Abstract
Purpose: To compare ablation zones created with equal amounts of 2.45 GHz microwave and 480 kHz radiofrequency (RF) energy in ex vivo liver and lung.
Methods: A total of 38 ablations were performed in ex vivo liver and lung for 10 min each. Nineteen RF ablations (nine liver, ten lung) were performed with a 480 kHz system (200 W max, impedance-based pulsing) and cooled electrode while measuring the average RF power applied. Nineteen microwave ablations (nine liver, ten lung) were then created using a cooled triaxial antenna to deliver 2.45 GHz at the same power level as in RF experiments. Ablation zones were then sectioned and measured for minimum, maximum and mean diameters, and circularity. Measurements were compared using t-tests, with P<0.05 indicating statistical significance.
Results: Mean diameters of microwave ablations were greater than RF ablations in both liver and lung (4.4±0.3 vs 3.3±0.2 cm in liver; 2.45±0.3 vs 1.6±0.5 cm in lungs; P<0.0005 all comparisons). There was no significant difference in the mean power applied during microwave or RF ablations in either organ (54.44±1.71 W vs 56.4±6.7 W in liver, P>0.05; 40±0.95 W vs 44.9±7.1 W in lung, P>0.05).
Conclusions: Using a single cooled applicator, microwave energy at 2.45 GHz produces larger ablations than an equivalent amount of 480 kHz RF energy in normal liver and lung. This was more apparent in lung, likely due to the high baseline impedance which limits RF, but not microwave power delivery.
Keywords: radiofrequency ablation, microwave ablation, liver, lung, thermal therapies, interventional oncology
INTRODUCTION
Thermal tumor ablation is a well established treatment option for unresectable primary and some secondary liver tumors and is being increasingly applied in other organs such as lung, kidney, and bone.1, 2, 3, 4, 5, 6, 7 Radiofrequency (RF) ablation is the most widespread thermal ablation modality worldwide, while microwave ablation has only been recently introduced to western countries.8, 9, 10
Current RF ablation systems are often limited by undesirably long ablation times (over 20 min for many procedures), deformation of the ablation zone by vascular structures, and the need for ground pads when using monopolar applicators.11, 12, 13, 14, 15 While increasing the RF power applied can increase ablation zone size, tissue temperatures during RF ablation are fundamentally limited to less than 105 °C; higher temperatures lead to dehydration and charring, which inhibit electrical current flow.16, 17 Techniques to avoid such charring, such as power ramping and pulsing, limit the average power applied and, consequently, reduce ablation zone size while increasing the probability of incomplete tumor destruction in large tumors.
Microwave ablation may be applied for the same indications as RF ablation but has several advantages in energy delivery. Most importantly, microwave propagation is not limited by charred tissue and, therefore, tissue temperatures can be elevated to very high levels (>150 °C) without impairing energy deposition.18, 19 High temperatures are more likely to overcome vascular mediated cooling20 and create larger and more lethal ablation zones with shorter treatment times.21 To date, the primary limitations of microwave ablation systems have been related to power distribution, with higher powers often requiring large antenna diameters (>14-gauge) to reduce shaft heating. Recent advantages in antenna cooling, however, can help reduce the amount of shaft heating and increase power delivery by small-diameter antennas.22
Direct comparisons of RF and microwave ablation systems are scarce, but recent studies have suggested that microwave ablation is capable of creating larger and more reproducible zones of ablation than RF ablation in liver, lungs, and kidney.23, 24, 25 While encouraging, these studies compared RF and microwave systems at dissimilar average applied powers, leaving open the possibility that the larger ablation zones associated with microwaves were simply due to greater total energy deposition. This disparity derives from the fact that the RF systems utilized in those studies relied on a pulsed energy delivery algorithm with less than 100% duty cycle that reduces average power to keep temperatures below 100 °C.26, 27 Reduced RF power applied is particularly evident in lung tissue where electrical impedance is inherently high.28, 29, 30, 31 In contrast, microwave heating is created by the propagation and absorption of electromagnetic energy in a lossy dielectric, such as biological tissue, and continues even in dehydrated or desiccated tissue with low electrical conductivity.32 Therefore, a second hypothesis to explain improved microwave performance in previous studies is that microwaves produce a more spatially efficient heating pattern. However, a recent computer simulation study suggested that direct heating provided by RF and microwave energy is not substantially different, leaving open the question of whether the larger microwave ablations observed in previous studies are only due to greater energy delivery.18 Therefore, the purpose of this study was to compare ablation zones created with equivalent amounts of energy. The null hypothesis of this study is that there will be no difference in the size of ablations created with 480 kHz RF current or 2.45 GHz microwaves when the total energy delivered by each system is equivalent. Rejection of this hypothesis would imply that microwaves produce a larger zone of direct heating.
