Abstract
Inflammation is an important environmental factor that promotes tumourigenesis and the progression of established cancerous lesions, and recent studies have started to dissect the mechanisms linking the two pathologies. These inflammatory and infectious conditions trigger immune and stromal cell release of soluble mediators which facilitate survival and proliferation of tumour cells in a paracrine manner. In addition, (epi-)genetic mutations affecting oncogenes, tumour-suppressor genes, chromosomal rearrangements and amplifications trigger the release of inflammatory mediators within the tumour microenvironment to promote neoplastic growth in an autocrine manner. These two pathways converge in tumour cells and result in activation of the latent signal transducer and activator of transcription 3 (Stat3) which mediates a transcriptional response favouring survival, proliferation and angiogenesis. The abundance of cytokines that activate Stat3 within the tumour microenvironment, which comprises of members of the interleukin (IL) IL6, IL10 and IL17/23 families, underpins a signaling network that simultaneously promotes the growth of neoplastic epithelium, fuels inflammation and suppresses the host's anti-tumour immune response. Accordingly, aberrant and persistent Stat3 activation is a frequent observation in human cancers of epithelial origin and is often associated with poor outcome.
Here we summarize insights gained from mice harbouring mutations in components of the Stat3 signaling cascade and in particular of gp130, the shared receptor for the IL6 family of cytokines. We focus on the various feed-back and feed-forward loops in which Stat3 provides the signaling node in cells of the tumour and its microenvironment thereby functionally linking excessive inflammation to neoplastic growth. Although these observations are particularly pertinent to gastrointestinal tumours, we suggest that the tumour's addiction to persistent Stat3 activation is likely to also impact on other epithelial cell-derived cancers. These insights provide clues to the judicious interference of the gp130/Stat3 signaling cascade in therapeutically targeting cancer.
Introduction
Chronic infection and the ensuing inflammation are among the most important epigenetic and environmental factors that contribute to tumourigenesis and the progression of established cancerous lesions [1]. Aberrant proliferation alone is insufficient to cause cancer, which requires both an initial mutagenizing event that triggers neoplastic behaviour, as well as a microenvironment that is rich in factors which support cellular survival, growth and promote angiogenesis. Many of these cytokines, angiogenic factors and chemokines are produced by activated stroma and immune cells which accumulate in situ during chronic inflammation [1]. As these factors not only exert profound effects on (neoplastic) epithelium, endothelial and mesenchymal cells, but also recruit immune cells, the cancer microenvironment shares many molecular features of a 'never healing wound'. In addition, tumour cells themselves acquire the ability to subvert the host's anti-tumourigenic innate and adaptive immune responses [2,3]. Accordingly, the risk of cancer development increases with the failure to appropriately resolve immune responses, which promote excessive tissue remodeling, loss of tissue architecture, and cellular stress on proteins and DNA.
Compelling evidence for a link between inflammation and cancer comes from several epidemiological studies. Chronic inflammation triggered by viral or bacterial infection increases the risk for the development of papilloma virus-associated cervical cancer [4,5], hepatitis B and C-associated hepatocellular carcinoma and Epstein Barr virus-associated lymphoproliferative disorder [6], and bacterial infections can promote metastasis following surgery [7]. In the gastrointestinal tract, Helicobacter pylori (H.pylori)-associated gastric cancer along with ulcerative colitis and Crohn's disease-associated colorectal cancer comprise major health issues. Besides familial adenomatous polyposis and the hereditary nonpolyposis colon cancer syndrome, ulcerative colitis accounts for one of the three highest risk groups for developing colorectal cancer [8,9]. Accordingly, the use of non-steroidal anti-inflammatory drugs (NSAIDs) and inhibitors of the rate limiting Cox-2 enzyme in the prostaglandin E2 pathway, not only inhibits chronic inflammation in patients with premalignant disease, but also reduces the risk of cancer of the colon, lung, stomach, esophagus and ovaries [10].
In recent years, studies in genetically modified mice have helped to dissect and characterize some of the underlying molecular events that link inflammation to cancer [11,12]. For instance, the development of colorectal cancer is increased in various knockout mouse models of inflammatory bowel disease [13-16], and epidemiological evidence links polymorphisms in the corresponding genes to increased inflammation and cancer susceptibility in humans. Perhaps the greatest insights, however, have been mutant mice carrying loss- and gain-of-function mutations in intracellular components where a number of oncogenic signalling cascades converge. In this review we focus on Stat3, because it provides a central signaling node for neoplastic cells to induce transcriptional responses which promote tumour growth. Stat3 is aberrantly activated in a majority of cancers of epithelial origin [17,18]. Moreover, Stat3 plays an important role in determining the outcome of the interaction between cancers and immune cells, both in terms of suppressing anti-tumour activities as well as facilitating a tumour promoting inflammatory microenvironment. These roles have recently been clarified in the gastrointestinal tract, where Stat3 has attracted attention for its capacity to functionally link inflammation to tumourigenesis (Figure 1).
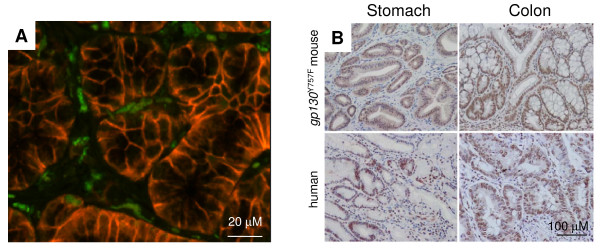
Figure 1.
Hematopoietic cell infiltration and STAT3 hyperactivation in gastrointestinal tumours. (A) Haematopoietic cell infiltration (green) in gastric tumours of gp130Y757F mutant mice visualized following adoptive transfer of bone marrow from GFP-transgenic mice. Metaplastic gastric epithelium is visualized following antibody binding to the intestine-specific epithelial cells surface marker gpA33 (red) [164]. (B) Immunohistological stain for activated STAT3 in sections of spontaneous arising gastric and CAC-induced colonic adenomas in gp130Y757F mutant mice, and from human gastric and colonic adenocarcinomas using an antibody directed against tyrosine phosphorylated STAT3.
Stat3 mode of action
All seven Stat proteins act as latent transcription factors that primarily mediate signalling from cytokine and growth factor receptors. Following their activation through phosphorylation on carboxy-terminally located conserved tyrosine residues and subsequent reciprocal SH2 domain interaction, Stat proteins form stable homo- and/or heterodimers in the cytoplasm [15]. Their subsequent nuclear translocation enables binding to DNA in a sequence-specific manner and results, usually in conjunction with other cofactors, in transcriptional regulation of target genes. Different Stat proteins show preferred specificity for individual cytokine family receptors. Stat1 primarily promotes growth arrest, apoptosis, and anti-tumour immunity downstream of type I and II interferons as demonstrated by the susceptibility of Stat1-deficient mice to develop tumours [19]. By contrast, Stat3 mediates activity of cytokines generally associated with systemic acute phase and cancer-promoting inflammatory responses. Stat3 can also be activated by other cancer-associated receptor tyrosine kinases, including those for epidermal growth factor and scatter factor c-Met [20-22]. Meanwhile, cellular transformation by the cytoplasmic tyrosine kinase c-src [23] or chromosomal translocation involving the anaplastic lymphoma kinase Alk is also dependent on Stat3 [24,25]. These cytoplasmic tyrosine kinases, often in conjunction with Jaks, are likely to mediate Stat3 activation subsequent to many other cancer-initiating, toxic insults, including UV-radiation, stress, and smoke [26,27].
