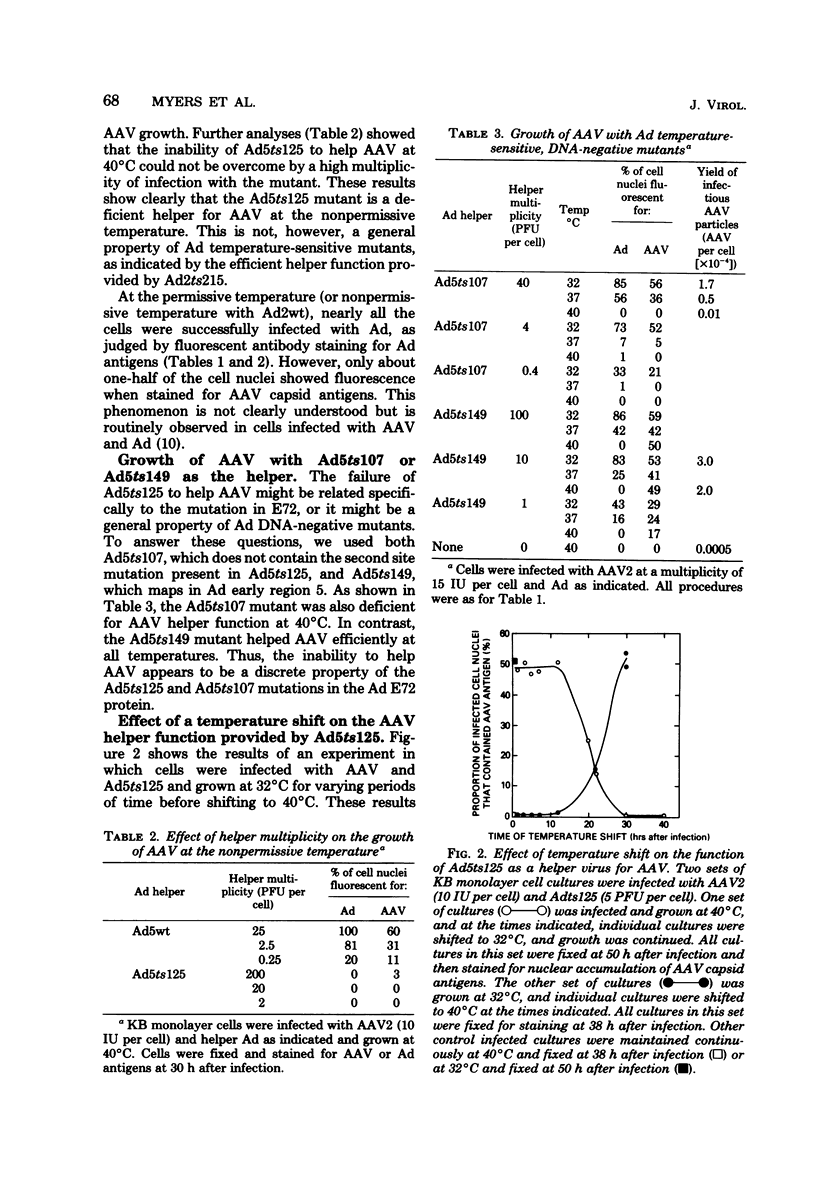
Adeno-associated virus (AAV) grows efficiently only in cells that are also infected with an adenovirus (Ad). We employed Ad mutants to determine which genes may be required for the AAV helper function. Two mutants of Ad type 5 (Ad5), Ad5ts125 and Ad5ts107, with temperature-sensitive lesions in the E72 DNA-binding protein coded by the Ad early region 2, were deficient for AAV helper functions at the nonpermissive temperature (40 degrees C). In contrast, Ad5ts149, with a temperature-sensitive lesion in the Ad early region 5, was an efficient helper of AAV at the nonpermissive temperature. In KB cells, with the Ad5ts125 or Ad5ts107 mutant as the helper, the accumulation of AAV capsid proteins and AAV particles was decreased by about two logs, whereas AAV DNA synthesis was decreased only severalfold. Cytoplasmic, polyadenylic acid-containing AAV RNA is composed of a set of overlapping, spliced RNAs having different 5' start points. With the ts125 helper at 40 degrees C there was a decreased accumulation of some but not all of these AAV RNAs. The Ad5 E72 protein may have an effect on transcription or more likely posttranscriptional processing of AAV RNA. These observations suggest additional pleiotropic effects of the multifunctional E72 protein and suggest further similarities in the actions of E72 and the simian virus 40 T-antigen.