MATERIALS AND METHODS
Tissue sample preparation
Four bovine livers and five sets of porcine lungs were obtained within 1 h of animal death and stored at 4 °C for no more than 72 h. Livers were then warmed to room temperature (20±1 °C) and sectioned into approximately 10×10×10 cm3 blocks free from vessels larger than 5 mm before ablations were performed. Lungs were warmed to room temperature (22±2 °C), left intact, and inflated with room temperature air through a cuffed tracheal tube. Airflow was adjusted as needed during the ablation procedures to maintain full insufflation.
Radiofrequency and microwave ablation technique
Treatment duration was fixed at 10 min for both RF and microwave ablations. RF ablations (n=9 in liver and n=10 in lungs) were performed by using a 480 kHz system that provided a maximum power output of 200 W and impedance-based power pulsing (CoolTip; Valleylab, Boulder, CO). The RF energy was delivered through a 17-gauge internally cooled electrode with 3 cm active length (CoolTip; Valleylab, Boulder, CO). The cooling water temperature was maintained at 4 °C with a flow rate of approximately 100 ml∕min by the peristaltic pump provided with the RF ablation system. The internal cooling mechanism prevents charring or desiccation of the tissue in contact with the surface of the electrode, increasing the duration of treatment and amount of power that can be applied through a single needle applicator.
Generator output voltage (±1 V), current (±0.01 A), power (±1 W), impedance (±1 Ω), and electrode tip temperature (±1 °C) were all monitored continuously during RF ablations. Tip temperature was also recorded at the end of each ablation after turning off coolant flow. The total energy delivered was calculated by integrating the output power over time. The average power delivered was then calculated as the total energy divided by treatment time (600 s) for each trial. The mean average power delivered of all RF trials was then used to determine the power setting of the microwave ablation system.
Microwave ablations (n=9 in liver and n=10 in lungs) were carried out using a prototype 17-gauge water-cooled triaxial antenna delivering power from a 2.45 GHz generator (Cober Muege, Norwalk, CT). Cooling water flow was similar to that used in the RF experiments, approximately 100 ml∕min. Energy was delivered from the generator to the antenna through a 1.5 m long coaxial cable (RG-400: Pasternack Enterprises, Irvine, CA). To assess how much of the generator power would be delivered to tissue, the combined power loss from the power delivery cable, connectors, and antenna cable were measured with a vector network analyzer (8753ES, Agilent, Santa Clara, CA) at 2.45 GHz. Using this measurement, we determined that the microwave generator output power should be 100 W for liver ablations and 80–90 W for lung ablation to deliver the same average power as in RF ablations (60 W for liver, 45 W for lung).
Output power and reflected power at the generator (±1 W) were recorded continuously during microwave ablations. The total power delivered to the tissue was then calculated as the generator output power minus power losses in the power delivery cables and antenna reflections.
Ablation zone measurements
Due to the different consistency of liver and lung parenchyma, we used a different slicing method for each tissue. For liver, ablation zones were sliced transverse to the electrode insertion track in approximately 5 mm increments using a Thompson blade immediately following the ablation procedure. Lung ablations were excised within an approximately 5×5×5 cm3 block and stored at −10 °C for at least 24 h, until solid, to facilitate sectioning. Frozen blocks of lung were sliced transverse to the electrode insertion track in 5 mm increments using a commercial meat slicer. Slices were placed onto a flatbed scanner (Epson Perfection V200; Long Beach, CA) and the digital images were saved electronically. One slice from each ablation zone containing the largest transverse diameters was selected and used for further measurements. Measurements of minimum, maximum and mean [(min+max)∕2] diameters, area, and circularity were then performed using the software IMAGEJ v1. 39e (National Institutes of Health, Bethesda, MD) based on the outer edge of the transition zone.33 Circularity was defined as the normalized ratio of the area to the square of the perimeter (4πA∕P2) and was used to identify the effects of tissue inhomogeneities on the ablation zone. Ideally, because of the axial symmetry of the ablation applicators, the ablation circularity would be that of a perfect circle or unity. Two investigators performed independent measurements on each sample, with the mean values used for statistical analysis.
Statistical analysis
Descriptive statistics were calculated for each measurement. Two-tailed Student’s t-tests were used to compare the measured average power, total energy, circularity, maximum, minimum, and mean diameter, and maximum, minimum, and mean diameter normalized to the delivered power (diameter∕power×100) between RF and microwave groups independently. Statistical analysis was performed using SAS, version 9.1.3 (SAS Institute Inc., Cary, NC) package SA (v9.1.3; VCU). P-values less than 0.05 were considered to indicate statistical significance.
