Abstract
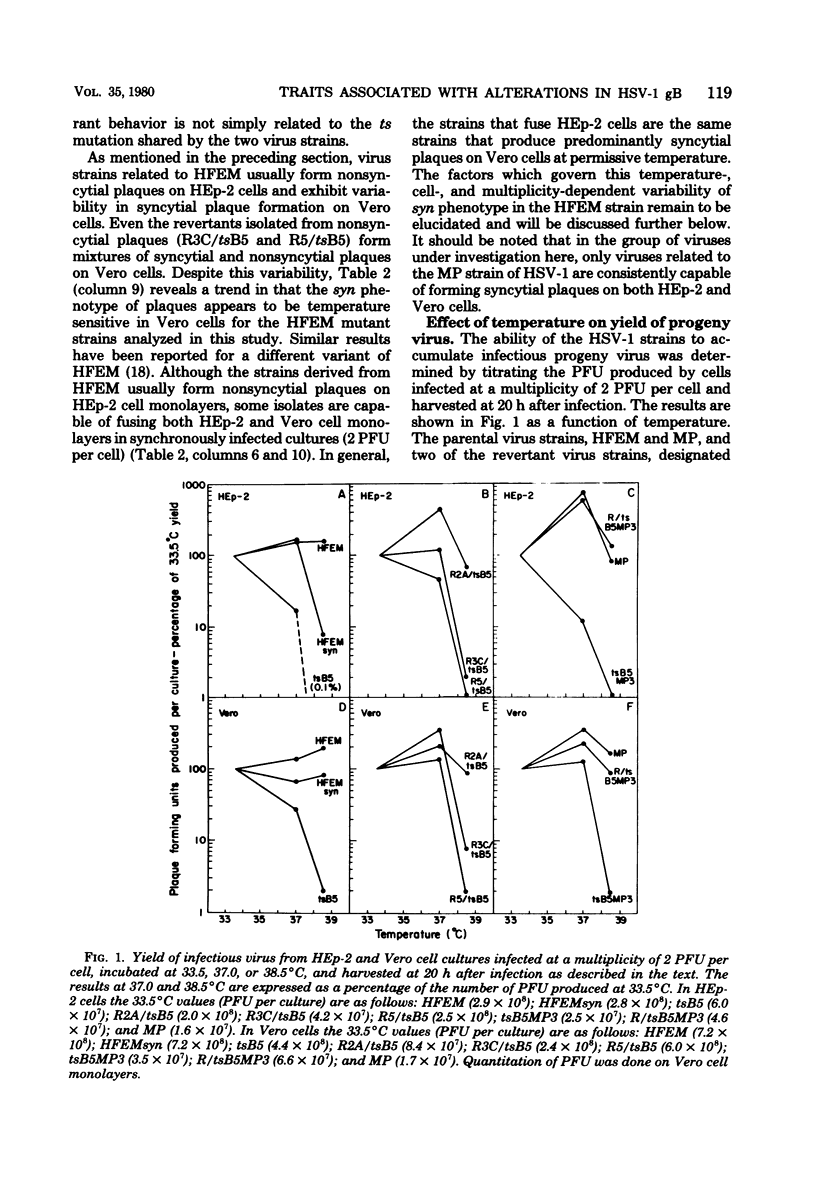
The tsB5 mutant of herpes simplex virus type 1 (HSV-1) strain HFEM was shown previously to be temperature sensitive for accumulation of the mature form of glycoprotein gB, for production or activity of a factor required in virus-induced cell fusion, and for production of virions with normal levels of infectivity. In addition, a previous study showed that virions produced by tsB5 at permissive temperature were more thermolabile than HFEM virions and contained altered gB that did not assume the dimeric conformation characteristic of HFEM. Results presented here demonstrate that, at permissive temperature, tsB5 differs from HFEM in another respect: plaques formed by tsB5 are syncytial on Vero cells (but not on HEp-2 cells), whereas plaques formed by HFEM are nonsyncytial on both cell types. In addition, our results indicate that tsB5 produces an oligomeric form of gB, but that it differs in electrophoretic mobility and stability from the gB dimers of HFEM. The major purpose of this study was to investigate the dependence of the various tsB5 mutant phenotypes on the temperature sensitivity of gB accumulation and on the alterations in oligomeric conformation of gB produced at permissive temperature. For this work the following HSV-1 strains related to tsB5 or HFEM were analyzed: (i) phenotypic revertants selected from tsB5 stocks for nonsyncytial plaque morphology on Vero cells or for ability to form plaques at restrictive temperature (38.5°C); (ii) a plaque morphology variant of HFEM selected for its syncytial phenotype on Vero cells; (iii) temperature-sensitive recombinants previously isolated from a cross between tsB5 and the non-temperature-sensitive syncytial strain HSV-1(MP); and (iv) a phenotypic revertant selected from one of the recombinant stocks for its ability to form plaques at 39°C. These strains were all compared with tsB5 and HFEM at three different temperatures in two different cell lines with respect to plaque formation, yield of infectious progeny, virus-induced cell fusion, and accumulation of gB. The results of our analyses on all the strains tested revealed the following correlations between mutant phenotypes and the accumulation and oligomeric conformation of gB. (i) There was a direct and quantitative relationship between the accumulation in infected cells of infectious progeny and of the mature form of gB, providing strong support for the hypothesis that this form of gB is necessary to the production of infectious virions. The oligomeric conformation of gB characteristic of HFEM is apparently not required for virion infectivity; nor was virion thermostability necessarily related to the presence of the HFEM-like