Full text
PDF

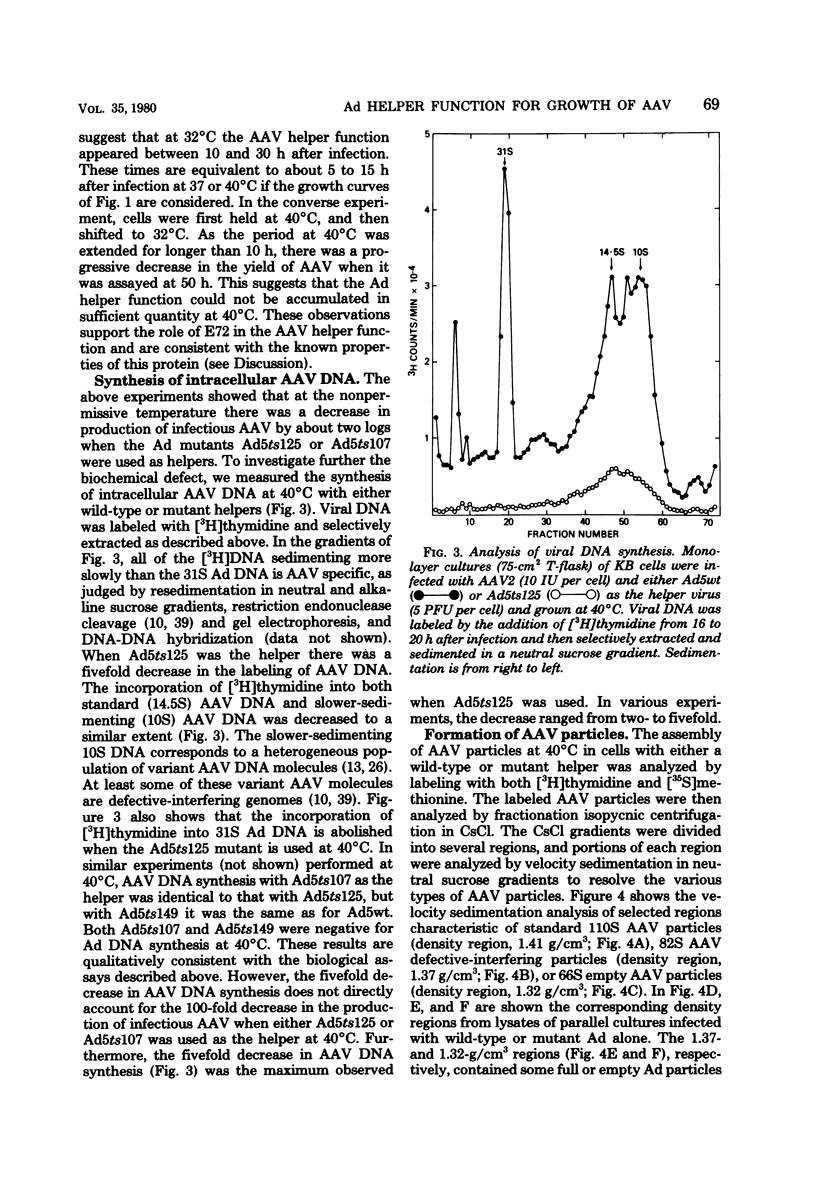
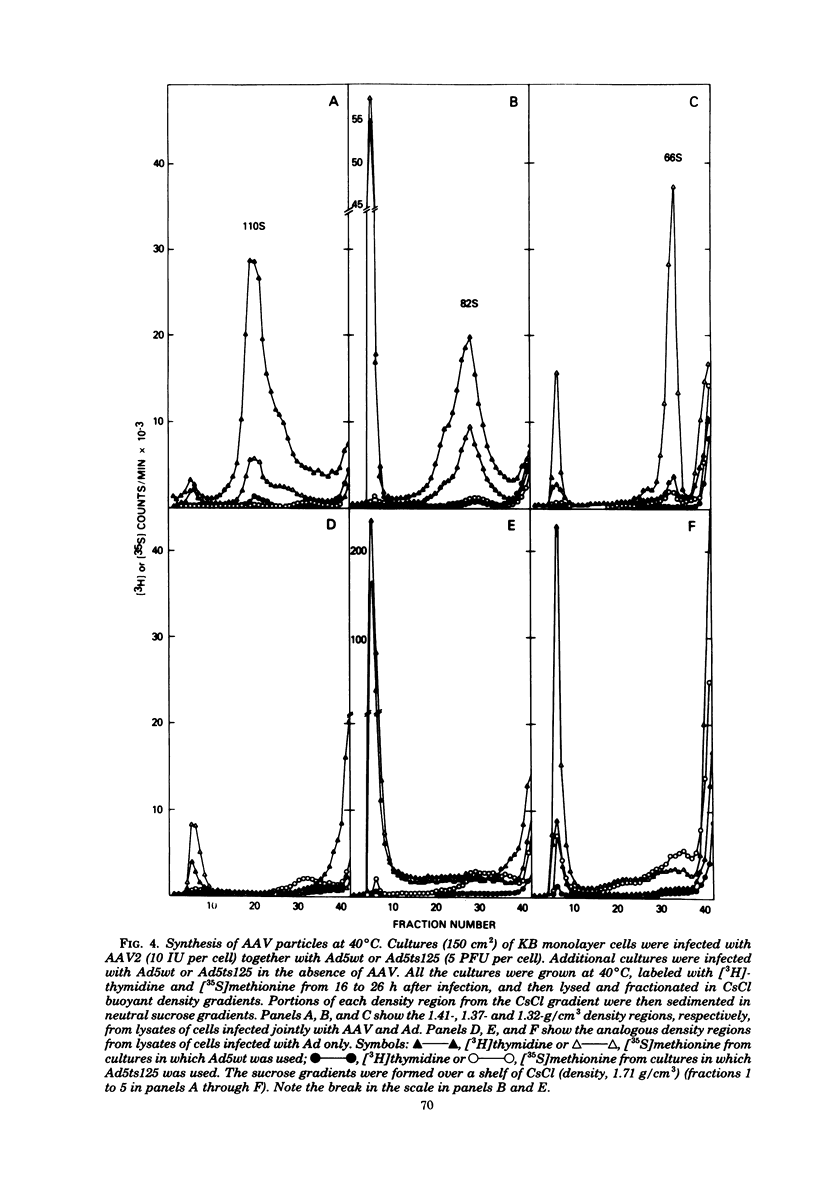
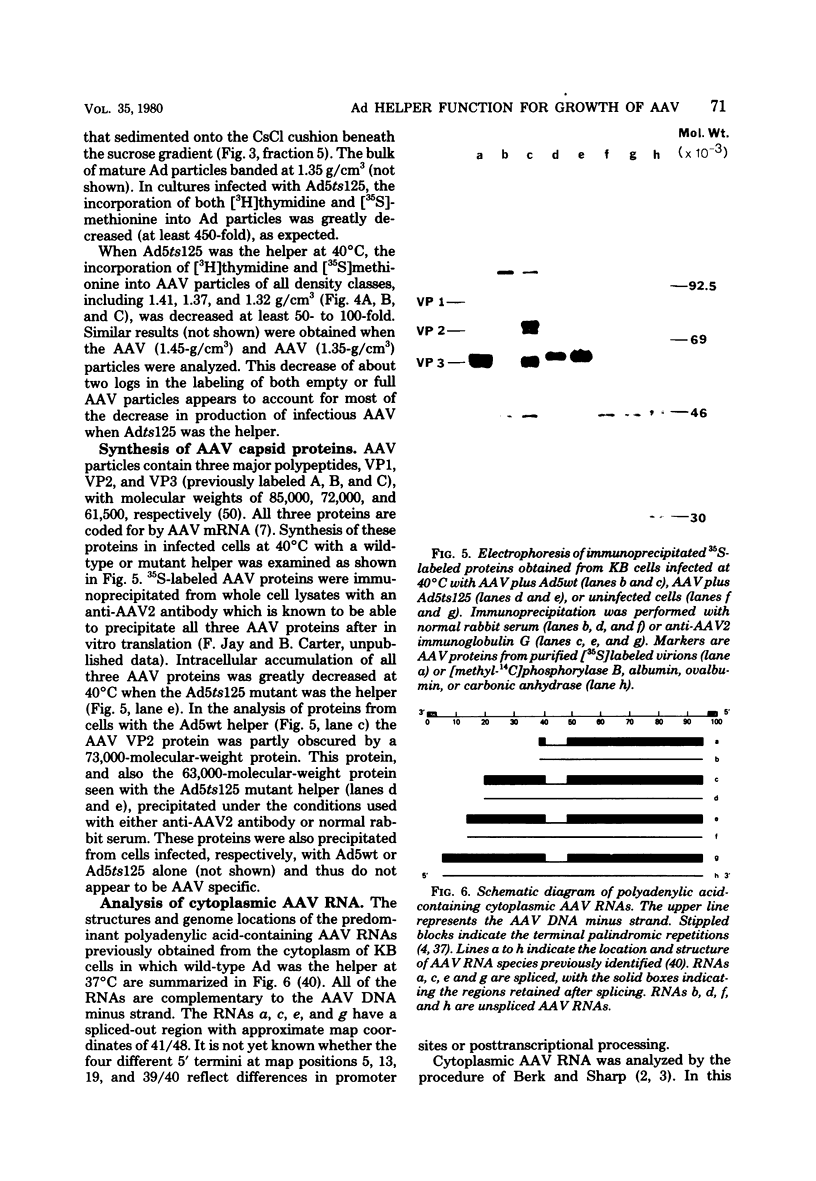
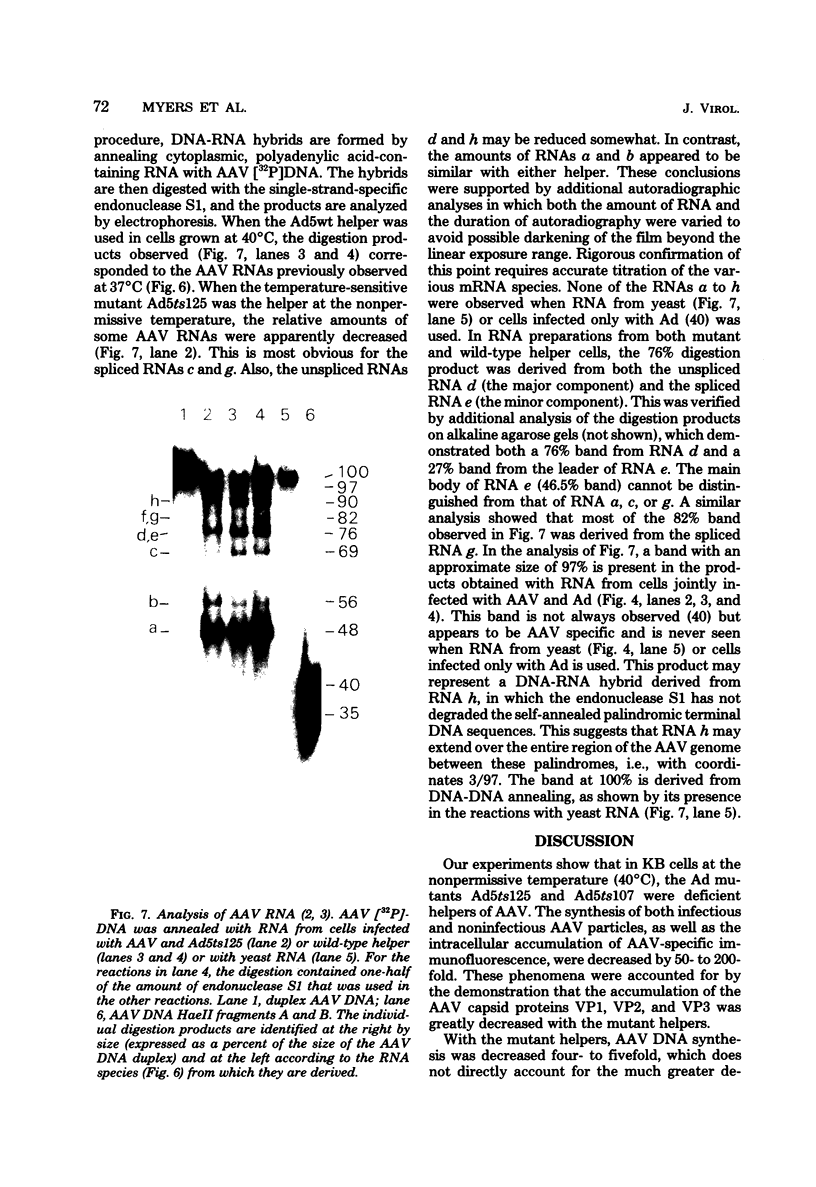



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Hauswirth W. W., Fife K. H., Lusby E. Adeno-associated virus DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):781–787. doi: 10.1101/sqb.1979.043.01.085. [DOI] [PubMed] [Google Scholar]
- Blanton R. A., Carter T. H. Autoregulation of adenovirus type 5 early gene expression. III. Transcription studies in isolated nuclei. J Virol. 1979 Feb;29(2):458–465. doi: 10.1128/jvi.29.2.458-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Straus S. E., Rose J. A. Mechanism of host restriction of adenovirus-associated virus replication in African green monkey kidney cells. J Gen Virol. 1979 Jun;43(3):663–672. doi: 10.1099/0022-1317-43-3-663. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Koczot F. J., Garrison J., Rose J. A., Dolin R. Separate helper functions provided by adenovirus for adenovirus-associated virus multiplication. Nat New Biol. 1973 Jul 18;244(133):71–73. doi: 10.1038/newbio244071a0. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Laughlin C. A., de la Maza L. M., Myers M. Adeno-associated virus autointerference. Virology. 