Abstract
Background
Ginsenosides such as Rb1, Rg3 and Rh2 are major bioactive components of Panax ginseng. This in vivo study investigates the metabolic pathways of ginsenosides Rb1, Rg3 and Rh2 orally administered to rats.
Methods
High performance liquid chromatography-mass spectrometry (LC-MS) and tandem mass spectrometry (MS-MS) techniques, particularly liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS), were used to identify the metabolites.
Results
Six metabolites of Rb1, six metabolites of Rg3 and three metabolites of Rh2 were detected in the feces samples of the rats. Rh2 was a metabolite of Rb1 and Rg3, whereas Rg3 was a metabolite of Rb1. Some metabolites such as protopanaxadiol and monooxygenated protopanaxadiol are metabolites of all three ginsenosides.
Conclusion
Oxygenation and deglycosylation are two major metabolic pathways of the ginsenosides in rat gastrointestinal tracts.
Background
Panax ginseng (Renshen) is used in Chinese medicines to treat various conditions such as debility, ageing, stress, diabetes, insomnia and sexual inadequacy [1-3]. The major bioactive components of P. ginseng are O-glycosides of the triterpen dammarane saponins known as ginsenosides [4,5] which exhibit properties such as anti-inflammation and anti-tumor [6-8]. Over 80 ginsenosides have been isolated from P. ginseng [9]. Rb1, Rg3 and Rh2 are three major ginsenosides with various bioactivities.
Rb1, which is the most abundant (0.22-0.62%) among all ginsenosides [5], protects against free radical damage, maintains normal cholesterol and blood pressure [10] and inhibits the induction phase of long-term potentiation by high frequency stimulation in the dentate gyrus of the brain [11]. Rb1 also rescues hippocampal neurons from lethal ischemic damage [12] and delays neuronal death from transient forebrain ischemia in vitro [13]. Rg3 is used as the major active component in an anti-tumor and anti-cancer drug in China [14]. The cytotoxicity of ginsenoside Rg3 against tumor cells increases when Rg3 is metabolized into Rh2 or protopanaxadiol [15]. The metabolic transformation of Rg3 into protopanaxadiol also increases the activity against Helicobacter pylori. Recently, in vitro biotransformation of ginsenosides was reported. The metabolites were identified by high-resolution tandem mass spectrometry. Degradation and bioconversion routes of the different ginsenosides at acidic (gastric) conditions and in the presence of intestinal microbiota were elaborated [16].
High performance liquid chromatography (HPLC) is a powerful chemical analysis technology that allows complex mixtures to be transformed into separated components. Mass spectrometry (MS) has progressed extremely rapidly during the last decade; especially in production, separation and ejection of ions, data acquisition and data reduction. Compared to other detectors, the advantages of the mass spectrometer are that in many cases it can provide absolute identification, not only structural information from the molecule under investigation but the molecular weight of the analyte.
Due to the specificity and sensitivity of LC-MS, especially in combination with MS-MS, it is powerful in identification of drug metabolites. Common biotransformation, e.g., oxidative reactions (hydroxylation), conjugation reactions to produces sulphates, glucuronides, glutathiones or other conjugates, hydrolysis of esters and amides, and reduction reactions, can be evaluated from just the knowledge of the molecular mass of the metabolites. Combination of the molecular-mass and possible biotransformation products, predicted by computer-aided molecular modeling approaches, enables the confirmation of metabolic pathways. Further confirmation and/or structure elucidation of metabolites is possible using MS-MS methods [17]. The identification of the metabolites of antihistamine compounds is feasible by using thermospray LC-MS and LC-MS-MS [18,19]. The present study aims to investigate the biotransformation of ginsenosides Rb1, Rg3 and Rh2 orally administered to rats by using LC-MS and MS-MS.
Methods
Chemicals
Ginsenosides Rb1, Rg3 and Rh2 (purity >99%) were provided by the Chinese Medicine Laboratory, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, China. HPLC-grade methanol was purchased from Acros Organics (USA). A Mili-Q Ultra-pure water system (Millipore, USA) was used to provide water for all the experiments. Other chemicals (analytical grade) were purchased from Sigma (USA).