RESULTS
The combined percent power loss from the microwave power delivery cable, connectors, and antenna cable was 34% (−1.9 dB). This loss was used in the calculation of the antenna reflection coefficient and power delivered to the tissue.
Liver
Applied power and ablation zone measurements from normal liver are summarized in Table 1. The mean initial impedance during RF ablations was 91.8±7.1 Ω. The mean final tip temperature was 81±4 °C. The mean reflection coefficient from microwave antennas was 8.5%.
Table 1.
Ablation measurements for radiofrequency and microwave ablation in liver tissue. All measurements given as mean±standard deviation.
| RF | MW | P-values | |
|---|---|---|---|
| Power (W) | 56.46±6.7 | 54.45±1.7 | 0.9314 |
| Energy (J) | 34 020±3968 | 32.669±1027 | 0.9314 |
| Maximum diameter (cm) | 3.51±0.3 | 4.60±0.3 | <0.0001 |
| Minimum diameter (cm) | 3.27±0.2 | 4.15±0.3 | <0.0001 |
| Mean diameter∕delivered power×100 | 6.07±0.7 | 8.03±1.4 | <0.0001 |
| Circularity | 0.968±0.016 | 0.934±0.030 | 0.0092 |
There was no statistical difference between the mean total energy delivered during RF or microwave ablations (32.7±1.0 kJ versus 34.0±4.0 kJ; P=0.38). Similarly, there was no significant difference in average power applied between microwave and RF ablations (54.4±1.7 W versus 56.5±6.7 W, respectively; P=0.38). At gross pathology, microwave ablations had a significantly greater mean diameter when compared to RF ablations (4.37±0.3 cm versus 3.39±0.2, respectively; P<0.0001; Figs. 12). RF ablations were statistically more circular than microwave ablations, though the difference was not practically significant (0.96 versus 0.93, P=0.009).
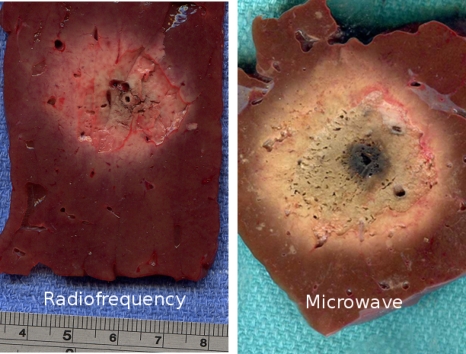
Figure 1.
Typical RF (left, 3.6 cm diameter) and microwave (right, 4.6 cm diameter) ablations created with an average power of about 60 W for 10 min in ex vivo bovine liver.
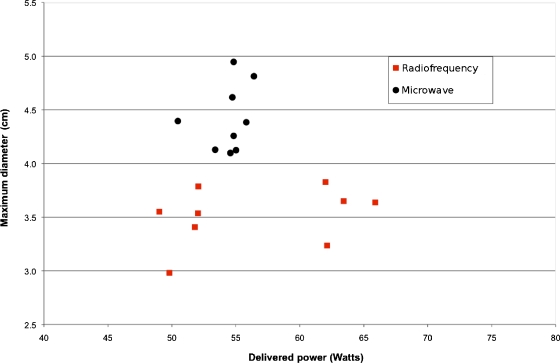
Figure 2.
Graph shows the relationship between delivered power and ablation size (maximum diameter) in ex vivo bovine liver. Microwave ablations (●) have a greater maximum diameter than radiofrequency ablations (◼) (mean difference 1.1 cm; p=0.0005). Note the greater variability in actual delivered power for radiofrequency ablations compared to microwave. There was no statistical difference in the delivered power between microwave and radiofrequency energies.
Lungs
Applied power and ablation zone measurements from normal lung are summarized in Table 2. The mean initial impedance during RF ablations was 223±36 Ω. The mean final tip temperature was 38±10 °C. The mean reflection coefficient from microwave antennas was 14%. The mean tip temperature after RF ablations in lung was significantly lower than in liver tissue (38±10 versus 81±4 °C, respectively; P<0.0001).
Table 2.