1979 Jan 30;92(2):449–462. doi: 10.1016/0042-6822(79)90149-1. [DOI] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Possible role of the 72,000 dalton DNA-binding protein in regulation of adenovirus type 5 early gene expression. J Virol. 1978 Feb;25(2):664–674. doi: 10.1128/jvi.25.2.664-674.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L. Post-transcriptional restriction of human adenovirus expression in monkey cells. J Virol. 1975 May;15(5):1256–1261. doi: 10.1128/jvi.15.5.1256-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Fowlkes D. M., Lord S. T., Linné T., Pettersson U., Philipson L. Interaction between the adenovirus DNA-binding protein and double-stranded DNA. J Mol Biol. 1979 Aug 5;132(2):163–180. doi: 10.1016/0022-2836(79)90389-9. [DOI] [PubMed] [Google Scholar]
- Friedman M. P., Lyons M. J., Ginsberg H. S. Biochemical consequences of type 2 adenovirus and Simian virus 40 double infections of African green monkey kidney cells. J Virol. 1970 May;5(5):586–597. doi: 10.1128/jvi.5.5.586-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Galos R. S., Williams J., Binger M. H., Flint S. J. Location of additional early gene sequences in the adenoviral chromosome. Cell. 1979 Aug;17(4):945–956. doi: 10.1016/0092-8674(79)90334-9. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Anderson C., Sharp P. A., Sambrook J. Conditional lethal mutants of adenovirus 2-simian virus 40 hybrids. I. Host range mutants of Ad2+ND1. J Virol. 