Administration of ginsenosides
Water soluble Rb1, Rg3 and Rh2 were administered to three groups (n = 3 in each group) of male Sprague Dawley rats (body weight 200-220 g; age 6-7 weeks) respectively at a dose of 100 mg/kg body weight with 2 ml dosing solution. The protocols of the animal study were fully complied with the University policy on the care and use of animals and with related codes of practice. The animal experiments were conducted with the licenses granted by Hong Kong Hygiene and Health Department. Rat feces samples were collected at such intervals: 0 to 120 hours for Rb1 (half-life 16.7 hours), 0 to 24 hours for Rg3 (half-life 18.5 minutes) and 0 to 48 hours for Rh2 (half-life 16 minutes)[20-22].
Feces sample preparation
Each feces sample of each rat was suspended in 150 ml of water and then extracted with n-butanol (100 ml × 3). The extract was dried and the residue was dissolved in 1 ml of methanol. After centrifugation at 12000 rpm for 20 minutes (Eppendorf Centrifuge 5415R, Hamburg, Germany), 2 μl of the supernatant was analyzed with LC-Ms and LC-MS-MS for the identification of the ginsenosides and their metabolites. The blank feces (baseline) were collected from the same Sprague Dawley rat prior to the administration of ginsenosides, prepared and analyzed with the same method as the experimental groups.
LC-ESI-MS analysis
HPLC separation was performed with a LC system coupled with an auto-sampler and a micro mode pump (HP1100, Agilent Technologies, USA). A reversed-phase column (Waters, Xterra MS-C8, 2.1 × 100 mm, 3.5 μm) was used to separate the ginsenosides and their metabolites. The auto-sampler was set at 10°C. Mobile phase consisted of two eluents: water (A) and methanol (B). Gradient elution was 40% B in 0-4 minutes, 40-90% B in 4-5 minutes, 90% B in 5-35 minutes, 90-40% B in 35-36 minutes and 40% B in 36-42 minutes at a flow rate of 100 μl/min. Effluent from the LC column was diverted to waste for the first 12 minutes following the injection, and then diverted to the MS ion source.