Functionally the most important Stat3 regulators are the IL6 and IL10 family of cytokines (Figure 2). The IL6 family of ligands is defined by its shared use of the gp130 receptor β-subunit. Binding of IL6 and IL11 to their respective IL6Rα and IL11Rα receptor subunits triggers gp130 homodimerisation, while the remaining IL-6 family ligands (comprising LIF, CNTF, CT-1, Oncostatin M and IL27) induce formation of heterodimeric gp130 receptor complexes [16]. Engagement of gp130 triggers activation of the associated Janus kinases Jak1, Jak2 and Tyk2 [28,29] and subsequent tyrosine phosphorylation of gp130. While the four membrane-distal residues in the cytoplasmic tail of gp130 are required and sufficient for subsequent activation of Stat3, and to a lesser extent of Stat1, an additional membrane-proximal phospho-tyrosine residue (Y757 in mouse, Y759 in human) enables activation of the Ras/ERK pathway via the tyrosine phosphatase Shp-2. The same phospho-tyrosine in gp130 also serves as the binding site for the negative regulator Socs3, which is transcriptionally induced by Stat3. Binding of Socs3 to the activated gp130 complex results in its proteosomal degradation, thereby maintaining Stat3 activity of a transient nature. Accordingly, tissue-specific Socs3 ablation in mice amplifies ligand-dependent gp130 signalling, while the Y757F tyrosine-to-phenylalanine substitution in the corresponding gp130Y757F knock-in mutant mice results in excessive activation of Stat3 and Stat1 [30,31]. Interestingly, in the context of gp130 mediated Stat activation, Stat1 and Stat3 are capable of regulating each other [32,33].
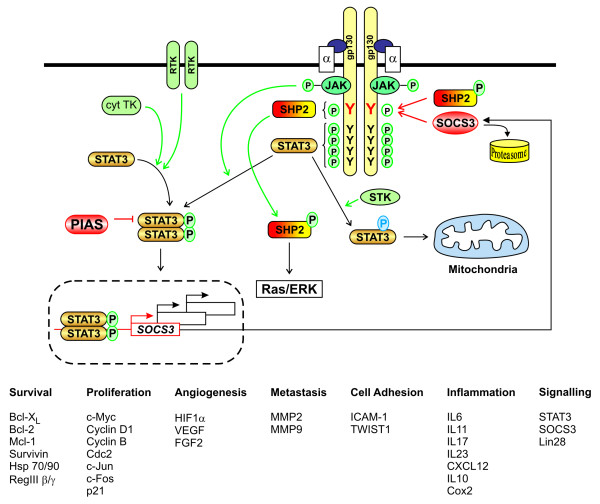
Figure 2.
Regulation of intracellular Stat3 signalling. Stat3 signalling is induced by various kinases (green) in a phosphorylation dependent manner (green arrows), and counteracted by several regulatory proteins (red). For instance, Stat3 activation occurs in response to gp130 homodimerization following binding of IL6 or IL11 (blue) to their specific transmembrane receptor α-subunits (white) [165]. Phosphorylation of four membrane distal tyrosine (Y, black) residues by constitutively associated JAK-family tyrosine kinases (TK) is sufficient to enable src-homology domain (SH)-2 mediated binding of STAT3 and, to a lesser extent, of STAT1. Once tyrosine phosphorylated, STAT1/3 form homo- and heterodimers, which translocate and trans-activate target genes, including the negative regulator SOCS3. STAT3 can also be phosphorylated by certain cytosolic TKs and receptor TK. Meanwhile, serine threonine kinases (STK) mediate serine-phosphorylation that maximizes the transcriptional activity of STAT3 and enables its mitochondrial targeting. Gp130 also engages the Ras/ERK pathway through binding of the tyrosine phosphatase SHP2 to the membrane proximal phospho-YxxV consensus sequence (red, where V is valine and × any amino acid). This phosphor-tyrosine also provides the binding site for SOCS3 to mediate proteasomal degradation of ligand-occupied receptor complexes. Gp130 signaling is also attenuated by the Y-phosphatase activity of SHP2, while cytoplasmic PIAS3 protein sequesters Y-phosphorylated STAT3 from homodimerization, nuclear translocation and target gene activation. Representative examples of different types of bona-fide Stat3 target genes are listed [166].
The IL10 family of cytokines shares the common IL10Rβ receptor subunit and comprises IL10, IL19, IL22 and IL24. IL10 confers broad anti-inflammatory responses in IL10Rα chain expressing cells, and these responses are amplified in a feed-forward loop encompassing Stat3-dependent transcriptional induction of Il10 [34]. Accordingly, mice lacking il10 or harbouring Stat3-deficient macrophages are characterized by excessive cytokine release and develop colitis [35,36]. IL22 is expressed during chronic inflammation by Th17, natural killer (NK) and Dendritic (DC) cells [37] and acts on IL-22R subunit expressing (intestinal) epithelial cells to induce IL10 and acute phase protein production [38]. Since the IL10-family receptor subunits lack Socs3 binding sites, IL10-mediated receptor engagement results in sustained Stat3 activation (Figure 3). Thus at least in macrophages, Socs3 provides the key molecular switch determining whether Stat3 promotes an inflammatory or anti-inflammatory response [39]. Accordingly, transient Stat3 activation by IL6 in wild-type macrophages promotes an inflammatory response, while sustained Stat3 activation by IL6 of gp130Y757F mutant macrophages suppresses the inflammatory gene response through the induction of transcriptional repressors [39,40]. Similarly, sustained gp130 and Stat3 activation in Socs3-deficient macrophages triggers a strong anti-inflammatory response [41-43] and expression of the canonical TGFβ signaling pathway inhibitor Smad7 [31].
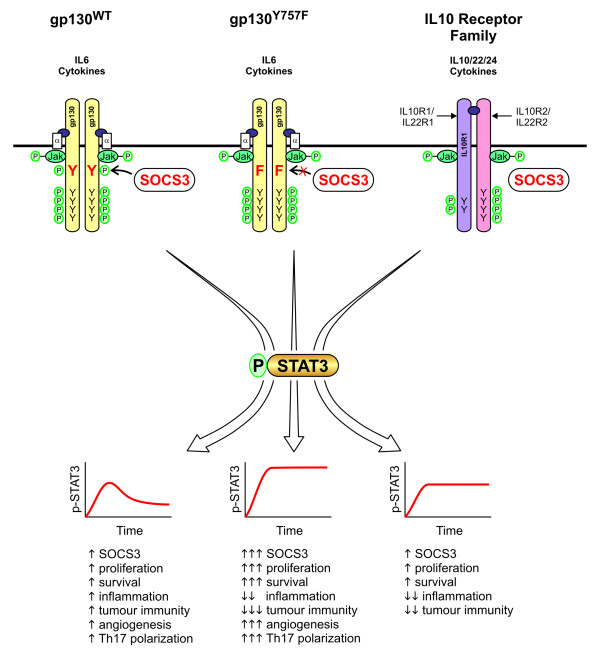
Figure 3.
Strength and duration of STAT3 activation determines cellular responses. STAT3 activation patterns and associated outcomes differ between IL6 and IL10 family cytokine-induced signaling. In response to binding of ligand (blue), signaling from gp130 is transient, because the phosphorylated, membrane proximal tyrosine residue (Y, red) provides a binding site for the negative regulator SOCS3. Although SOCS3 is also induced in response to activation of the IL10 receptor family, STAT3 signaling remains sustained, because the IL10 receptor chains lack the corresponding YxxV motif. Similarly, signaling from the mutant gp130Y757F receptor is sustained due to phenylalanine (F) substitution of the Y757 residue (Y759 in human GP130), resulting in exaggerated activation of its down-stream signaling molecules. The range of target genes activated differs between acute and sustained STAT3 activation, most evident in macrophages where the former promotes and the latter suppresses inflammatory response.