1974 Jun;13(6):1237–1244. doi: 10.1128/jvi.13.6.1237-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Shiroki K., Shimojo H. Complementation of adeno-associated virus growth with temperature-sensitive mutants of human adenovirus types 12 and 5. J Gen Virol. 1975 Nov;29(2):239–242. doi: 10.1099/0022-1317-29-2-239. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Ito M. The potentiation of type 1 adeno-associated virus by temperature-sensitive conditional-lethal mutants of CELO virus at the restrictive temperature. Virology. 1971 Jul;45(1):317–320. doi: 10.1016/0042-6822(71)90141-3. [DOI] [PubMed] [Google Scholar]
- Ito M., Suzuki E. Adeno-associated satellite virus growth supported by a temperature-sensitive mutant of human adenovirus. J Gen Virol. 1970 Dec;9(3):243–245. doi: 10.1099/0022-1317-9-3-243. [DOI] [PubMed] [Google Scholar]
- Jerkofsky M. Enhancement of the replication of human adenovirus in simian cells by a series of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jun;65(2):579–582. doi: 10.1016/0042-6822(75)90063-x. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G. Genetic evidence for SV40 gene function in enhancement of replication of human adenovirus in simian cells. Nature. 1974 Apr 12;248(449):590–592. doi: 10.1038/248590a0. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Anderson C. W. Block to multiplication of adenovirus serotype 2 in monkey cells. J Virol. 