MS experiments were performed on a quadruple-time of flight (Q-TOF) tandem mass spectrometer API Q-STAR Pulsar I (Applied Biosystems, USA). Negative or positive ion mode in electrospray ionization (ESI) was used to analyze ginsenosides and their metabolites in rat feces samples. The following parameters of the turbo-ionspray for positive ion mode were used: ionspray voltage 5500 V, declustering potential 1 (DP1) 90 V, focusing potential (FP) 265 V and declustering potential 2 (DP2) 10 V, collision energy (CE) 55 eV for MS-MS analysis. For negative ion mode, the parameters were: ionspray voltage -4200 V, declustering potential 1 (DP1) -90 V, focusing potential (FP) -265 V and declustering potential 2 (DP2) 10 V, collision energy (CE) -60 eV for MS-MS analysis. For both positive and negative ion mode, the ion source gas 1 (GS1), gas 2 (GS2), curtain gas (CUR) and collision gas (CAD) were 20, 15, 25 and 3, respectively. The temperature of GS2 was set at 400°C.
Results and Discussion
Metabolites of Rb1 in rat feces
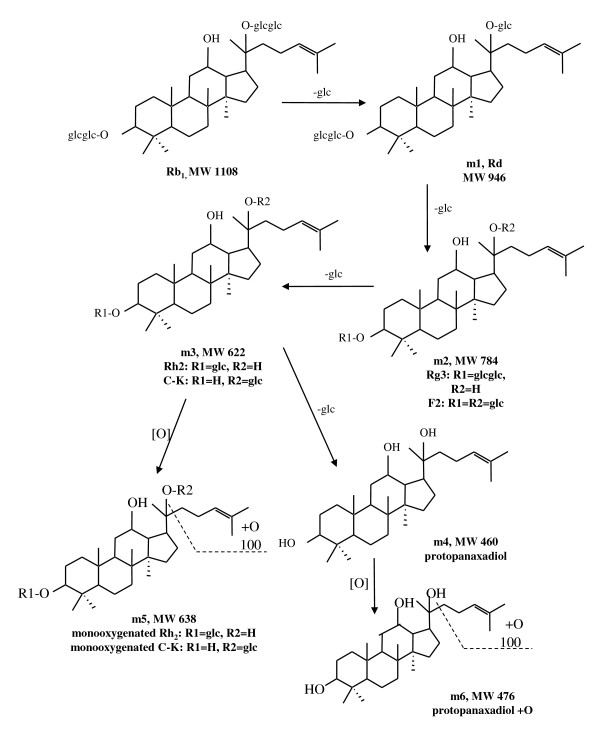
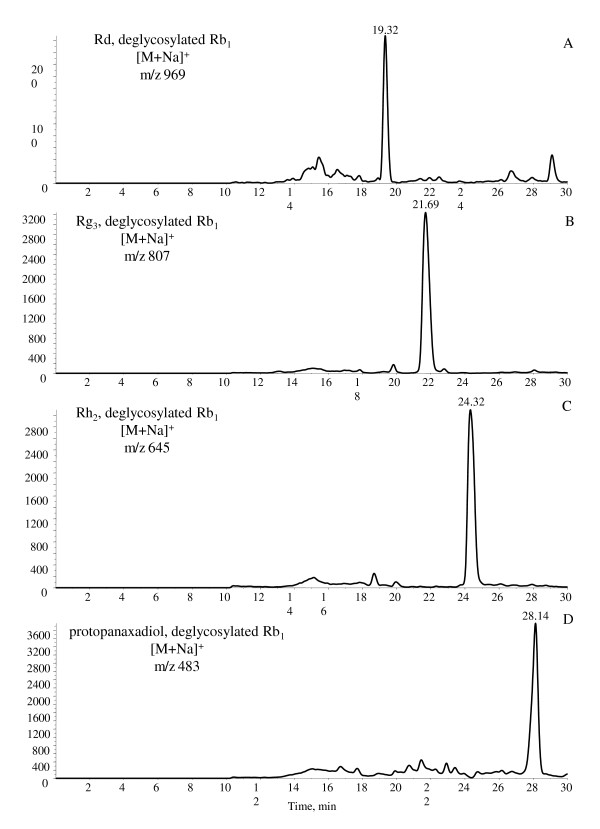
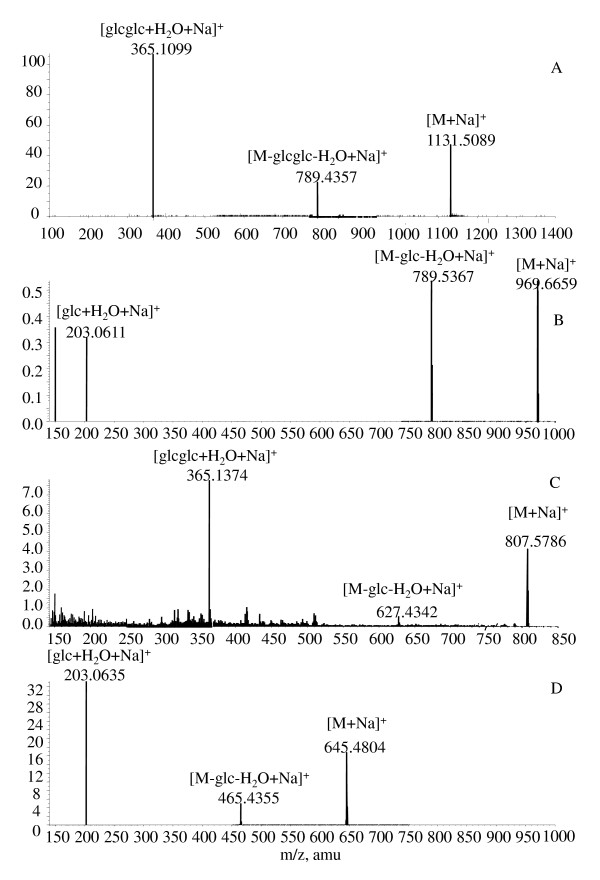
The parent Rb1 and direct oxygenated metabolites of Rb1 were not detected in the feces samples. These results suggested that Rb1 might have largely metabolized in the gastrointestinal tracts in rats. Six metabolites were detected in rat feces samples collected 0-120 hours after Rb1 was orally administered (Figure 1). The metabolites were detected from the LC-MS analyses and confirmed by the results from the LC-MS-MS experiments in positive ESI mode [18]. A total of four deglycosylated metabolites were identified, namely Rd, Rg3, Rh2 and protopanaxadiol (Figure 2). Analysis of [M + Na]+ ions (Figure 3) indicated that the metabolites shared similar MS-MS fragmentation pattern with the parent Rb1. The fragmentation patterns of the metabolites, produced from the [M + Na]+ ions at m/z 969, m/z 807, and m/z 645 respectively, were compared with that of Rb1. The deglycosylated metabolites of Rb1 showed the same fragment patterns as Rb1, i.e. the glucose moiety and water were lost from the molecular ion and the corresponding sodium-adduct daughter ions at m/z 789 and m/z 203 for Rd, m/z 627 and m/z 365 for Rg3 and m/z 465 and m/z 203 for Rh2 were produced.