Ablation measurements for radiofrequency and microwave ablation in lung tissue. All measurements given as mean±standard deviation.
| RF | MW | P-values | |
|---|---|---|---|
| Power (W) | 44.86±7.1 | 40.03±0.9 | 0.1230 |
| Energy (J) | 27 031.928±4276 | 24 022.704±573 | 0.0892 |
| Maximum diameter (cm) | 1.69±0.6 | 2.65±0.4 | 0.0005 |
| Minimum diameter (cm) | 1.46±0.5 | 2.25±0.2 | 0.0002 |
| Mean diameter∕delivered power×100 | 3.51±1 | 6.11±0.7 | <0.0001 |
| Circularity | 0.913±0.052 | 0.907±0.051 | 0.9118 |
The mean average power delivered during microwave and RF ablations in lung were not significantly different (40.0±0.9 W versus 44.8±7.1 W, respectively; P=0.1230). However, at gross pathology, microwave ablations had a significantly greater mean diameter when compared to RF ablations (2.45±0.32 cm versus 1.57±0.5 cm, respectively; P<0.0003; Table 2, Figs. 34). No difference in circularity was observed between microwave and RF ablations in lung (0.92±0.1 versus 0.91±0.1, respectively; P>0.05). Microwave energy delivery was more consistent in both liver and lung tissues, as shown by the lower standard deviation in average power delivered when compared to RF energy (Tables 1, 2).
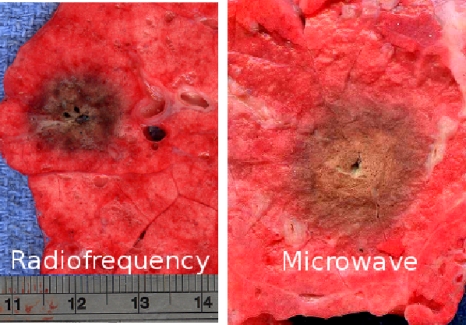
Figure 3.
Typical RF (left, 1 cm diameter) and microwave (right, 2.1 cm diameter) ablations created at a power of about 40 W for 10 min in ex vivo porcine lung tissue.
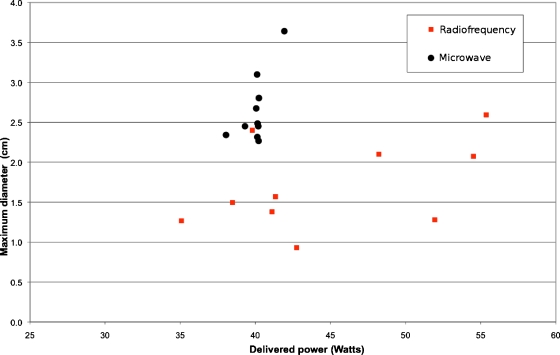
Figure 4.
Graph shows the relationship between delivered power and ablation size (maximum diameter) in ex vivo porcine lungs. Microwave ablations (●) have a greater maximum diameter than radiofrequency ablations (◼) (mean difference 0.96 cm, p<0.0001) even though the mean power delivered was slightly lower.
DISCUSSION
This study evaluated ablation zones created with 480 kHz RF and 2.45 GHz microwave energy in ex vivo liver and lung using cooled, needlelike applicators. While there was no difference in the power applied by either system in each tissue, microwaves created significantly larger ablation zones in both liver and lung. In previous preclinical studies, differences between microwave and RF ablations were attributed to either (1) increased average power deposition with microwaves due to an insensitivity to charring, which permits continuous power delivery, or (2) deeper penetration of microwave energy, resulting in greater volume heating compared to RF energy.18, 19, 23, 24, 25 The present study held average delivered power and applicator size and form constant, yet microwave ablations were larger, implying that microwave energy was spatially distributed into a larger volume than RF.
This result is aligned with previous, largely theoretical proposals on RF and microwave heating differences. Earlier analyses have predicted that the zone of active microwave heating can extend into a 2 cm diameter surrounding the antenna, and experimental data have demonstrated substantial temperature elevations (in excess of 150 °C) inside microwave ablations.19, 34, 35, 36 In contrast, during RF ablation, active tissue heating is limited due to the high impedance of heated, desiccated, and charred tissue. Moreover, temperatures in the zone of active RF heating are limited to less than 105 °C to prevent desiccation and charring, both of which inhibit the application of additional RF energy.27 The RF ablation then grows concentrically by thermal conduction, which is often vulnerable to heat sinks and local heterogeneities, especially at the periphery of the ablation zone where the vast majority of tumor recurrences are located.14, 37, 38, 39
The difference between RF and microwave ablation is more apparent in lung tissue. Previous studies have shown that normal lung parenchyma is characterized by both relatively low electrical and thermal conductivities compared to normal liver tissue (0.303 versus 0.527 W∕mK, respectively).40 Both of these factors limit energy deposition and tissue heating during lung RF, making it particularly difficult to achieve ablative margins. Our study confirms that as expected from theory, microwave energy propagation is not substantially influenced by air-filled alveoli while RF current flow is reduced by the high electrical impedance of lung tissue.31, 41 As a result, microwave ablations were significantly larger in lung. This finding also substantiates previous results from an in vivo porcine lung model and has clinical significance given the relatively high recurrence rates for RF ablation in lung.10, 23, 29, 39, 41, 42, 43, 44
Only a few other studies have directly compared RF and microwave ablation, drawing different conclusions depending on the settings and optimization of each particular system. None of these previous studies controlled for the total amount of power delivered.23, 24, 25, 45, 46 Similarly, the results of the present study should not be construed to apply to all microwave or RF ablation systems or clinical scenarios. Of note, the present study did not include multitined or multiple electrode devices designed to spatially distribute RF energy into a larger volume of tissue.27, 47, 48, 49 Nor did we investigate other microwave antenna designs, including deployable applicators, that may have broader heating patterns.8, 50, 51, 52 Instead, we chose to control for applicator size and form as well as power, using only needlelike applicators.
