Abstract
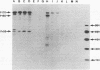
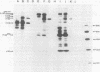
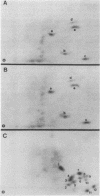
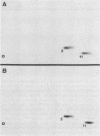
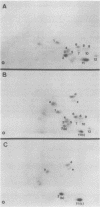
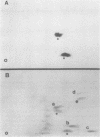
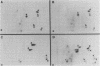
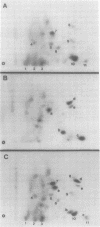
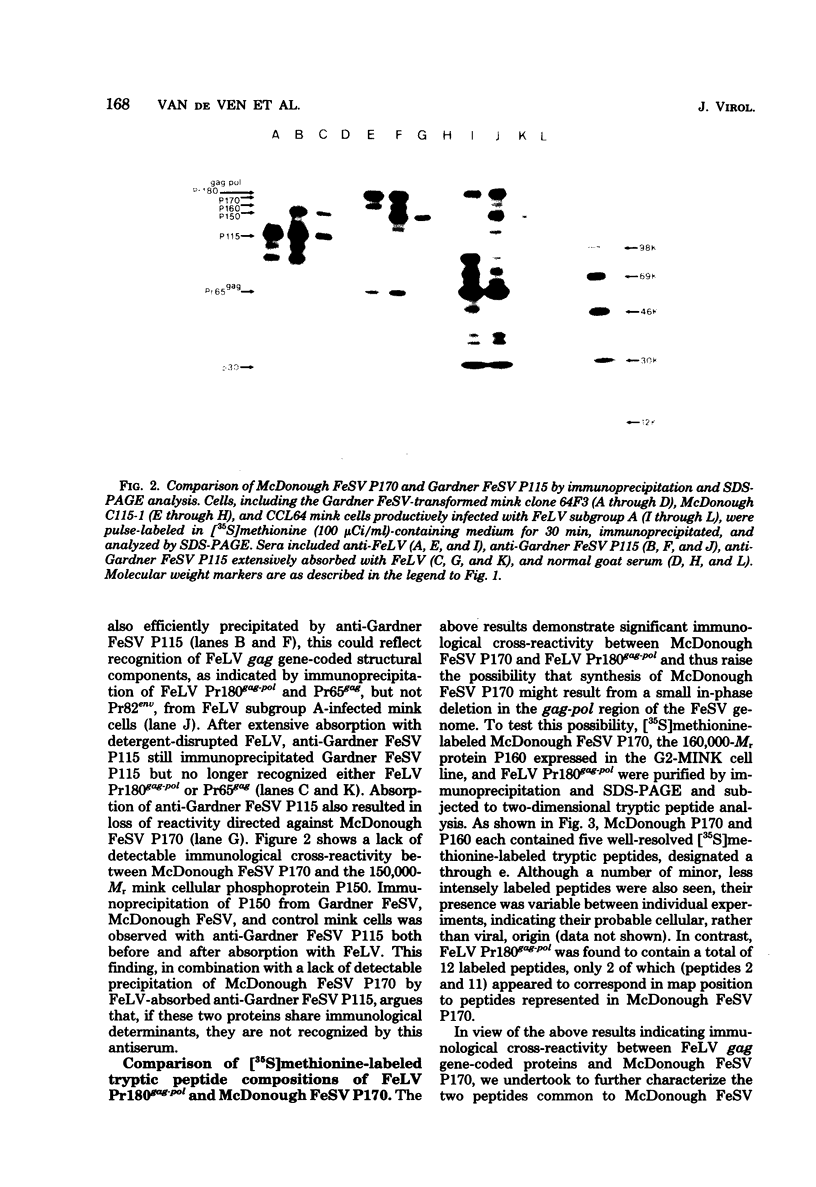
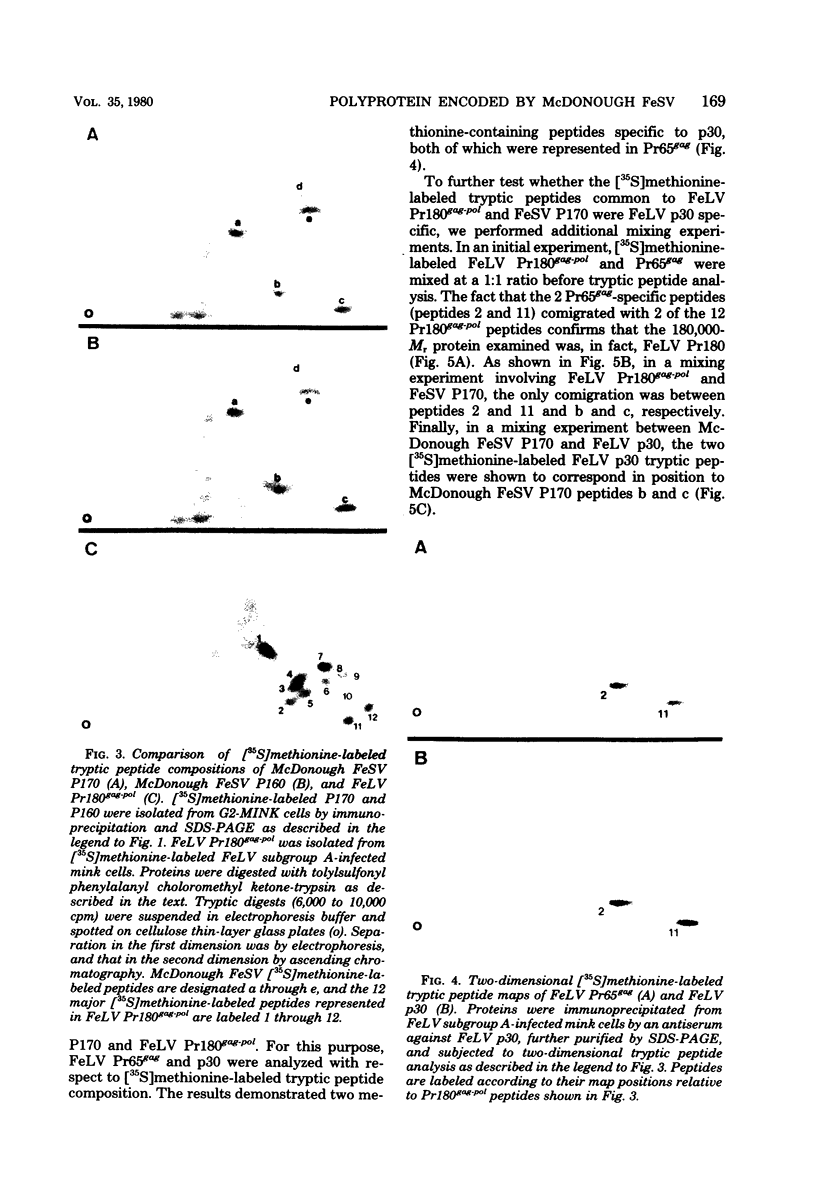
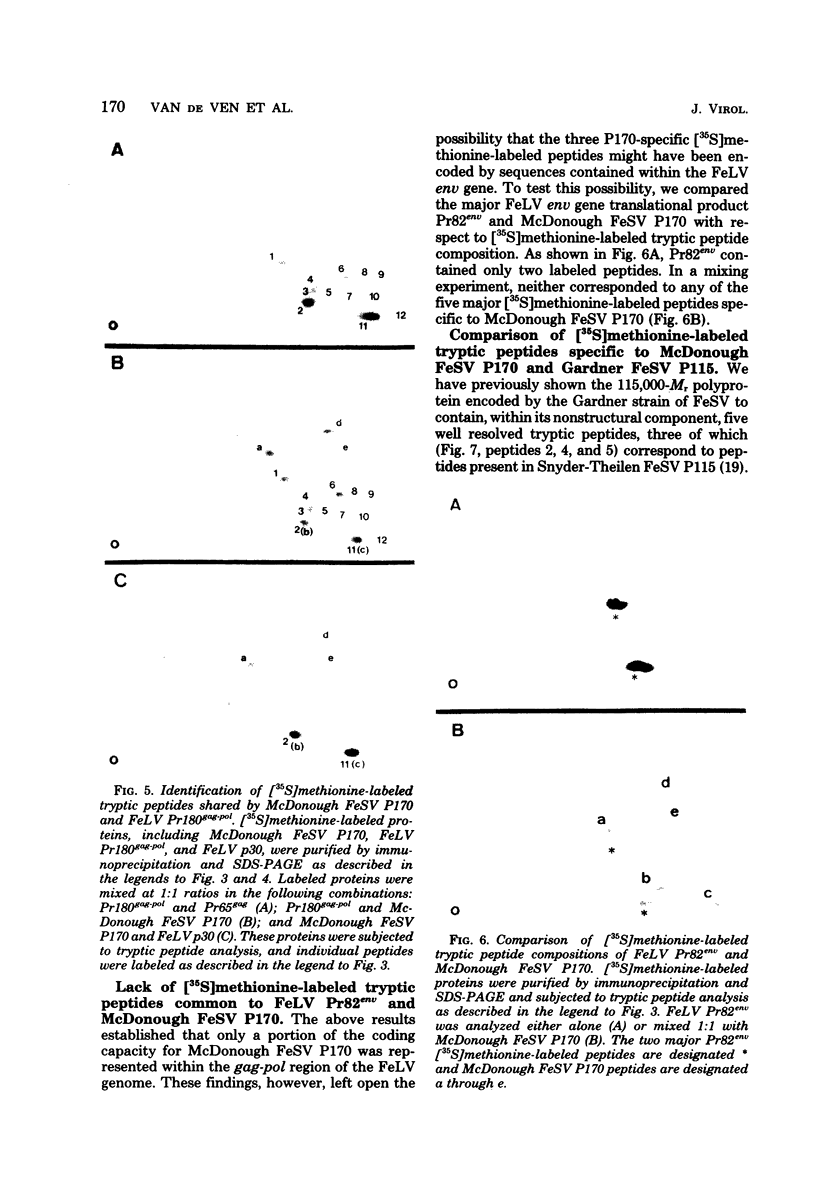
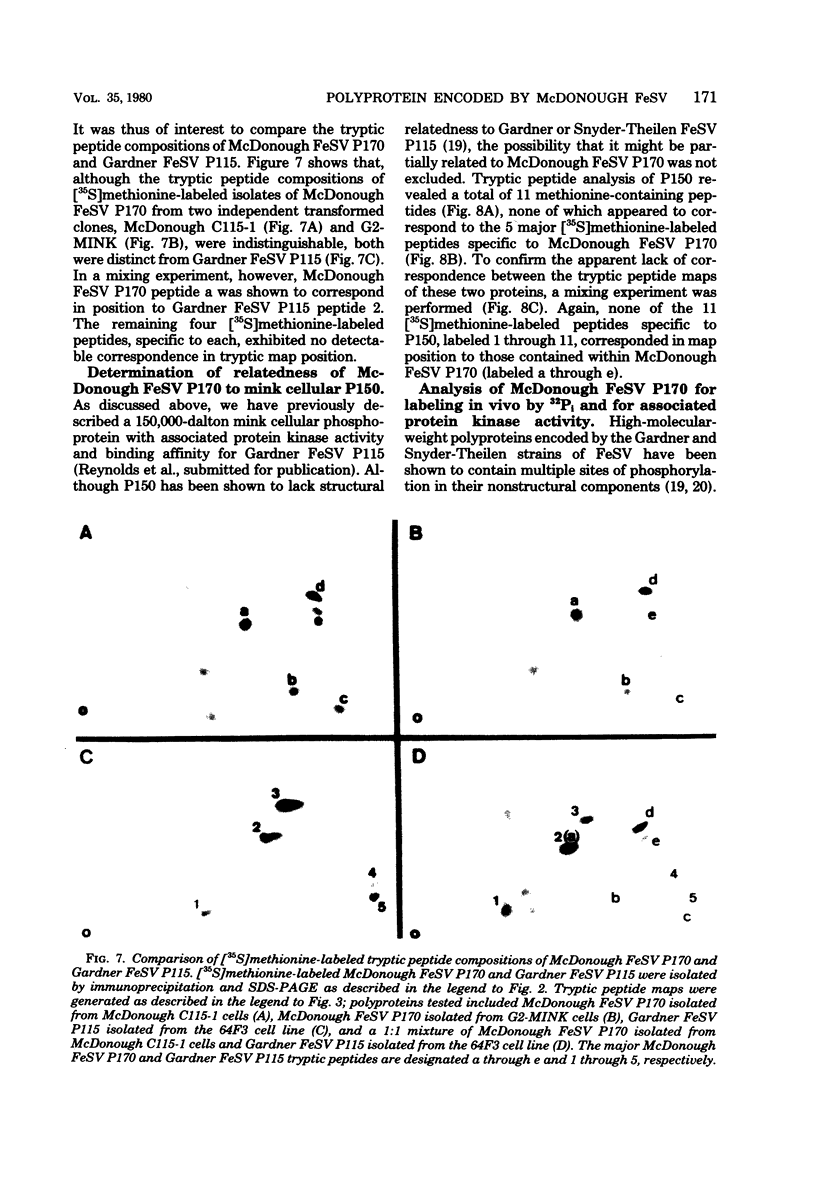
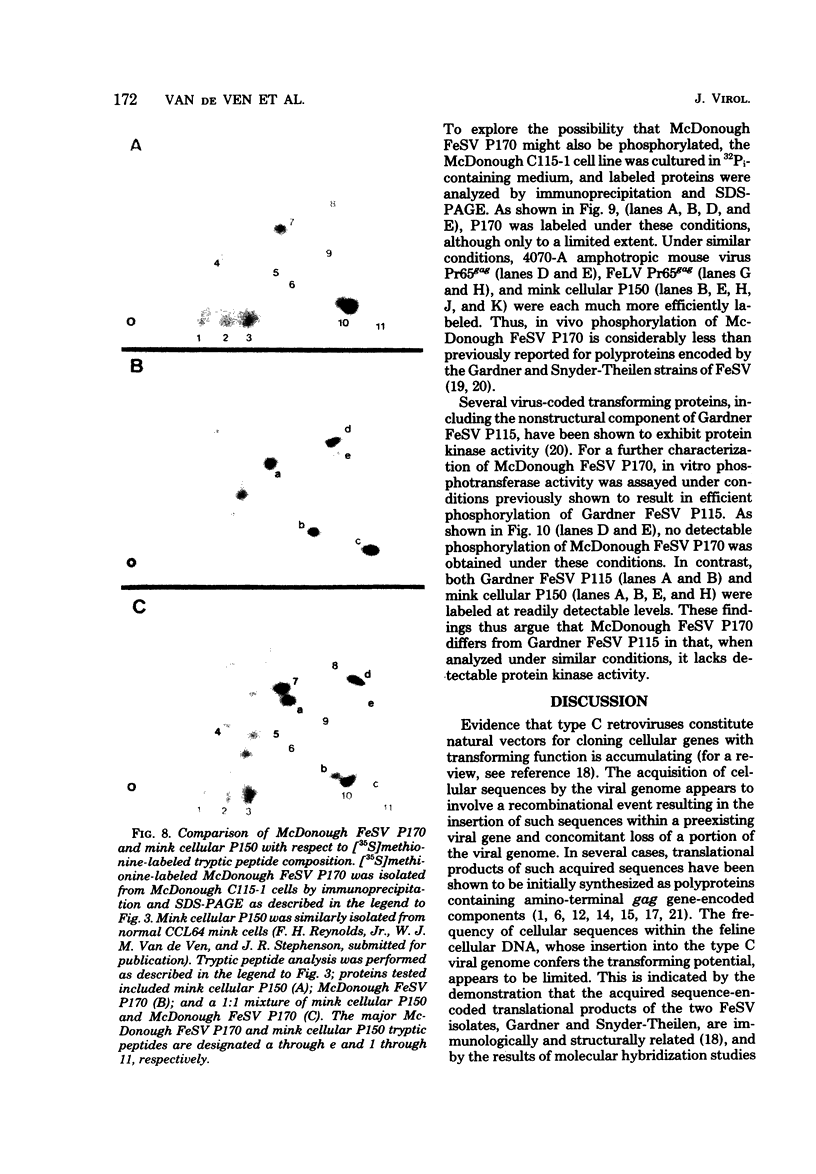
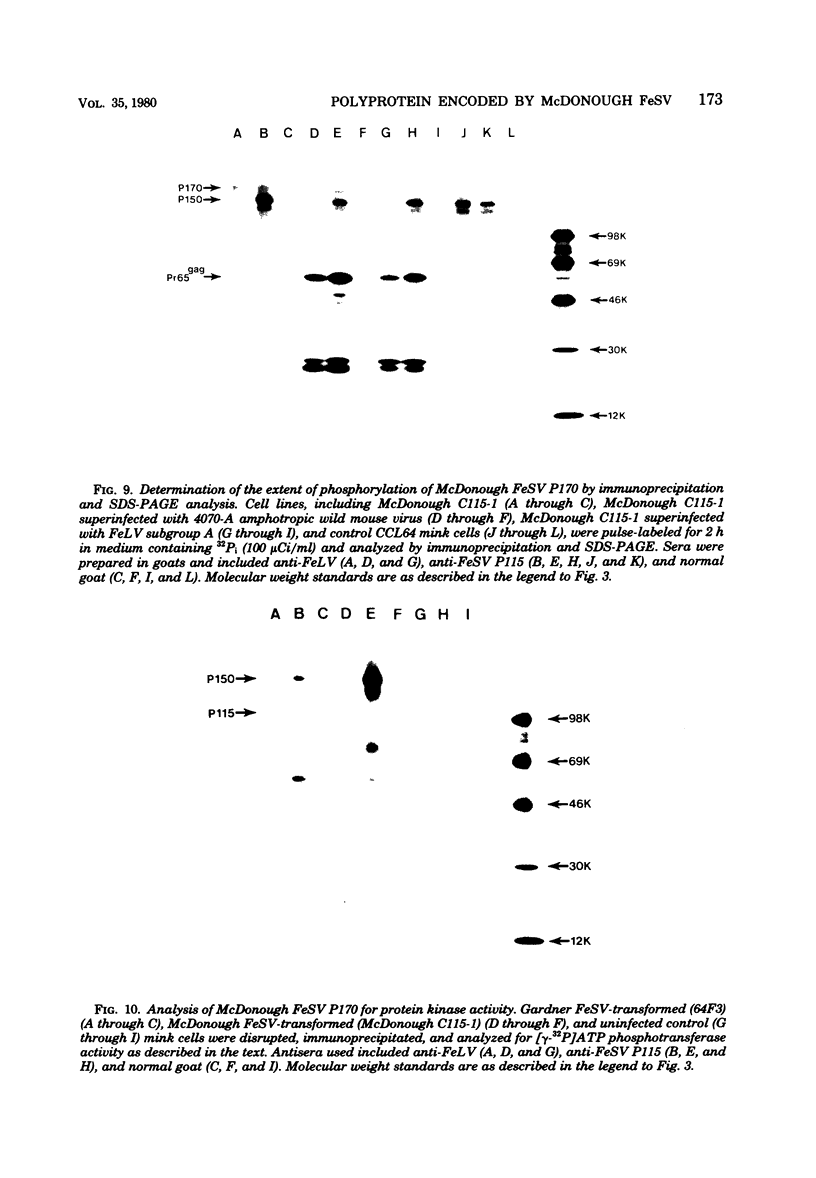
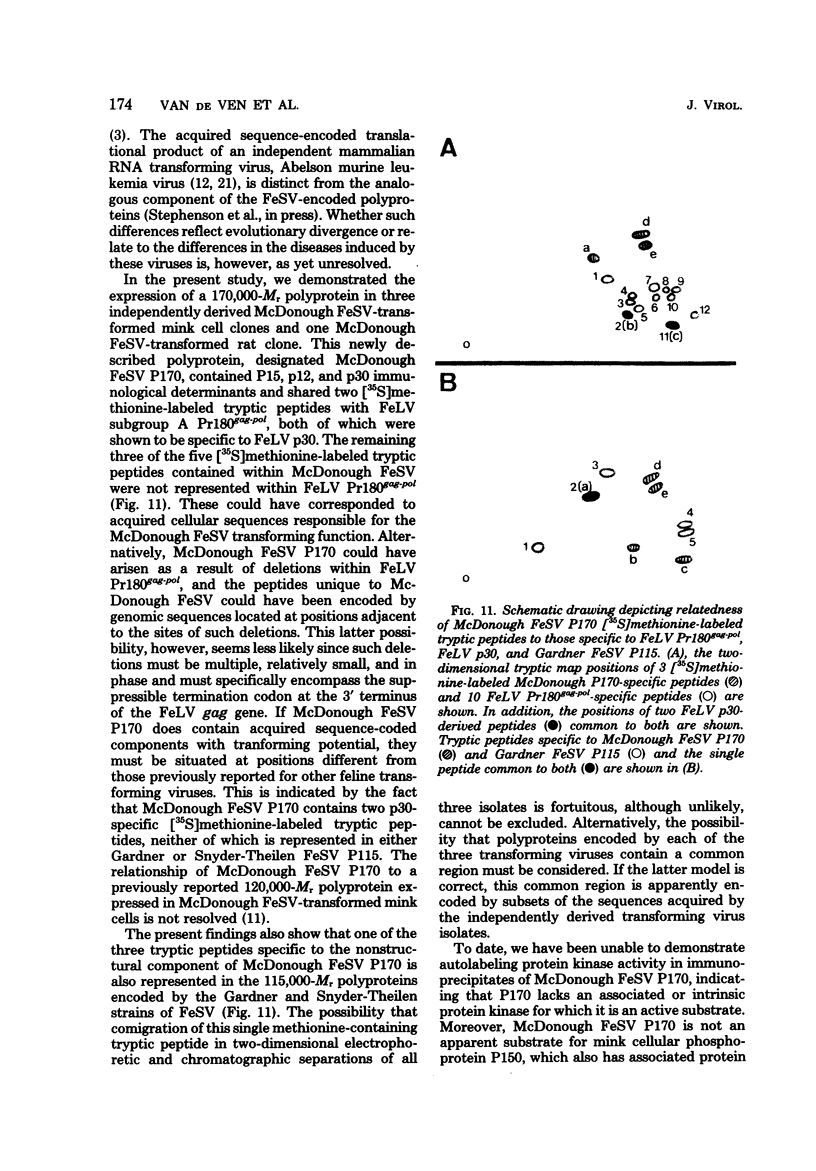