Since Stat3 occupies a central node for many converging signaling pathways, excessive Stat3 activity in tumours can result from oversupply of (IL6-family) cytokines and other growth factors within the tumour microenvironment. Besides these paracrine (or cell-extrinsic) pathways, activation of (proto-)oncogenes, inactivation of tumour-suppressor genes, chromosomal rearrangement/amplification and other genetic events in neoplastic cells either directly trigger Stat3 activation, or the release of inflammatory mediators as part of an autocrine (or cell-intrinsic) pathway. Remarkably, there is no genetic evidence for constitutively activating mutations within STAT3 itself. However, a variety of cancers harbour activating point mutations in Jak2 [44] and gp130 in-frame deletion mutations, which mediate ligand-independent activation of Stat3, are found in hepatocellular carcinomas [45]. Excessive activation of Stat3 can also result from impairment mutations affecting any of the negative regulatory proteins which limit the extent of Stat3 activation under physiological conditions [46]. For instance, epigenetic silencing of the negative regulator SOCS3 occurs in epithelial cancers [47], while other cancers show somatic mutations in Stat3-inactivating phosphatases T and δ [48,49]. Owing to their capacity to inactivate upstream tyrosine kinases or to sequester phosphorylated Stat3 from de novo Stat-dimers, mutagenic alterations in the cytosolic tyrosine phosphatases CD45 [50,51], SHP1 and SHP2 [50,51], or the SUMO E3 ligase Pias3 [52] and Grim19 [53] are also expected to result in excessive activation of Stat3-dependent target genes.
Cellular outcomes of Stat3 activation
A decade ago, Hanahan and Weinberg have suggested that the malignant growth characteristics of cancer cells requires six essential alterations in cellular physiology, namely self-sufficiency in growth signals, insensitivity to growth inhibiting signals, evasion of apoptosis, unlimited cellular replication, sustained angiogenesis, and tissue invasion and metastasis [54]. They argued that each change represents a new capability acquired during tumour development which overcomes rate limiting steps for anti-cancer defence mechanisms in normal cells. Stat3 promotes at least three of these hallmarks (proliferation, survival and angiogenesis) and often more when investigated in specific cell types.
Stat3 inhibits apoptosis by up-regulating the pro-survival Bcl-2 proteins Bcl-XL, Mcl-1 and Bcl-w [55-58]. In epithelial cells, Stat3 also induces other proteins that indirectly suppress apoptosis, including the chaperone protein Hsp70 [59] and the C-type lectin-type RegIIIβ, which are both overexpressed in human colon cancer and inflammatory bowel disease [60,61] (Figure 2). In conjunction with c-jun, Stat3 inhibits the extrinsic apoptosis pathways through transcriptional repression of the FAS death receptor [62]. Stat3-mediated induction of survivin not only suppresses apoptosis, but also promotes mitogenic progression through binding to cdc2 [63,64]. However, Stat3 promotes proliferation primarily by stimulating transcription of cyclinB1, cdc2, c-myc and cyclinD1 [55,65,66], along with the induction of the immediate early genes c-jun and c-fos [67] and repression of the cell cycle inhibitor p21 [68]. Accordingly, Stat3 promotes the G1/S phase transition of the cell cycle in gastric, colon and squamous cell carcinoma, as well as in bladder cancer cells [65,68-70]. By contrast, Stat3 ablation in intestinal epithelium in vivo or in tumour cell lines in vitro resulted in cell cycle arrest in the G2/M transition and is associated with histone H3 phosphorylation-associated mitotic arrest [68].
Among the angiogenic factors, VEGF and HIF1α stand out as prominent transcriptional targets for Stat3 [71,72], and a requirement for Stat3 has been proposed for functionality of HIF1α [73]. Accordingly, Stat3 is required for endothelial cell survival and their arrangement into new vascular structures [74], while nuclear Stat3 correlates with enhanced VEGF expression and microvessel density in gastric cancer [75,76]. Since Stat3 inhibition also blocks VEGF expression in tumours characterized by aberrant activation of Src [77], therapeutic targeting of Stat3 may inhibit neovascularisation in tumours associated with excessive signaling through epidermal growth factor receptor. Stat3 may also promote neovascularisation by mediating endothelial cell responses to other growth factors, including granulocyte-macrophage stimulating factor [78]. Excessive activation of Stat3 correlates with tumour invasion and metastasis in a variety of cancers [17,18] and high level of tyrosine-phosphorylated STAT3 is a pertinent feature in colon and gastric cancers associated with adverse outcomes [79] (Figure 1). Finally, Stat3 is part of the transcriptional network that mediates epithelial-to-mesenchymal (EMT) transformation in glioblastoma [80] and promotes metastasis by induction of the extracellular matrix-degrading metalloproteinases, including MMP-2 and MMP-9 [81].
Experimental carcinogenesis
To understand the function of Stat3 during carcinogenesis, it is helpful to divide (experimental) carcinogenesis into three distinct stages with an irreversible, genetic alteration providing the tumour initiating event. Subsequent tumour promotion occurs as a consequence of expansion of these genetically altered, pre-neoplastic cells which is frequently associated with an inflammatory response within the tumour microenvironment. Further tumour progression and growth coincides with the acquisition of additional (epi-)genetic changes, which ultimately enable the primary tumour to spread to distant metastatic sites. These sequential carcinogenesis processes can be experimentally recapitulated in mice using two-hit models employing 7,12-dimethylbenz(a) anthracene (DMBA) and 12-O-tetradecanoylphorbo-13-acetate (TPA) in the skin [82], or the azoxymethane (AOM) plus the polysaccharide dextran sodium sulfate (DSS) in the colitis-associated cancer (CAC) model of the colon [68]. In a hepatocellular carcinoma model, a two stage strategy is used by injecting diethylenitrosamine (DEN) as the tumour initiator and phenobarbitol as the promoter [83].
Stat3 in epithelial cancer cells
In the CAC model, inflammation triggered through prolonged administration of DSS reveals the mutagenic effect of prior exposure to the colonotropic mutagen AOM. DSS-mediated epithelial damage and impairment of epithelial barrier function enables commensal microbes to activate resident macrophages and release inflammatory cytokines, such as IL1, TNFα and IL6. In the absence of epithelial Stat3 expression, this results in the formation of occasional low-grade intraepithelial neoplastic lesions, while epithelial Stat3 proficiency enables progression of these lesions into advanced tubular tumors [68,84]. Conversely, excessive Stat3 activation, through epithelial-specific Socs3 ablation or introduction of the Socs3-binding deficient gp130Y757F mutation, results in increased tumour burden both in terms of tumour size as well as incidence [68,85]. Similar findings were obtained in the skin, where keratinocyte-specific Stat3 ablation abrogated skin tumour development [86], while keratinocyte-specific expression of the artificial, transcriptionally constitutive active Stat3C mutant, promoted the formation of squamous cell carcinoma in situ [82]. In either situation, Stat3 suppressed apoptosis of (mutagenized) stem and progenitor cells in the bulge region of the skin or the intestinal crypt, thereby curbing either their chance to be mutated or to subsequently expand [86] (Phesse T, Buchert M, Ernst M: Epistatic interaction between aberrant Wnt and Stat3 signaling during intestinal tumorigenesis, submitted). Consistent with these observations, systemic ablation of the il6 gene conferred a partial protective effect against tumour promotion in the CAC model, since IL6 enhances survival, proliferation and possibly cellular migration of enterocytes and their transformed counterparts that originated from the intestinal stem or transiently amplifying cell compartments [87,88]. Excessive abundance of IL6 also exacerbates colitis by suppressing apoptosis of infiltrating T-cells through "trans-signaling", whereby shedding of the extracellular domain from IL6Rα-proficient epithelium provides a soluble, ligand-binding receptor subunit for IL6 to activate gp130 in IL6Rα-deficient T-cells [89]. Thus, administration of either neutralizing IL6Rα antibodies or soluble gp130Fc suppressed enterocyte-specific Stat3 activation and proliferation, and reduced tumor incidence [90]. Concomitant overexpression of IL6 and IL6Rα in double transgenic mice is sufficient to induce hepatocellular carcinomas [91] and administration of Hyper-IL6, but not IL6, increased colonic tumours in CAC-challenged wild-type mice [84]. Due to the capacity of Hyper-IL6, a fusion protein between IL6 and IL6Rα [87,88], to activate gp130 receptors independently of the presence of the ligand-binding IL6Rα subunit, these observations suggest that cancer-initiating cells may not always express sufficient IL6Rα subunits to respond to IL6. In genetic complementation studies, we found functional redundancy between the IL6 and IL11 signaling in intestinal epithelium, where both cytokines were equally potent in conferring Stat3-dependent, epithelial resistance to DSS-induced apoptosis and colitis [68]. Consistent with these observations, IL11 administration protected against radiation-induced mucositis, suggesting that IL11 signaling may play an important role in the maintenance of intestinal epithelium [92]. Genetic deficiency for the ligand binding IL-11Rα subunit completely abrogates gastric tumour formation in gp130Y757F mice, and mono-allelic il11ra ablation delayed the onset and reduced overall gastric tumour burden [32]. However, unlike the observations in the colon, gastric tumourigenesis in gp130Y757F mice occurred independently of IL6 [32]. Meanwhile, systemic reduction of Stat3 expression in gp130Y757F;Stat3+/- mice not only prevented gastric tumour formation [31], but also reduced their susceptibility to colonic tumourigenesis in the CAC model [93]. Surprisingly, Stat1 gene inactivation also partially reduces gastric tumourigenesis in gp130Y757F mice [32], despite its general function in mediating IFNγ-dependent anti-tumour immunity [19]. However, therapeutic application of Stat3-antisense oligonucleotides or IL11 antagonists to gp130Y757F mice, suggest that growth and maintenance of gastric tumours remains dependent on the continuous activation of Stat3 [31,93].