1975 Dec;16(6):1650–1668. doi: 10.1128/jvi.16.6.1650-1668.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979 Aug;17(4):957–966. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughlin C. A., Myers M. W., Risin D. L., Carter B. J. Defective-interfering particles of the human parvovirus adeno-associated virus. Virology. 1979 Apr 15;94(1):162–174. doi: 10.1016/0042-6822(79)90446-x. [DOI] [PubMed] [Google Scholar]
- Laughlin C. A., Westphal H., Carter B. J. Spliced adenovirus-associated virus RNA. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5567–5571. doi: 10.1073/pnas.76.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., van der Vliet P. C., Sussenbach J. S. The replication of papovavirus and adenovirus DNA. Curr Top Microbiol Immunol. 1976;73:67–124. doi: 10.1007/978-3-642-66306-2_3. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Ratner J. Analysis of adeno-associated satellite virus DNA. Biochim Biophys Acta. 1973 Mar 19;299(2):189–195. doi: 10.1016/0005-2787(73)90341-9. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Carter B. J. Assembly of adeno-associated virus. Virology. 1980 Apr 15;102(1):71–82. doi: 10.1016/0042-6822(80)90071-9. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Oda K. The alteration of ribosomes for mRNA selection concerned with adenovirus growth in SV40-infected simian cells. Virology. 