Figure 1.
Deglycosylated and oxygenated metabolic pathways of Rb1 orally administered to rats.
Figure 2.
MS spectra of Rb1 orally administered to rats. (A) Rd and its deglycosylated metabolites, m/z 969; (B) Rg3, m/z 807; (C) Rh2, m/z 645; (D) protopanaxadiol, m/z 483.
Figure 3.
LC-MS-MS spectra of ginsenosides. (A) Rb1 and its deglycosylated metabolites; (B) Rd; (C) Rg3; (D) Rh2.
The deglycosylated metabolites were also confirmed by the LC-MS analysis of authentic standards of Rd, Rg3, Rh2 and protopanaxadiol. Moreover, the LC-MS-MS analysis indicated that these deglycosylated metabolites were subsequently oxygenated in digestive tracts. Thus, deglycosylation and subsequent oxygenation are the major metabolic pathways of orally administered Rb1 in rats. Figure 1 illustrates the proposed metabolic pathways of Rb1.
Metabolites of Rg3 in rat feces
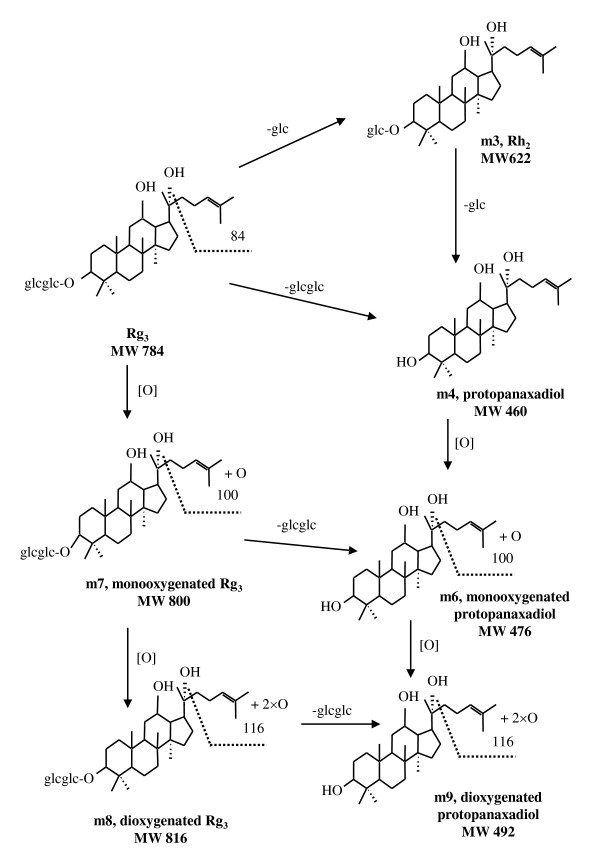
Six metabolites were detected in rat feces samples collected 0-24 hours after Rg3 was orally administered. The same LC-MS and MS-MS method as for Rb1 was used to detect major deglucosylated and further oxygenated metabolites of Rg3. The MS-MS results were similar to those for Rb1. Rh2 and protopanaxadiol as the deglucosylated products were also confirmed by reference standards. Figure 4 summarizes the major metabolites of Rg3 detected in the rat feces samples and the metabolic pathway in rat gastrointestinal tracts. After the oral administration, oxygenation and deglycosylation appeared to be the major metabolic pathways of ginsenosides. Metabolites were detected for the parent Rg3 and its deglucosylated metabolites including the mono- and deoxygenated products of protopanaxadiol.
Figure 4.
Metabolic pathways of Rg3 orally administered to rats.