Is excessive Stat3 activation in epithelial cells sufficient to trigger de novo tumour formation? In models akin to (onco-)gene amplification, enforced transgenic expression of constitutive active STAT3C confers tumourigenic capacity in a 3T3 xenograph model. Overexpression of STAT3C in vivo also induced broncho-alveolar adenocarcinomas [94] and the formation of squamous cell carcinoma in situ [82] when expressed in alveolar II epithelial cells or keratinocytes, respectively. Significantly, bronchoalveolar adenocarcinomas in STAT3C transgenic mice were preceded by inflammatory cell infiltrates and tumour development was associated with excessive secretion of inflammatory cytokines, including IL6 [94]. Even though there is no evidence for tumour-specific amplification of the STAT3 locus in humans, excessive activation of endogenous Stat3 reproducibly promotes gastric adenoma formation in gp130Y757F mice at a very young age. Tumour initiation and growth in this model correlates with bacterial load, because prophylactic antimicrobial treatment delayed the occurrence of these tumours [95]. Surprisingly, tumour development in gp130Y757F mice is restricted to the glandular stomach despite systemic hyperactivation of endogenous Stat3. Consistent with this finding, we also observed that enforced, ligand-independent activation of endogenous Stat3 in the epithelium of the small and large intestine failed to confer tumour development in transgenic mice [96]. Since the gp130Y757F germline mutation also impairs expression of the stomach-specific tumour suppressor gene tff1 [30], and since all colonic tumours in CAC-challenged gp130Y757F mice harbour mutagen-induced oncogenic conversions of β-catenin, excessive activation of endogenous Stat3 may only promote tumour growth in conjunction with preexisting tumour-initiating mutation(s). However, these observations may also predicate the (co-)existence of cell type-specific Stat3 threshold levels required for neoplastic transformation, akin to those described for the canonical Wnt pathway [97,98].
While epithelial Stat3 activity is dispensable during normal development and tissue homeostasis of the adult intestine, reduction of Stat3 expression, by either ablating il6 [84] or depleting the capacity of gp130 to activate Stat3, increases susceptibility to acute colitis and impairs intestinal wound healing [30]. In humans STAT3 represents one of the disease loci for Crohn's and inflammatory bowel disease (IBD) [99], and most likely relates to the capacity of Stat3 to promote intestinal barrier function and integrity in response to IL6, IL11 and IL22 exposure. Expression of IL22 during chronic inflammation provides a directional signal from immune cells to epithelium, as immune cells lack IL-22R (Figure 4). Sustained activation of Stat3 in (intestinal) epithelium, brought about by activation of the Socs3-unresponsive IL10R, IL22R or gp130Y757F receptors, induces an anti-microbial response. This comprises induction of mucins, lipocalin-2, RegIIIβ, RegIIIγ, and β-defensins to buffer the epithelium against an inappropriate innate immune response elicited by commensal bacteria and to prevent gastrointestinal inflammation and colitis [100-102]. Accordingly, experimental delivery of IL22 to mice with DSS-induced colitis reduced inflammatory infiltrates and promoted the mucosal healing response by goblet cells [103,104]. Thus, deficiency in Stat3, IL6 or IL11 signaling increases the susceptibility to colonic mucositis in CAC-challenged mice, but safeguards against excessive proliferation, survival and angiogenic activity of mutagenized cells. By contrast, the very mechanisms that confer resistance to colitis in Stat3 proficient epithelium also promote tumourigenesis, including IL22-dependent induction of tumour-promoting inducible nitric-oxide synthase [105].
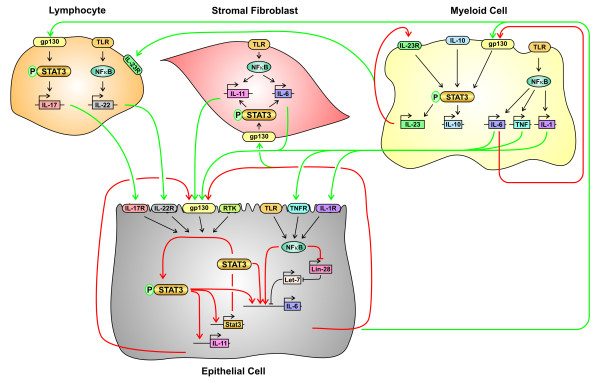
Figure 4.
Stat3 links inflammation to cancer. Inflammation and cancer are functionally linked by intrinsic, Stat3-dependent autocrine feedback loops in neoplastic epithelium (red arrows) and extrinsic, feedforward and often reciprocal interactions between tumour, inflammatory and stromal cells that collectively make up the microenvironment (green arrows) [167]. The ubiquitous expression of gp130 and the capacity of STAT3 to stimulate its own transcription as well as that of gp130 ligands (in particular IL6 and IL11) also provides numerous amplification loops between the different cell types. Furthermore, limited responsiveness to IL6 and IL11 imposed by restricted expression of the ligand-specific receptor α-subunits can be overcome by IL6-transsignaling [90]. Excessive cell-intrinsic STAT3 activation is also triggered by oncogenic events from (epi-)genetic activation of positive regulators (i.e. receptor (-associated) TKs) and loss of function mutations of negative regulators (i.e. SOCS3). Expression of IL6 is also directly induced by NF-κB and indirectly through a feedback mechanism including lin28 and the let7-microRNA, while latent Stat3 in conjunction with NF-κB triggers non-canonical IL6 expression. Epithelial NF-κB and STAT3 are activated in response to the abundantly present inflammatory cytokines IL1, TNFα and IL6 which are released from TLR-activated myeloid cell (macrophages), with IL6 and IL11 also contributed by tumour-associated stromal fibroblast and myoepithelial cells. Meanwhile, release of IL17 and IL22 from mature Th17 cells provide an additional extrinsic link which results in excessive STAT3 activation in tumour cells.
Since Stat3 hyperactivation is frequently fueled by excessive IL6, an important autocrine amplification loop arises from the capacity of phosphorylated Stat3 to induce its own transcription, where de novo Stat3 protein in turn directly promotes expression of il6 without a requirement for tyrosine phosphorylation (Figure 4) [106]. The functional relevance of these autocrine and paracrine feed-forward loops, originally proposed in multiple myeloma [107] and comprising the IL6/gp130/Stat3 cascade, has recently been extended to solid tumours, including lung adenocarcinoma [20], prostate cancer [108], ovarian carcinoma [109] and Ras-transformed cancer cells [110]. In Ras-transformed cancer cells, serine phosphorylated Stat3 may also aid tumour growth by promoting metabolic functions in mitochondria possibly through its association with Grim19 [111], and stimulation of the electron transport chain in a transcription independent way [112].
