Abstract
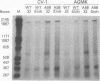
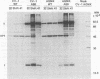
Examination of the simian virus 40 early mRNA's from infected AGMK or CV-1 cells showed that the ratio of large T- to small t-antigen mRNA's increased with an increased incubation temperature. In tsA58 mutant-infected cells, an increased incubation temperature resulted in the overproduction of early RNAs'; however, the ratio of the early mRNA's was the same, at any temperature, in both wild-type- and tsA58-infected cells. Thus, the thermally induced alteration in the early mRNA ratios was apparently not affected by the tsA mutation or by the overproduction of early RNA in tsA mutant-infected cells. Time course studies at various temperatures showed that, although the ratio of large T- to small t-antigen mRNA's increased with temperature, at any one temperature it was consistent from early to late times of infection. Furthermore, the ratio of the early mRNA's adjusted in temperature shift experiments. Thus, the ratio of the early mRNA's appeared to be intrinsic to the thermodynamic environment of the cell. The thermally induced alterations in the early mRNA's were reflected at the protein level by parallel changes in the ratio of large T- to small t-antigens. These data suggest a level of gene expression control which may function at the stage of splicing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Dhar R., Khoury G. A small RNA induced late in simian virus 40 infection can associate with early viral mRNAs. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1379–1383. doi: 10.1073/pnas.77.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K., Tegtmeyer P., Anthony D. D. Relationship of replication and transcription of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1927–1930. doi: 10.1073/pnas.70.7.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Nucleotide sequence of a fragment of SV40 DNA that contains the origin of DNA replication and specifies the 5' ends of "early" and "late" viral RNA. IV. Localization of the SV40 DNA complementary to the 5' ends of viral mRNA. J Biol Chem. 1977 Jan 10;252(1):368–376. [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Synthesis and characterization of late lytic simian virus 40 RNA from transcriptional complexes. Virology. 1977 May 1;78(1):150–161. doi: 10.1016/0042-6822(77)90087-3. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Jay G., Jay F. T., Friedman R. M., Levine A. S. Simian virus 40-specific ribosome-binding proteins induced by a nondefective adenovirus 2-simian virus 40 hybrid. J Virol. 1977 Sep;23(3):692–699. doi: 10.1128/jvi.23.3.692-699.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Shiu R. P., Jay F. T., Levine A. S., Pastan I. Identification of a transformation-specific protein induced by a Rous sarcoma virus. Cell. 1978 Mar;13(3):527–534. doi: 10.1016/0092-8674(78)90326-4. [DOI] [PubMed] [Google Scholar]
- Khoury G., Carter B. J., Ferdinand F. J., Howley P. M., Brown M., Martin M. A. Genome localization of simian virus 40 RNA species. J Virol. 1976 Mar;17(3):832–840. doi: 10.1128/jvi.17.3.832-840.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P., Dhar R., Lai C. J. Processing and expression of early SV40 mRNA: a role for RNA conformation in splicing. Cell. 1979 Sep;18(1):85–92. doi: 10.1016/0092-8674(79)90356-8. [DOI] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Dhar R., Khoury G. Mapping the spliced and unspliced late lytic SV40 RNAs. Cell. 1978 Aug;14(4):971–982. doi: 10.1016/0092-8674(78)90351-3. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L., Windmueller H. G. Accelerated clearance of low-density and high-density lipoproteins and retarded clearance of E apoprotein-containing lipoproteins from the plasma of rats after modification of lysine residues. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1746–1750. doi: 10.1073/pnas.76.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Ferguson J., Davis R. W., Stark G. R. T antigen binds to simian virus 40 DNA at the origin of replication. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1605–1609. doi: 10.1073/pnas.72.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Levine A. J., Khoury G. Evidence for non-spliced SV40 RNA in undifferentiated murine teratocarcinoma stem cells. Nature. 1979 Jul 26;280(5720):335–338. doi: 10.1038/280335a0. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Baygell P., Livingston D. M. Thermolabile T (tumor) antigen from cells transformed by a temperature-sensitive mutant of simian virus 40. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4351–4355. doi: 10.1073/pnas.72.11.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]