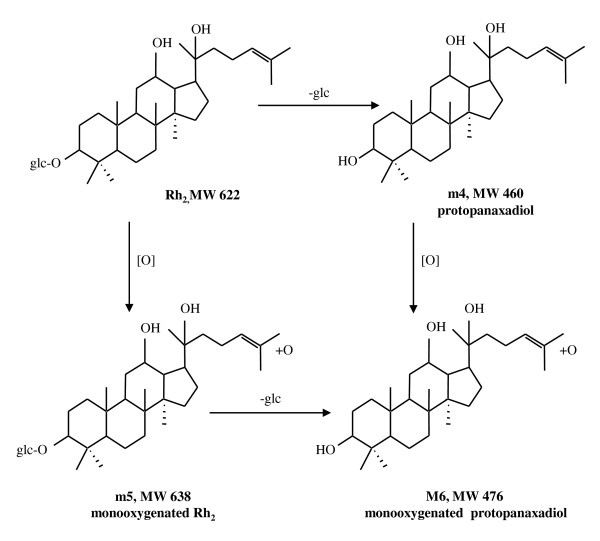
Metabolites of Rh2 in rat feces
Three major metabolites were detected in rat feces samples collected 0-48 hours after Rh2 was orally administered. The LC-MS and MS-MS method in positive ESI mode was used to detect and confirm the metabolites respectively. Oxygenated products, such as mono-oxygenated protopanaxadiol, were also identified. Deglycosylation and oxygenation were the major metabolic pathways of Rh2. Figure 5 illustrates the proposed metabolic pathway of Rh2 in rat gastrointestinal tracts.
Figure 5.
Metabolic pathways of Rh2 orally administered to rats.
Conclusion
Oxygenation and deglycosylation are two major metabolic pathways of the ginsenosides in rat gastrointestinal tracts. Furthermore, Rh2 is a metabolite of Rb1 and Rg3, whereas Rg3 is a metabolite of Rb1. Some metabolites such as protopanaxadiol and monooxygenated protopanaxadiol are metabolites of all three ginsenosides.
Abbreviations
HPLC: High performance liquid chromatography; LC-MS: High performance liquid chromatography coupled with mass spectrometry; MS-MS: Tandem mass spectrometry; LC-MS-MS: High performance liquid chromatography coupled with tandem mass spectrometry; ESI: Electric-spray ionization; Q-TOF: Quadruple-time of flight; DP: Declustering potential; CE: Collision energy; EP: Focusing potential; GS: source gas; CUR: Curtain gas; CAD: Collision gas; LC-ESI-MS: Liquid chromatography electrospray ionization mass spectrometry.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TXQ designed the experimental study, conducted the animal and LC-MS experiments and performed the analysis. ZWC conceived the study. All authors read and approved the final manuscript.
Contributor Information
Tianxiu Qian, Email: txqian88@yahoo.com.
Zongwei Cai, Email: zwcai@hkbu.edu.hk.
Acknowledgements
This work was supported by earmarked grants HKBU2154/04 M from the University Grants Committee (RGC) of Hong Kong.
References
- Crellin JK, Philpott J. A reference guide to medicinal plants: herbal medicine past and present. Vol. 2. Durham: Duke University Press; 1990. [Google Scholar]
- Chang MS, Lee SG, Rho HM. Transcriptional activation of Cu/Zn superoxide dismutase and catalase genes by panaxadiol ginsenosides extracted from Panax ginseng. Phytother Res. 1999;13:641–644. doi: 10.1002/(SICI)1099-1573(199912)13:8<641::AID-PTR527>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Lewis R, Wake G, Court G, Court JA, Pickering AT, Kim YC, Perry EK. Non-ginsenoside nicotinic activity in ginseng species. Phytother Res. 1999;13:59–64. doi: 10.1002/(SICI)1099-1573(199902)13:1<59::AID-PTR423>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Karikura M, Miyase T, Tanizawa H, Taniyama T, Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and -Rb2 in the digestive tract of rats. Chem Pharm Bull (Tokyo) 1991;39(9):2357–2361. doi: 10.1248/cpb.39.2357. [DOI] [PubMed] [Google Scholar]
- Takino Y. Studies on the pharmacodynamics of ginsenoside-Rg1, -Rb1 and -Rb2 in rats. Yakugaku Zasshi (Japanese) 1994;114(8):550–564. [PubMed] [Google Scholar]
- Wu JY, Gardner BH, Murphy CI, Seals JR, Kensil CR, Recchia J, Beltz GA, Newman GW, Newman MJ. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol. 1992;148(5):1519–1525. [PubMed] [Google Scholar]