While there is ample evidence for IL6 in promoting tumour activity on epithelium, the role played by the other family members is less well defined. We have identified a prominent role for non-haematopoietic IL11 rather than (myeloid-derived) IL6 in promoting gastric tumour formation in the gp130Y757F mouse model [32]. IL11 expression correlates with development of intestinal-type gastric adenocarinoma in humans, and IL11Rα expression is linked to cancer depth and venous vessel invasion [113]. Since IL11 is expressed in epithelial and stromal cells, and its gene is transcriptionally activated by Stat3 [32], it remains to be established whether IL11 may also provide an autocrine and paracrine feed forward mechanism that, akin to IL6, fuels Stat3-dependent progression of tumours other than those of the stomach.
Stat3 in myeloid cells
Many of the inflammatory cytokines found in the tumour microenvironment are derived from activated myeloid cells, in particular neutrophils, DC, mast cells and macrophages, where a tightly controlled Toll-like receptor (TLR)-pathway regulates the innate immune response. Excessive TLR signaling can promote tumourigenesis, since ablation of the adaptor molecule MyD88 reduced intestinal tumourigenesis in ApcMin;MyD88-/- compound mutant mice [114]. Indeed, it has been speculated that debris from dying neoplastic cells may elicit TLR-dependent activation of macrophages or Kupfer cells in the liver [115] and engage the transcription factor NF-κB pathway through activation of its catalytic subunit IKKβ and culminating in induction of TNFα, IL-1 and IL-6 [116]. Thus, systemic administration of an IKKβ-specific inhibitor reduced Stat3 activation and IL6-target gene expression and ameliorated disease in colitis-prone IL10-deficient mice [117]. Similarly, myeloid-specific ablation of IKKβ inhibited tumour promotion and malignant cell proliferation in tobacco smoke- or oncogenic K-Ras-induced lung cancers [118], and reduced tumour size and multiplicity in the colon of CAC-challenged mice [11,119]. Indeed, high levels of IL6 in the tumour microenvironment are associated with the progression of colorectal [120], pancreatic [121], lung [122] and prostate cancer [123]. Furthermore, the incidence of hepatocellular carcinoma in humans, or in DEN- and CAC-induced tumours in mice, is less prominent in females due to the capacity of estrogen to suppress IL6 transcription [115,124]. The genetic ablation of il6 diminishes tumour burden in ApcMin and in CAC-challenged wild-type mice [84,125], DEN-induced liver [115] and in a tobacco smoke-associated lung cancer model [118]. Although gastric tumourigenesis in gp130Y757F mice occurred independently of IL6 [32], we found that MyD88-deficiency reduced their tumour burden (Jarnicki A, Puoczki T, Ernst M: A mouse model for innate immune cell-mediated gastric tumorigenesis, submitted), consistent with our observation that excessive Stat3-activation increases Tlr4 expression and susceptibility of these mice to lipopolysaccharide-induced septic shock (Jenkins B, Jarnicki A, Thiem S, Ernst M: Systemic alteration of IL6-mediated Stat3 signaling increases susceptibility to endotoxemia in mice, submitted).
Aberrant Stat3 activation in tumour cells promotes the secretion of immunomodulatory factors, which selectively reduce the Th1 dominated anti-tumour response [126]. In response to tumour-derived IL10 and VEGF, for instance, excessive Stat3 activity in myeloid cells inhibits maturation and activation within the DC lineage, favours polarization and activation of tumour associated macrophages (TAM), and reduces cytotoxic activity of neutrophils and NK cells [127]. The physical contact between tumour and antigen presenting cells also directly activates Stat3 and triggers a tolerogenic DC phenotype [128]. The capacity of Stat3 to modulate the anti-tumour immune response in macrophages and DCs partly depends on the heterodimeric IL12 cytokine family, which directs the outcome of inflammatory processes. Activation of tissue macrophages and DCs, for instance, results in production of IL12 (comprising IL12a/IL12b heterodimers) and subsequent INFγ-dependent Th1 and CTL anti-tumour responses. Meanwhile, IL10-mediated sustained Stat3 activation in TAMs represses IL12 expression and promotes production of IL23 (comprising IL23a/IL12b heterodimers), which helps to propagate the Th17 T-cell subset [129]. These findings reiterate the critical role played by Socs3 in maintaining an inflammatory, anti-tumourigenic environment characterized by IL12 expression that is converted to a tumour promoting cytokine profile when Socs3 is unable to abate gp130 signaling following engagement of the IL10 family receptor components. Accordingly, administration of Stat3 antagonists reduces tumour burden even in xenograph models where the primary tumour is not sensitive to inhibition of Stat3, suggesting that Stat3 inhibition provides a beneficial "bystander" effect on tumour cell killing that is associated with extensive tumour-specific lymphocyte infiltration [130]. Furthermore, Stat3-deficient myeloid derived suppressor cells fail to promote the formation of vessel-like structures in vitro, because induction of the pro-angiogenic factors VEGF, bFGF, IL-1β, MMP9, CCL2 and CXCL2 is Stat3 dependent [74]. Although, these observations suggest that excessive Stat3 activation within the myeloid cell lineages indirectly enhances tumour progression by subverting anti-tumour immunity, the contribution of myeloid Stat3 activation to the growth of tumours that are driven by persistent epithelial Stat3 activation remains less well understood. Systemic Stat3 inhibition, for instance, reduced gastric tumour burden even in gp130Y757F mice that had undergone adoptive bone marrow transfer with wild-type cells [32].
Stat3 in lymphoid cells
The Th17 subset of T-cells secrete large amounts of IL17A, which induces the angiogenic factors VEGF and TGFβ in fibroblasts and endothelial cells [131], and both IL17 and IL23 promote tumourigenesis [132,133]. Stat3 is indispensible for the development of the Th17 cell lineage, as it enables expression of the transcription factor RoRγt, which facilitates IL6-mediated polarization of naïve CD4 cells, and transcriptionally induces the IL-17a gene [134]. Thus, excessive Stat3 activity enforces differentiation into Th17 cells even in the context of Th1 polarising anti-tumour conditions [135], and genetic interference with the IL6/gp130 pathway selectively blocks Th17 cell polarization [136]. Although polarization of naïve CD4 to Th17 as well as Treg cells requires tumour-associated TGFβ in mice, only Th17 differentiation requires Stat3 activity. Accordingly, the extent of lymphocytic Stat3 activation directly shapes the overall tumour immune response including the Treg's capacity to deprive Th17 cells from essential activation cues [137]. Importantly, IL17 and IL23 alongside IL22 and cell-autonomous acting IL21, all promote and stabilize the Th17 phenotype and sustain inflammation [138] through various Stat3-dependent feed-forward loops within the tumour, stromal and haematopoietic cells of the microenvironment [133] (Figure 4). The existence of these networks are corroborated by findings that exposure of pre-neoplastic epithelium of ApcMin mice to the enterotoxic Bacteroides fragilis promotes colon tumourigenesis through an IL17-/Stat3-dependent mechanism [139]. Although H.pylori-associated gastritis coincides with a marked mucosal induction of IL17 and IL23 [140], and these cytokines are also elevated in gastric cancer bearing gp130Y757F mice (Putoczki T, Ernst M: A role for IL17 in a mouse model of gastric cancer, submitted), the latter tumours also develop in gp130Y757F;Rag-/- mice in the absence of adaptive immune cells [141]. Indeed, the gp130-family cytokine IL-27 may promote an anti-tumour response by suppressing Th17 cell polarization and favouring Th1 differentiation through its capacity to activate Stat1 [142].