In this study, we demonstrated the expression of a 170,000-Mr polyprotein in each of several McDonough feline sarcoma virus (FeSV)-transformed mink cell clones and one McDonough FeSV-transformed rat clone. This polyprotein designated McDonough FeSV P170, contained feline leukemia virus (FeLV) p15, p12, and p30 immunological determinants and shared two of its five [35S]methionine-labeled tryptic peptides with FeLV Pr180gag-pol. Both of these peptides were shown to be specific to the p30 component of Pr180gag-pol. The remaining McDonough FeSV P170 methionine-containing peptides were not represented within either FeLV Pr180gag-pol or Pr82env. Of interest, of the three peptides specific to the nonstructural component of McDonough FeSV P170, one was also represented in the 115,000-Mr polyproteins encoded by the Gardner and Snyder-Theilen strains of FeSV. These findings raise the possibility that the nonstructural components of polyproteins encoded by each of the three independently derived feline transforming viruses contained both common and unique regions. Moreover, if the sequences encoding these components are involved in transformation, as appears to be the case, our findings establish that the position of their insertion within the gag-pol region of the FeLV genome can vary among individual isolates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Garma P. S., Log T. Defectiveness of a feline sarcoma virus demonstrable by the acquisition of a new envelope antigenic type by phenotypic mixing. Virology. 