A second limitation is the unperfused ex vivo model used in this study. Prior studies have demonstrated that microwave ablation is less influenced by vascular perfusion than RF, with some studies actually noting improved performance in vivo for some microwave devices.20, 50 Therefore, it is likely that the differences between microwave and RF performance noted in this study would also be observed in vivo.
In summary, our study demonstrates that for a given amount of energy delivery, larger zones of coagulation can be achieved with single applicator 2.45 GHz microwave energy compared to 480 kHz RF energy in ex vivo tissues. This finding suggests that the broader spatial heating offered by 2.45 GHz microwaves at the applied energy level is more efficient for tissue ablation than 480 kHz RF energy, and emphasizes the concept that energy is not the only important factor in tissue heating; how the energy is distributed spatially matters as well. This knowledge may help guide microwave system development and optimization for clinical tumor ablation. For example, our finding that microwaves utilize a larger volume of direct heating indicates that antenna designs with broader heating patterns may be preferable. Since microwave ablations were larger for a given application time, it is also reasonable that microwaves may reduce ablation times required by current RF ablation systems. Further technology development and clinical trials are needed to understand if the potential benefits of microwave ablation will translate to a clinical setting.
References
- Gillams A. R., “Image guided tumour ablation,” Cancer Imaging 5, 103–109 (2005). 10.1102/1470-7330.2005.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter T. C., Laesekee P. F., and Lee F. T., “Focal tumor ablation: A new era in cancer therapy,” Ultrasound Q. 22, 195–217 (2006). 10.1097/01.ruq.0000232614.79547.b6 [DOI] [PubMed] [Google Scholar]
- Livraghi T., Meloni F., DiStasi M., Rolle E., Solbiati L., Tinelli C., and Rossi S., “Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice?,” Hepatology (Baltimore) 47, 82–89 (2008). 10.1002/hep.21933 [DOI] [PubMed] [Google Scholar]
- Gillams A., “Tumour ablation: Current role in the liver, kidney, lung and bone,” Cancer Imaging 8, S1–S5 (2008). 10.1102/1470-7330.2008.9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerich D. and Laeseke P. F., “Thermal tumour ablation: Devices, clinical applications and future directions,” Int. J. Hyperthermia 21, 755–760 (2005). 10.1080/02656730500226423 [DOI] [PubMed] [Google Scholar]
- Bandi G., Hedican S. P., and Nakada S. Y., “Current practice patterns in the use of ablation technology for the management of small renal masses at academic centers in the United States,” Urology 71, 113–117 (2008). 10.1016/j.urology.2007.08.023 [DOI] [PubMed] [Google Scholar]
- Pennathur A., Abbas G., Schuchert M., Landreneau R. J., and Luketich J. D., “Radiofrequency ablation for the treatment of lung neoplasm,” Expert Rev. Med. Devices 5, 613–621 (2008). 10.1586/17434440.5.5.613 [DOI] [PubMed] [Google Scholar]
- Iannitti D. A., Martin R. C., Simon C. J., Hope W. W., Newcomb W. L., McMasters K. M., and Dupuy D., “Hepatic tumor ablation with clustered microwave antennae: The US Phase II Trial,” HPB (Oxford) 9, 120–124 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. C., Scoggins C. R., and McMasters K. M., “Microwave hepatic ablation: Initial experience of safety and efficacy,” J. Surg. Oncol. 96, 481–486 (2007). 10.1002/jso.20750 [DOI] [PubMed] [Google Scholar]
- Wolf F. J., Grand D. J., Machan J. T., DiPetrillo T. A., Mayo-Smith W. W., and Dupuy D. E., “Microwave ablation of lung malignancies: Effectiveness, CT findings, and safety in 50 patients,” Radiology 247, 871–879 (2008). 10.1148/radiol.2473070996 [DOI] [PubMed] [Google Scholar]