Crosstalk of Stat3 with NF-κB and Wnt/β-catenin pathways
While Stat3 provides a major molecular link between the inflammatory response and epithelial tumourigenesis, some of its functions are also shared with NF-κB. Like Stat3, canonical activation of NF-κB induces genes that encode anti-apoptotic functions (incl. Bcl-XL, Gadd45b, Bfl1, Sod2, etc. [11,119]) to facilitate survival of (neo-plastic) cells. Therefore, inhibition of canonical NF-κB activating through ablation of the IKKβ gene in the intestinal epithelium decreased tumour incidence (but not size) in the colon of CAC-challenged mice [11,119]. Epithelial NF-κB activation results from the rich abundance of IL1β, TNFα and TLR-agonists in the tumour microenvironment, and IL1β, TNFα and many other cytokines and chemokines (i.e. IL6, CXCL2, CCL2 and CCL20) are transcriptional targets for NF-κB [119,143]. The intimate link between inflammation-associated hyper-activation of NF-κB and Stat3 has recently been extended by a further feed-forward loop, whereby NF-κB induction of the RNA binding protein Lin28 blocks processing of the let-7 microRNA (Figure 4) and thereby de-represses transcription of il6 [144]. It also has been suggested that Stat3 signaling prolongs nuclear retention of canonically activated NF-κB through RelA/p50 acetylation and associated interference with its nuclear export [145]. Importantly NF-κB and Stat3-mediated signaling converge on the EMT process where IL6-mediated Stat3 activation promotes EMT through transcriptional induction of the E-cadherin repressor snail [146], while activation of NF-κB promotes posttranslational stabilization of the Snail protein [147]. Unphosphorylated Stat3 can also cooperate with the NF-κB pathway by competing with IKKβ for binding to unphosphorylated NF-κB, and this complex activates genes, such as rantes and il8, independent of their binding sites for NF-κB and/or Stat3 [148].
While NF-κB and Stat3 cooperatively enhance survival of (neo)plastic cells through transcription of shared survival genes, the molecular mechanisms underlying functional cooperation between the aberrantly activated Stat3 and Wnt/β-catenin pathways are less clear. Evidence for the latter comes from the observation that all colonic tumours in the CAC-challenged gp130Y757F mice harbour activating mutations in β-catenin, and that gp130Y757F;ApcMin mice show increased tumour multiplicity [68,93], while enterocyte-specific Stat3 ablation reduced tumour incidence in ApcMin mice [33]. Although the two pathways share transcriptional responsiveness of proliferative target genes, such as c-myc and cyclinD1, IL11 administration and excessive Stat3 activation also facilitates survival of epithelial cells with the capacity to repopulate the intestine after radiation damage [92] (Phesse T, Buchert M, Ernst M: Epistatic interaction between aberrant Wnt and Stat3 signaling during intestinal tumorigenesis, submitted). Similarly, Stat3 promotes survival of tissue stem cells and suppresses their differentiation [144,149] in mutagen challenged skin models and in mouse embryonic stem (ES) cells. In the fruitfly, the genes dome, hop and Stat92E (orthologues of mammalian gp130, Jak and Stat3, respectively) are required to reinstate gut homeostasis following apoptosis, enteric infection, or c-jun kinase (JNK)-mediated stress signaling [150]. In mammals the gene encoding intestinal Krüppel-like factor (Iklf/Klf5) is a target for gp130-signalling, promotes ES cell pluripotency [151] and mediates epithelial hyperplasia in the intestine [152]. Stat3 may therefore increase the pool of "stem" cells susceptible to tumour-inducing mutation, including loss-of-heterozygosity in ApcMin mice. Moreover, the failure to eliminate cyclin D1 in situations of sustained Stat3 activation may not only bypass the DNA replication checkpoint response [153], but also facilitate aberrant chromosome segregation triggered in the absence of functional Apc protein [154].
Targeting Stat3 activity
The preclinical observations cited above suggest that the growth and maintenance of many tumours, including some that are not caused by aberrant activation of Stat3, have become addicted to its continuous activation. However, systemic deletion of Stat3 is incompatible with embryonic development, and tissue-specific Stat3 ablation in adult mice triggers enterocolitis, impairs T-cell migration and ultimately causes Th1 autoimmunity [155]. Similarly, a dominant-negative mutation in STAT3 reduces its activity in human CD4 cells by approximately 75% and is associated with Hyper-IgE syndrome [156]. The latter finding is consistent with genetic observations obtained in compound mutant mice where reduction of Stat3 by more than 50% of its activity results in pathological outcomes [157]. However, systemic Stat3 haploinsufficiency suppresses growth of gastrointestinal tumours, without interfering with physiological responses during adult, fecund life [32,33]. These observations raise the exciting prospect for a therapeutic window, in which partial interference with Stat3 signaling may selectively affect tumours without the need to specifically target tumour (or tumour-associated immune) cells.
Soluble ligands have been extensively targeted by antibody-mediated therapies, and antibodies directed against IL6 and IL6Rα show promising results in the treatment of rheumatoid arthritis and other chronic inflammatory diseases. However, due to extensive redundancy among cytokines that activate Stat3, direct inhibition of Stat3 (activity) may show additional therapeutic benefits. Traditionally, pharmaceutical efforts have concentrated on targeting tyrosine kinases, and several inhibitors with specificity against Stat3-activating kinases, including EGF receptor, c-src, and Jak2, are either already in the clinic or undergoing preclinical testing [158]. These approaches are likely to be complemented by future developments of drugs that inhibit Stat3 directly. Indeed, a number of natural compounds and their derivatives, including curcumin, curcubitacins, resveratrol as well as indirubin and platinum complexes, have been shown to interfere with Stat3 activity. Their inhibitory activity most likely arises from a combination of binding directly to Stat3 as well as interfering with other cellular processes, and although compounds such as STA-21, S31-M2001 or S3I-201 suppress the growth of breast cancer, myeloma and melanoma cell lines in xenograph model, the clinical utility of these molecules still awaits confirmation. Other approaches include peptidometics and small molecules that target Stat3 dimerization, double-stranded decoy oligonucleotide to compete with Stat3 binding to target genes [159,160], as well as suppression of transcription and translation through the development of antisense-oligonuclotides [32] and small inhibitory RNA [161].
As we learn more about the underlying changes resulting from aberrant activation of Stat3, we will gain better insights into which of the aforementioned approaches may be most suitable to a particular situation. It is worthwhile to consider whether Stat3-driven tumours also develop addictions to non-oncogene pathways that are amenable to therapeutic interference [162]. Simultaneous targeting of such pathways in tumour cells, perhaps in conjunction with antibody-based strategies to curb cytokine-mediated activation of Stat3 (and NF-κB) in immune cells may hold therapeutic potential.
Conclusions
While a link between inflammation and cancer has been known for more than a century, we now start to unravel underlying mechanisms by which chronic inflammation promotes many human cancers. Compelling recent evidence suggests that Stat3, alongside with NF-κB, acts as the signaling node which provide the functional link by which aberrant activation of inflammatory cells within the tumor microenvironment triggers an epithelial survival and growth response that promotes overgrowth of neoplastic cells. The skewed anti-inflammatory gene response elicited by prolonged Stat3 activation in myeloid cells, on the other hand, curbs the immune system's anti-tumour response, while excessive Stat3 activation in inflammatory Th17 T-cells further fuels tumour growth and angiogenesis. Persistent activation of STAT3, most prominently observed in the epithelial and immune cells that constitute the tumour invasive front, often results from autocrine and paracrine production of IL6-family cytokines by the tumour and associated stroma [143]. IL6 provides an important link between obesity, aging, chronic inflammation and cancer [163], and a wealth of genetic models now permits detailed dissection of the contribution of individual signaling components within specific cell types. A comprehensive understanding of the gp130/Stat3 signaling cascade holds great promise to identify and validate therapeutic targets that simultaneously restrict the effect of tumour promoting inflammation while restoring anti-tumour immunity.
Competing interests
The research in the laboratory of M.E. is supported in part by a financial contribution from CSL Ltd.
Authors' contributions
All authors have contributed to the writing of this paper.
Contributor Information
Andrew Jarnicki, Email: Andrew.Jarnicki@ludwig.edu.au.
Tracy Putoczki, Email: Tracy.Putoczki@ludwig.edu.au.
Matthias Ernst, Email: Matthias.Ernst@ludwig.edu.au.
Acknowledgements
The authors would like to thank all members of the laboratory of M.E. for sharing unpublished data and helpful discussions. The authors wish to apologize for having been unable to cite all relevant contribution made to the subject matter of this review.