1975 Sep;67(1):85–93. doi: 10.1016/0042-6822(75)90406-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Carter B. J., Westphal H. Vero cells injected with adenovirus type 2 mRNA produce authentic viral polypeptide patterns: early mRNA promotes growth of adenovirus-associated virus. Proc Natl Acad Sci U S A. 1980 Feb;77(2):931–935. doi: 10.1073/pnas.77.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Maizel J. V., Jr, Inman J. K., Shatkin A. J. Structural proteins of adenovirus-associated viruses. J Virol. 1971 Nov;8(5):766–770. doi: 10.1128/jvi.8.5.766-770.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Ginsberg H. S., Rose J. A. DNA-minus temperature-sensitive mutants of adenovirus type 5 help adenovirus-associated virus replication. J Virol. 1975 Jan;17(1):140–148. doi: 10.1128/jvi.17.1.140-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H., Moritsugu Y. Isolation and a preliminary characterization of temperature-sensitive mutants of adenovirus 31. Virology. 1972 Aug;49(2):426–438. doi: 10.1016/0042-6822(72)90495-3. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet P. C., Levine A. J., Ensinger M. J., Ginsberg H. S. Thermolabile DNA binding proteins from cells infected with a temperature-sensitive mutant of adenovrius defective in viral DNA synthesis. J Virol. 1975 Feb;15(2):348–354. doi: 10.1128/jvi.15.2.348-354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]