oligomeric form of gB. (ii) The previously reported correlation between temperature sensitivity of gB accumulation and virus-induced cell fusion was confirmed for tsB5 and extended to other virus strains, and coordinate reversion of these traits was also demonstrated, providing support for the hypothesis that gB has a role in virus-induced cell fusion. At 37°C, intermediate between permissive and restrictive temperatures, some of the mutants and partial revertants induced cell fusion despite reduced accumulations of the mature form of gB, suggesting that the amount of mature gB present did not determine the extent of fusion and that other forms of gB as well as other factors should be investigated with regard to the process of cell fusion. (iii) Some of the mutants and partial revertants could form plaques at 38.5°C despite reduced ȧccumulations of gB and infectious progeny, indicating that the cell-to-cell transmission of viral infection may be at least in part independent of these factors.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- FARNHAM A. E. The formation of microscopic plaques by herpes simplex virus in HeLa cells. Virology. 1958 Oct;6(2):317–327. doi: 10.1016/0042-6822(58)90085-0. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshevsky U., Becker Y. Surface glycopeptides in the envelope of herpes simplex virions. Virology. 1972 Oct;50(1):277–279. doi: 10.1016/0042-6822(72)90371-6. [DOI] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980 May;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B. Polykaryocytosis. Cold Spring Harb Symp Quant Biol. 1962;27:327–342. doi: 10.1101/sqb.1962.027.001.031. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Studies of the determinant antigens of viable cells. I. A method, and its application in tissue culture studies, for enumeration of killed cells, based on the failure of virus multiplication following injury by cytotoxic antibody and complement. J Immunol. 1961 Dec;87:714–727. [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M. G. Mode of intercellular transfer of herpes virus. Nature. 1958 Nov 29;182(4648):1525–1526. doi: 10.1038/1821525a0. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Spear P. G. Membrane proteins specified by herpes simplex viruses. IV. Conformation of the virion glycoprotein designated VP7(B2). J Virol. 1979 Mar;29(3):1159–1167. doi: 10.1128/jvi.29.3.1159-1167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Carter V. C., Timbury M. C. Collaborative complementation study of temperature-sensitive mutants of herpes simplex virus types 1 and 2. J Virol. 1978 Sep;27(3):490–504. doi: 10.1128/jvi.27.3.490-504.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]