T.P. is supported by Cure Cancer Australia and Cancer Australia, and M.E. is a Fellow of the National Health and Medical Research Council (NHMRC) Australia. This work was supported in part by research grants from the NHMRC.
References
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papovaviruses and human tumors. Haematol Blood Transfus. 1983;28:289–292. doi: 10.1007/978-3-642-68761-7_55. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Meru N, Delecluse HJ. Epstein-Barr virus infection and human malignancies. Int J Exp Pathol. 2001;82:149–170. doi: 10.1111/j.1365-2613.2001.iep190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmey JH, Bucana CD, Lu W, Byrne AM, McDonnell S. Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. Int J Cancer. 2002;101:415–422. doi: 10.1002/ijc.10632. [DOI] [PubMed] [Google Scholar]
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman M, Boyle P. Chemoprevention of colorectal cancer. Gut. 1998;43:578–585. doi: 10.1136/gut.43.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Simpson SJ, Brown LF, Comiskey M, de Jong YP. Development of colonic adenocarcinomas in a mouse model of ulcerative colitis. Inflamm Bowel Dis. 1998;4:196–202. doi: 10.1097/00054725-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Gao SP, Mark KG, Leslie K, Pao W, Motoi N. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311–319. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- Schlessinger K, Levy DE. Malignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3. Cancer Res. 2005;65:5828–5834. doi: 10.1158/0008-5472.CAN-05-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Bowman TL, Niu G, Yu H, Minton S. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Oates A, Dunn AR. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J Biol Chem. 1996;271:30136–30143. doi: 10.1074/jbc.271.47.30136. [DOI] [PubMed] [Google Scholar]
- Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. Embo J. 1995;14:1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M. Stat3 is a negative regulator of intestinal tumor progression in ApcMin mice. Gastroenterology. 2010;138:1003–1011. doi: 10.1053/j.gastro.2009.11.049. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/S1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- Alonzi T, Newton IP, Bryce PJ, Di Carlo E, Lattanzio G. Induced somatic inactivation of STAT3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine. 2004;26:45–56. doi: 10.1016/j.cyto.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- El Kasmi KC, Smith AM, Williams L, Neale G, Panopoulos AD. Cutting edge: A transcriptional repressor and corepressor induced by the STAT3-regulated anti-inflammatory signaling pathway. J Immunol. 2007;179:7215–7219. doi: 10.4049/jimmunol.179.11.7215. [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- Morgan KJ, Gilliland DG. A role for JAK2 mutations in myeloproliferative diseases. Annu Rev Med. 2008;59:213–222. doi: 10.1146/annurev.med.59.061506.154159. [DOI] [PubMed] [Google Scholar]
- Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh CJ, Hilton DJ. Negative regulation of cytokine signaling. J Leukoc Biol. 2001;70:348–356. [PubMed] [Google Scholar]
- He B, You L, Uematsu K, Zang K, Xu Z. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci USA. 2003;100:14133–14138. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeriah S, Brennan C, Meng S, Singh B, Fagin JA. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc Natl Acad Sci USA. 2009;106:9435–9440. doi: 10.1073/pnas.0900571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Guo A, Yu J, Possemato A, Chen Y. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci USA. 2007;104:4060–4064. doi: 10.1073/pnas.0611665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- Kim H, Hawley TS, Hawley RG, Baumann H. Protein tyrosine phosphatase 2 (SHP-2) moderates signaling by gp130 but is not required for the induction of acute-phase plasma protein genes in hepatic cells. Mol Cell Biol. 1998;18:1525–1533. doi: 10.1128/mcb.18.3.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CD, Liao J, Liu B, Rao X, Jay P. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- Maximo V, Lima J, Soares P, Silva A, Bento I. GRIM-19 in Health and Disease. Adv Anat Pathol. 2008;15:46–53. doi: 10.1097/PAP.0b013e31815e5258. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- Stephanou A, Brar BK, Knight RA, Latchman DS. Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and Bcl-x promoters. Cell Death Differ. 2000;7:329–330. doi: 10.1038/sj.cdd.4400656. [DOI] [PubMed] [Google Scholar]
- Zushi S, Shinomura Y, Kiyohara T, Miyazaki Y, Kondo S. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer. 1998;78:326–330. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sikora A, Grzesiuk E. Heat shock response in gastrointestinal tract. J Physiol Pharmacol. 2007;58(Suppl 3):43–62. [PubMed] [Google Scholar]
- Macadam RC, Sarela AI, Farmery SM, Robinson PA, Markham AF. Death from early colorectal cancer is predicted by the presence of transcripts of the REG gene family. Br J Cancer. 2000;83:188–195. doi: 10.1054/bjoc.2000.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velden JJ van der, van Marion AM, Kremer B, Straetmans JM, Henquet CJ. Erythema nodosum as an early sign of Crohn's disease. Int J Dermatol. 2007;46(Suppl 3):27–29. doi: 10.1111/j.1365-4632.2007.03507.x. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB. Cooperation between STAT3 and c-jun suppresses Fas transcription. Mol Cell. 2001;7:517–528. doi: 10.1016/S1097-2765(01)00199-X. [DOI] [PubMed] [Google Scholar]
- O'Connor DS, Grossman D, Plescia J, Li F, Zhang H. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci USA. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Morishita R, Yamamoto K, Taniyama Y, Aoki M. Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and AKT in endothelial cells. Hypertension. 2001;37:581–586. doi: 10.1161/01.hyp.37.2.581. [DOI] [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Kanda N, Seno H, Konda Y, Marusawa H, Kanai M. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- Chen CL, Cen L, Kohout J, Hutzen B, Chan C. Signal transducer and activator of transcription 3 activation is associated with bladder cancer cell growth and survival. Mol Cancer. 2008;7:78. doi: 10.1186/1476-4598-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Le X, Zheng L, Wang L, Frey JA. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- Kim HL, Cassone M, Otvos L Jr, Vogiatzi P. HIF-1alpha and STAT3 client proteins interacting with the cancer chaperone Hsp90: therapeutic considerations. Cancer Biol Ther. 2008;7:10–14. doi: 10.1158/1535-7163.MCT-07-0192. [DOI] [PubMed] [Google Scholar]
- Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Ahn MJ, Park CK, Han HX, Kwon SJ. Phospho-Stat3 expression and correlation with VEGF, p53, and Bcl-2 in gastric carcinoma using tissue microarray. Apmis. 2006;114:619–625. doi: 10.1111/j.1600-0463.2006.apm_401.x. [DOI] [PubMed] [Google Scholar]
- Gong W, Wang L, Yao JC, Ajani JA, Wei D. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386–1393. doi: 10.1158/1078-0432.CCR-04-0487. [DOI] [PubMed] [Google Scholar]
- Laird AD, Li G, Moss KG, Blake RA, Broome MA. Src family kinase activity is required for signal tranducer and activator of transcription 3 and focal adhesion kinase phosphorylation and vascular endothelial growth factor signaling in vivo and for anchorage-dependent and -independent growth of human tumor cells. Mol Cancer Ther. 2003;2:461–469. [PubMed] [Google Scholar]
- Valdembri D, Serini G, Vacca A, Ribatti D, Bussolino F. In vivo activation of JAK2/STAT-3 pathway during angiogenesis induced by GM-CSF. Faseb J. 2002;16:225–227. doi: 10.1096/fj.01-0633fje. [DOI] [PubMed] [Google Scholar]
- Jackson CB, Giraud AS. STAT3 as a prognostic marker in human gastric cancer. J Gastroenterol Hepatol. 2009;24:505–507. doi: 10.1111/j.1440-1746.2009.05822.x. [DOI] [PubMed] [Google Scholar]
- Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- Chan KS, Sano S, Kataoka K, Abel E, Carbajal S. Forced expression of a constitutively active form of Stat3 in mouse epidermis enhances malignant progression of skin tumors induced by two-stage carcinogenesis. Oncogene. 2008;27:1087–1094. doi: 10.1038/sj.onc.1210726. [DOI] [PubMed] [Google Scholar]