1977 May 1;78(1):336–339. doi: 10.1016/0042-6822(77)90106-4. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Deobagkar D. N., Stephenson J. R. Isolation and characterization of a feline sarcoma virus-coded precursor polyprotein. Competition immunoassay for nonstructural components. J Biol Chem. 1978 Dec 25;253(24):8894–8901. [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline leukemia virus: biochemical and immunological characterization of gag gene-coded structural proteins. J Virol. 1977 Sep;23(3):599–607. doi: 10.1128/jvi.23.3.599-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline sarcoma virus-coded polyprotein: enzymatic cleavage by a type C virus-coded structural protein. J Virol. 1979 Feb;29(2):649–656. doi: 10.1128/jvi.29.2.649-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Porzig K. J., Barbacid M., Aaronson S. A. Biological properties and translational products of three independent isolates of feline sarcoma virus. Virology. 1979 Jan 15;92(1):91–107. doi: 10.1016/0042-6822(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Sen A., Todaro G. J., Sliski A., Essex M. Pseudotypes of feline sarcoma virus contain an 85,000-dalton protein with feline oncornavirus-associated cell membrane antigen (FOCMA) activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1505–1509. doi: 10.1073/pnas.75.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., van de Ven W. J., Reynolds F. H., Jr Type C retroviruses as vectors for cloning cellular genes with probable transforming function. J Natl Cancer Inst. 1979 Nov;63(5):1111–1119. [PubMed] [Google Scholar]
- Van de Ven W. J., Khan A. S., Reynolds F. H., Jr, Mason K. T., Stephenson J. R. Translational products encoded by newly acquired sequences of independently derived feline sarcoma virus isolates are structurally related. J Virol. 1980 Mar;33(3):1034–1045. doi: 10.1128/jvi.33.3.1034-1045.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Stephenson J. R. The nonstructural components of polyproteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinase activity. Virology. 1980 Feb;101(1):185–197. doi: 10.1016/0042-6822(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]