- Ahmed M., Liu Z., Humphries S., and Goldberg S. N., “Computer modeling of the combined effects of perfusion, electrical conductivity, and thermal conductivity on tissue heating patterns in radiofrequency tumor ablation,” Int. J. Hyperthermia 24, 577–588 (2008). 10.1080/02656730802192661 [DOI] [PubMed] [Google Scholar]
- Goldberg S. N., Hahn P. F., Tanabe K. K., Mueller P. R., Schima W., Athanasoulis C. A., Compton C. C., Solbiati L., and Gazelle G. S., “Percutaneous radiofrequency tissue ablation: Does perfusion-mediated tissue cooling limit coagulation necrosis?,” J. Vasc. Interv. Radiol. 9, 101–111 (1998). 10.1016/S1051-0443(98)70491-9 [DOI] [PubMed] [Google Scholar]
- Dos Santos I., Haemmerich D., Pinheiro Cda S., and da Rocha A. F., “Effect of variable heat transfer coefficient on tissue temperature next to a large vessel during radiofrequency tumor ablation,” Biomed. Eng. Online 11, 7–21 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D. S., Raman S. S., Limanond P., Aziz D., Economou J., Busuttil R., and Sayre J., “Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors,” J. Vasc. Interv. Radiol. 14, 1267–1276 (2003). [DOI] [PubMed] [Google Scholar]
- Goldberg S. N., Solbiati L., Halpern E. F., and Gazelle G. S., “Variables affecting proper system grounding for radiofrequency ablation in an animal model,” J. Vasc. Interv. Radiol. 11, 1069–1075 (2000). 10.1016/S1051-0443(07)61341-4 [DOI] [PubMed] [Google Scholar]
- Solazzo S. A., Ahmed M., Liu Z., Hines-Peralta A. U., and Goldberg S. N., “High-power generator for radiofrequency ablation: Larger electrodes and pulsing algorithms in bovine ex vivo and porcine in vivo settings,” Radiology 242, 743–750 (2007). 10.1148/radiol.2423052039 [DOI] [PubMed] [Google Scholar]
- Brace C. L., Laeseke P. F., Sampson L. A., Frey T. M., Mukherjee R., and Lee F. T., “Radiofrequency ablation with a high-power generator: Device efficacy in an in vivo porcine liver model,” Int. J. Hyperthermia 23, 387–394 (2007). 10.1080/02656730701397858 [DOI] [PubMed] [Google Scholar]
- Schramm W., Yang D., and Haemmerich D., “Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation,” in IEEE Engineering in Medicine and Biology Society Conference Proceedings, 2006, Vol. 1, pp. 5013–5016. [DOI] [PubMed]
- Skinner M. G., Iizuka N. M., Kolios M. C., and Sherar M. D., “A theoretical comparison of energy sources–microwave, ultrasound and laser–for interstitial thermal therapy,” Phys. Med. Biol. 43, 3535–3547 (1998). 10.1088/0031-9155/43/12/011 [DOI] [PubMed] [Google Scholar]
- Yu N. C., Raman S. S., Kim Y. J., Lassman C., Chang X., and Lu D. S., “Microwave liver ablation: Influence of hepatic vein size on heat-sink effect in a porcine model,” J. Vasc. Interv. Radiol. 19, 1087–1092 (2008). 10.1016/j.jvir.2008.03.023 [DOI] [PubMed] [Google Scholar]
- Brace C. L., Laeseke P. F., Sampson L. A., Frey T. M., van der Weide D. W., and Lee F. T., “Microwave ablation with a single small-gauge triaxial antenna: In vivo porcine liver model,” Radiology 242, 435–440 (2007). 10.1148/radiol.2422051411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang M., Lu M. D., Xie X. Y., Xu H. X., Mo L. Q., Liu G. J., Xu Z. F., Zheng Y. L., and Liang J. Y., “Liver cancer: Increased microwave delivery to ablation zone with cooled-shaft antenna—Experimental and clinical studies,” Radiology 242, 914–924 (2007). 10.1148/radiol.2423052028 [DOI] [PubMed] [Google Scholar]
- Brace C. L., Hinshaw J. L., Laeseke P. F., Sampson L. A., and Lee F. T., “Pulmonary thermal ablation: Comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model,” Radiology 251, 705–711 (2009). 10.1148/radiol.2513081564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. S., Sampson L. A., Warner T. F., Mahvi D. M., and Lee F. T., “Radiofrequency versus microwave ablation in a hepatic porcine model,” Radiology 236, 132–139 (2005). 10.1148/radiol.2361031249 [DOI] [PubMed] [Google Scholar]