- Sato K, Mochizuki M, Saiki I, Yoo YC, Samukawa K, Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17(5):635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18(9):1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- Dou D, Chen Y, Ren J. Ocotillone-type ginsenoside from leaves of Panax ginseng. J Chin Pharm Sci. 2002;11(4):119–121. [Google Scholar]
- Li X, Guo R, Li L. Pharmacological variations of Panax ginseng C.A. Meyer during processing. Zhongguo Zhong Yao Za Zhi. 1991;16(1):62. [PubMed] [Google Scholar]
- Wang XY, Zhang JT. Effect of ginsenoside Rb1 on long-term potentiation in the dentate gyrus of anaesthetized rats. J Asian Nat Prod Res. 2003;5(1):1–4. doi: 10.1080/10286020290029009. [DOI] [PubMed] [Google Scholar]
- Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M. Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res. 1997;28(3):191–200. doi: 10.1016/S0168-0102(97)00041-2. [DOI] [PubMed] [Google Scholar]
- Wen TC, Yoshimura H, Matsuda S, Lim JH, Sakanaka M. Ginseng root prevents learning disability and neuronal loss in gerbils with 5-minute forebrain ischemia. Acta Neuropathol. 1996;91(1):15–22. doi: 10.1007/s004010050387. [DOI] [PubMed] [Google Scholar]
- Huang JY, Sun Y, Fan QX, Zhang YQ. Efficacy of Shenyi Capsule combined with gemcitabine plus cisplatin in treatment of advanced esophageal cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2009;7(11):1047–51. doi: 10.3736/jcim20091105. [DOI] [PubMed] [Google Scholar]
- Bae EA, Han MJ, Choo MK, Park SY, Kim DH. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25(1):58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- Kong H, Wang M, Venema K, Maathuis A, Heijden R van der, Greef J van der, Xu G, Hankemeier T. Bioconversion of red ginseng saponins in the gastro-intestinal tract in vitro model studied by high-performance liquid hromatography-high resolution Fourier transform ion cyclotron resonance mass spectrometry. J Chromatogr A. 2009;1216(11):2195–2203. doi: 10.1016/j.chroma.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Yost RA, Perchalski RJ, Brotherton HO, Johnson JV, Budd MB. Pharmaceutical and clinical analysis by tandem mass spectrometry. Talanta. 1984;31(10 Pt 2):929–35. doi: 10.1016/0039-9140(84)80223-4. [DOI] [PubMed] [Google Scholar]
- Korfmacher WA, Holder CL, Betowski LD, Mitchum RK. Characterization of doxylamine and pyrilamine metabolites via thermospray/mass spectrometry and tandem mass spectrometry. Biomed Environ Mass Spectrom. 1988;15(9):501–8. doi: 10.1002/bms.1200150907. [DOI] [PubMed] [Google Scholar]
- Lay JO Jr, Getek TA, Kelly DW, Slikker W Jr, Korfmacher WA. Fast-atom bombardment and thermospray mass spectrometry for the characterization of two glucuronide metabolites of methapyrilene. Rapid Commun Mass Spectrom. 1989;3(3):72–5. doi: 10.1002/rcm.1290030306. [DOI] [PubMed] [Google Scholar]
- Qian T, Cai Z, Wong RNS, Jiang ZH. Liquid chromatography-mass spectrometric analysis of rat samples for in vivo metabolism and pharmacokinetic studies of ginsenoside Rh2. Rapid Commun Mass Spectrom. 2005;19:3549–3554. doi: 10.1002/rcm.2232. [DOI] [PubMed] [Google Scholar]
- Qian T, Cai Z, Wong RNS, Mak NK, Jiang ZH. In vivo metabolic and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B. 2005;816:223–232. doi: 10.1016/j.jchromb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Qian T, Jiang ZH, Cai Z. High performance liquid chromatography coupled with tandem mass spectrometry applied for in vivo metabolic study of ginsenoside Rb1. Anal Biochem. 2006;352(1):87–96. doi: 10.1016/j.ab.2006.02.025. [DOI] [PubMed] [Google Scholar]