- Ward JM, Diwan BA, Ohshima M, Hu H, Schuller HM. Tumor-initiating and promoting activities of di(2-ethylhexyl) phthalate in vivo and in vitro. Environ Health Perspect. 1986;65:279–291. doi: 10.2307/3430195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26:4833–4841. doi: 10.1038/sj.onc.1210286. [DOI] [PubMed] [Google Scholar]
- Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- Schneider DT, Lemburg P, Sprock I, Heying R, Gobel U. Introduction of the oncological pediatric risk of mortality score (O-PRISM) for ICU support following stem cell transplantation in children. Bone Marrow Transplant. 2000;25:1079–1086. doi: 10.1038/sj.bmt.1702403. [DOI] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Maione D, Di Carlo E, Li W, Musiani P, Modesti A. Coexpression of IL-6 and soluble IL-6R causes nodular regenerative hyperplasia and adenomas of the liver. Embo J. 1998;17:5588–5597. doi: 10.1093/emboj/17.19.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RJ, Peng RY, Fu KF, Gao YB, Han RG. Effect of recombinant human interleukin-11 on expressions of interleukin-11 receptor alpha-chain and glycoprotein 130 in intestinal epithelium cell line-6 after neutron irradiation. World J Gastroenterol. 2006;12:3055–3059. doi: 10.3748/wjg.v12.i19.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Phesse T, Jenkins B, Buchert M, McKenzie B. Linking inflammation to cancer-A novel role for Stat3. Cytokine. 2010;48:44. doi: 10.1016/j.cyto.2009.07.365. [DOI] [Google Scholar]
- Li Y, Du H, Qin Y, Roberts J, Cummings OW. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M. STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology. 2006;131:1073–1085. doi: 10.1053/j.gastro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Putoczki T, Thiem S, Jarnicki AG, Jenkins B, McKenzie B. IL-11 mediated Stat3 activation in inflammation and cancer. Cytokine. 2010;48:7. doi: 10.1016/j.cyto.2009.07.032. [DOI] [Google Scholar]
- Gaspar C, Franken P, Molenaar L, Breukel C, Valk M van der. A targeted constitutive mutation in the APC tumor suppressor gene underlies mammary but not intestinal tumorigenesis. PLoS Genet. 2009;5:e1000547. doi: 10.1371/journal.pgen.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert M, Athineos D, Abud HE, Burke ZD, Faux MC. Genetic dissection of differential signaling threshold requirements for the Wnt/beta-catenin pathway in vivo. PLoS Genet. 1000;6:e1000816. doi: 10.1371/journal.pgen.1000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium TWTCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin' through a glass onion. Eur J Immunol. 2008;38:3265–3268. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- Mejias-Luque R, Linden SK, Garrido M, Tye H, Najdovska M. Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors. Oncogene. 2010. [DOI] [PubMed]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziesche E, Bachmann M, Kleinert H, Pfeilschifter J, Muhl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem. 2007;282:16006–16015. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]
- Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- Catlett-Falcone R, Dalton WS, Jove R. STAT proteins as novel targets for cancer therapy. Signal transducer an activator of transcription. Curr Opin Oncol. 1999;11:490–496. doi: 10.1097/00001622-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Akimoto K, Nagashima Y, Kojima Y, Sasaki T. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc Natl Acad Sci USA. 2009;106:16369–16374. doi: 10.1073/pnas.0907044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich A, Medina L, Piura B, Segal S, Huleihel M. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res. 2007;27:267–272. [PubMed] [Google Scholar]
- Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Yoshizaki A, Izumida S, Suehiro T, Miura S. Expression of interleukin-11 (IL-11) and IL-11 receptor alpha in human gastric carcinoma and IL-11 upregulates the invasive activity of human gastric carcinoma cells. Int J Oncol. 2007;30:825–833. [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2007;Chapter 14(Unit 14):12. doi: 10.1002/0471142735.im1412s77. [DOI] [PubMed] [Google Scholar]
- Eckmann L, Nebelsiek T, Fingerle AA, Dann SM, Mages J. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci USA. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ogata H, Nishigaki R, Broide D, Karin M. Tobacco Smoke Promotes Lung Tumorigenesis by Triggering IKKβ- and JNK1-Dependent Inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Esfandi F, Mohammadzadeh Ghobadloo S, Basati G. Interleukin-6 level in patients with colorectal cancer. Cancer Lett. 2006;244:76–78. doi: 10.1016/j.canlet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Okada S, Okusaka T, Ishii H, Kyogoku A, Yoshimori M. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–15. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- Hoheisel G, Izbicki G, Roth M, Chan CH, Reichenberger F. Proinflammatory cytokine levels in patients with lung cancer and carcinomatous pleurisy. Respiration. 1998;65:183–186. doi: 10.1159/000029256. [DOI] [PubMed] [Google Scholar]
- Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001;159:2159–2165. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- Gur-Wahnon D, Borovsky Z, Liebergall M, Rachmilewitz J. The induction of APC with a distinct tolerogenic phenotype via contact-dependent STAT3 activation. PLoS One. 2009;4:e6846. doi: 10.1371/journal.pone.0006846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int Immunol. 2007;19:695–702. doi: 10.1093/intimm/dxm045. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso R, Pallone F, Monteleone G. Emerging role of IL-23/IL-17 axis in H pylori-associated pathology. World J Gastroenterol. 2007;13:5547–5551. doi: 10.3748/wjg.v13.i42.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett M, Judd LM, Jenkins B, La Gruta NL, Grail D. Differential regulation of gastric tumor growth by cytokines that signal exclusively through the coreceptor gp130. Gastroenterology. 2005;129:1005–1018. doi: 10.1053/j.gastro.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Herrmann A, Deng JH, Kujawski M, Niu G. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liao X, Agarwal MK, Barnes L, Auron PE. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Ni S, He S, Yoon D, Feng GS. Stat3 promotes the development of erythroleukemia by inducing Pu.1 expression and inhibiting erythroid differentiation. Oncogene. 2009;28:3349–3359. doi: 10.1038/onc.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Guo G, Wray J, Eyres I, Nichols J. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- McConnell BB, Klapproth JM, Sasaki M, Nandan MO, Yang VW. Kruppel-like factor 5 mediates transmissible murine colonic hyperplasia caused by Citrobacter rodentium infection. Gastroenterology. 2008;134:1007–1016. doi: 10.1053/j.gastro.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields BJ, Hauser C, Bukczynska PE, Court NW, Tiganis T. DNA replication stalling attenuates tyrosine kinase signaling to suppress S phase progression. Cancer Cell. 2008;14:166–179. doi: 10.1016/j.ccr.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Dikovskaya D, Schiffmann D, Newton IP, Oakley A, Kroboth K. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176:183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma NK, Dourlat J, Davies AM, Long A, Liu WQ. STAT3-stathmin interactions control microtubule dynamics in migrating T-cells. J Biol Chem. 2009;284:12349–12362. doi: 10.1074/jbc.M807761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- Shen Y, Schlessinger K, Zhu X, Meffre E, Quimby F. Essential role of STAT3 in postnatal survival and growth revealed by mice lacking STAT3 serine 727 phosphorylation. Mol Cell Biol. 2004;24:407–419. doi: 10.1128/MCB.24.1.407-419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- Groner B, Lucks P, Borghouts C. The function of Stat3 in tumor cells and their microenvironment. Semin Cell Dev Biol. 2008;19:341–350. doi: 10.1016/j.semcdb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell. 2009;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone CN, White SJ, Tebbutt NC, Clay FJ, Ernst M. Analysis of the regulation of the A33 antigen gene reveals intestine-specific mechanisms of gene expression. Journal of Biological Chemistry. 2002;277:34351–34359. doi: 10.1074/jbc.M204865200. [DOI] [PubMed] [Google Scholar]
- Ernst M, Jenkins B. Acquiring signalling specificity from the cytokine receptor gp130. Trends in Genetics. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Reviews Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]