- Laeseke P. F., Lee F. T., Sampson L. A., van der Weide D. W., and Brace C. L., “Microwave ablation versus radiofrequency ablation in the kidney: High-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes,” J. Vasc. Interv. Radiol. 20, 1224–1229 (2009). 10.1016/j.jvir.2009.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. N., Stein M. C., Gazelle G. S., Sheiman R. G., Kruskal J. B., and Clouse M. E., “Percutaneous radiofrequency tissue ablation: Optimization of pulsed-radiofrequency technique to increase coagulation necrosis,” J. Vasc. Interv. Radiol. 10, 907–916 (1999). 10.1016/S1051-0443(99)70136-3 [DOI] [PubMed] [Google Scholar]
- Pereira P. L., Trübenbach J., Schenk M., Subke J., Kroeber S., Schaefer I., Remy C. T., Schmidt D., Brieger J., and Claussen C. D., “Radiofrequency ablation: In vivo comparison of four commercially available devices in pig livers,” Radiology 232, 482–490 (2004). 10.1148/radiol.2322030184 [DOI] [PubMed] [Google Scholar]
- Nomori H., Imazu Y., Watanabe K., Ohtsuka T., Naruke T., Kobayashi T., and Suemasu K., “Radiofrequency ablation of pulmonary tumors and normal lung tissue in swine and rabbits,” Chest 127, 973–977 (2005). 10.1378/chest.127.3.973 [DOI] [PubMed] [Google Scholar]
- Lee J. M., Youk J. H., Kim Y. K., Han Y. M., Chung G. H., Lee S. Y., and Kim C. S., “Radio-frequency thermal ablation with hypertonic saline solution injection of the lung: Ex vivo and in vivo feasibility studies,” Eur. Radiol. 13, 2540–2547 (2003). 10.1007/s00330-003-1876-x [DOI] [PubMed] [Google Scholar]
- Gananadha S. and Morris D. L., “Saline infusion markedly reduces impedance and improves efficacy of pulmonary radiofrequency ablation,” Cardiovasc. Intervent Radiol. 27, 361–365 (2004). 10.1007/s00270-003-0112-z [DOI] [PubMed] [Google Scholar]
- Solazzo S. A., Liu Z., Lobo S. M., Ahmed M., Hines-Peralta A. U., Lenkinski R. E., and Goldberg S. N., “Radiofrequency ablation: Importance of background tissue electrical conductivity—An agar phantom and computer modeling study,” Radiology 236, 495–502 (2005). 10.1148/radiol.2362040965 [DOI] [PubMed] [Google Scholar]
- Brace C. L., Laeseke P. F., van der Weide D. W., and Lee F. T., “Microwave ablation with a triaxial antenna: Results in ex vivo bovine liver,” IEEE Trans. Microwave Theory Tech. 53, 215–220 (2005). 10.1109/TMTT.2004.839308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. N., Grassi C. J., Cardella J. F., Charboneau J. W., Dodd G. D., Dupuy D. E., Gervais D., Gillams A. R., Kane R. A., Lee F. T., Livraghi T., McGahan J., Phillips D. A., Rhim H., and Silverman S. G., “Image-guided tumor ablation: Standardization of terminology and reporting criteria,” Radiology 235, 728–739 (2005). 10.1148/radiol.2353042205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Converse M. C., Mahvi D. M., and Webster J. G., “Measurement and analysis of tissue temperature during microwave liver ablation,” IEEE Trans. Biomed. Eng. 54, 150–155 (2007). 10.1109/TBME.2006.884647 [DOI] [PubMed] [Google Scholar]
- Brace C. L., van der Weide D. W., Lee F. T., Laeseke P. F., and Sampson L., “Analysis and experimental validation of a triaxial antenna for microwave tumor ablation,” IEEE MTT-S Int. Microwave Symp. Dig. 3, 1437–1440 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Nan Q., Li L., and Liu Y., “Numerical study on thermal field of microwave ablation with water-cooled antenna,” Int. J. Hyperthermia 25, 108–115 (2009). 10.1080/02656730802587720 [DOI] [PubMed] [Google Scholar]
- Lu D. S., Raman S. S., Vodopich D. J., Wang M., Sayre J., and Lassman C., “Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: Assessment of the ‘heat sink’ effect,” AJR, Am. J. Roentgenol. 78, 47–51 (2002). [DOI] [PubMed] [Google Scholar]
- Lam V. W., Ng K. K., Chok K. S., Cheung T. T., Yuen J., Tung H., Tso W. K., Fan S. T., and Poon R. T., “Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: Analysis of risk factors and prognostic factors,” Ann. Surg. Oncol. 15, 782–790 (2008). 10.1245/s10434-007-9733-9 [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Ikeda K., Someya T., Akuta N., Suzuki F., Tsubota A., Suzuki Y., Saitoh S., Arase Y., and Kumada H., “Stepwise hook extension technique for radiofrequency ablation therapy of hepatocellular carcinoma,” Oncology 63, 139–144 (2002). 10.1159/000063808 [DOI] [PubMed] [Google Scholar]
- Duck F. A., Physical Properties of Tissue: A Comprehensive Reference Book (Academic, London, 1990). [Google Scholar]
- Morrison P. R., van Sonnenberg E., Shankar S., Godleski J., Silverman S. G., Tuncali K., Jaklitsch M. T., and Jolesz F. A., “Radiofrequency ablation of thoracic lesions: Part 1, experiments in the normal porcine thorax,” AJR, Am. J. Roentgenol. 184, 375–380 (2005). [DOI] [PubMed] [Google Scholar]
- Lencioni R., Crocetti L., Cioni R., Suh R., Glenn D., Regge D., Helmberger T., Gillams A. R., Frilling A., Ambrogi M., Bartolozzi C., and Mussi A., “Response to radiofrequency ablation of pulmonary tumours: A prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study),” Lancet Oncol. 9, 621–628 (2008). 10.1016/S1470-2045(08)70155-4 [DOI] [PubMed] [Google Scholar]
- Simon C. J., Dupuy D. E., DiPetrillo T. A., Safran H. P., Grieco C. A., Ng T., and Mayo-Smith W. W., “Pulmonary radiofrequency ablation: Long-term safety and efficacy in 153 patients,” Radiology 243, 268–275 (2007). 10.1148/radiol.2431060088 [DOI] [PubMed] [Google Scholar]
- Zhu J. C., Yan T. D., and Morris D. L., “A systematic review of radiofrequency ablation for lung tumors,” Ann. Surg. Oncol. 15, 1765–1774 (2008). 10.1245/s10434-008-9848-7 [DOI] [PubMed] [Google Scholar]
- Shibata T., Iimuro Y., Yamamoto Y., Maetani Y., Ametani F., Itoh K., and Konishi J., “Small hepatocellular carcinoma: Comparison of radio-frequency ablation and percutaneous microwave coagulation therapy,” Radiology 223, 331–337 (2002). 10.1148/radiol.2232010775 [DOI] [PubMed] [Google Scholar]
- Ohmoto K., Yoshioka N., Tomiyama Y., Shibata N., Kawase T., Yoshida K., Kuboki M., and Yamamoto S., “Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas,” J. Gastroenterol. Hepatol 24, 223–227 (2009). 10.1111/j.1440-1746.2008.05596.x [DOI] [PubMed] [Google Scholar]
- Lee J. M., Han J. K., Chang J. M., Chung S. Y., Kim S. H., Lee J. Y., and Choi B. I., “Radiofrequency ablation in pig lungs: In vivo comparison of internally cooled, perfusion and multitined expandable electrodes,” Br. J. Radiol. 79, 562–571 (2006). 10.1259/bjr/51844219 [DOI] [PubMed] [Google Scholar]
- Shibata T., Shibata T., Maetani Y., Isoda H., and Hiraoka M., “Radiofrequency ablation for small hepatocellular carcinoma: Prospective comparison of internally cooled electrode and expandable electrode,” Radiology 23, 346–353 (2006). [DOI] [PubMed] [Google Scholar]
- Meijerink M. R., van den Tol P., van Tilborg A. A., van Waesberghe J. H., Meijer S., and van Kuijk C., “Radiofrequency ablation of large size liver tumours using novel plan-parallel expandable bipolar electrodes: Initial clinical experience,” Eur. J. Radiol. 17 (2009). [DOI] [PubMed] [Google Scholar]
- Hines-Peralta A. U., Pirani N., Clegg P., Cronin N., Ryan T. P., Liu Z., and Goldberg S. N., “Microwave ablation: Results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver,” Radiology 239, 94–102 (2006). 10.1148/radiol.2383050262 [DOI] [PubMed] [Google Scholar]
- Yu N. C., Lu D. S., Raman S. S., Dupuy D. E., Simon C. J., Lassman C., Aswad B. I., Ianniti D., and Busutti R. W., “Hepatocellular carcinoma: Microwave ablation with multiple straight and loop antenna clusters—Pilot comparison with pathologic findings,” Radiology 239, 269–275 (2006). 10.1148/radiol.2383041592 [DOI] [PubMed] [Google Scholar]
- Meredith K., Lee F., Henry M. B., Warner T., and Mahvi D., “Microwave ablation of hepatic tumors using dual-loop probes: Results of a phase I clinical trial,” J. Gastrointest Surg. 9, 1354–1360 (2005). 10.1016/j.gassur.2005.07.028 [DOI] [PubMed] [Google Scholar]