Abstract
We performed qualitative and histoquantitative investigations of tissue restoration after implanting polyglycolide (PGA), polydioxanone (PDS), polylevolactide (PLLA), and stainless steel pins in the intramedullary canal of rabbit femurs. The effect of bioabsorbable devices on healing of a cortical bone defect was also assessed. The cortical bone defect was created in the right femur of 80 rabbits. Bioabsorbable and metallic pins in 60 and two metallic pins alone were implanted in 20 intramedullary canals; 80 left femurs served as intact controls. Follow-up times were 3, 6, 12, 24, and 52 weeks. At all time points, collagenous connective tissue, including bone trabeculae, surrounded the implant at the tissue–implant interface, replacing hematopoiesis and fat of the intramedullary canal. The groups did not differ in the area and trabecular bone area fraction of the resulting callus. Residual fragments of PGA and PDS were observed at 24 weeks, and complete degradation occurred within 52 weeks. PGA, PDS, PLLA, and metallic implants induced a bony and fibrous walling-off response in the intramedullary cavity. No inflammation was observed. Complete tissue restoration did not occur within the follow-up, even after complete degradation of PGA and PDS, which had shorter degradation times than PLLA. The cortical bone healing effect was not different between bioabsorbable pins and metallic wires. Thus, these polymers had no specific osteostimulatory or osteoinhibitory properties compared to stainless steel. Within the follow-up period, there were no significant differences in biocompatibility between the implants and no adverse inflammatory foreign-body reactions.
Keywords: Tissue restoration, Tissue response, Bioabsorbable implant, Biodegradation, Biocompatibility, Bone fixation device
Bioabsorbable polymers are popular alternatives to metallic bone fixation devices. An extensive line of bioabsorbable devices is available as pins, nails, rods, screws, plugs, and tacks; and their clinical application in the fixation of some small fragment fractures, osteotomies, and osteochondral fragments is established [1–4]. In clinical orthopedic surgery, the most frequently used devices are made of polyglycolide (PGA) or polylevolactide (PLLA) [5–12]. Pins made of polydioxanone (PDS), a well-known suture material with good biocompatibility [13], have also been successfully employed for the fixation of first metatarsal osteotomies [14–16].
Earlier reports on the bone tissue response to these compounds are limited to mainly descriptions of the host-bone tissue response in cancellous bone, and only a few studies have investigated the use and related tissue response of these bioabsorbable devices when implanted in cortical bone. Implantation of polylactide and PGA devices in rabbit cancellous bone is reported to induce an osteostimulatory or osteoconductive response at the tissue–implant interface [17–19]. Studies of cortical bone implantation have primarily focused on PLLA alone or have evaluated the fixation properties and safety of PLLA vs. metallic implants [19–24]. To our knowledge, no previous studies have investigated the biological behavior of PLLA, PGA, and PDS vs. metallic implants in cortical bone healing in the same study setting.
We performed a qualitative and histoquantitative study to evaluate tissue restoration at the tissue–implant interface in the intramedullary canal of a rabbit femur after implantation of PGA, PDS, PLLA, and stainless steel pins under identical conditions. The effects of bioabsorbable pins on the healing of a cortical bone defect in rabbit femur and the tissue response were also assessed and compared with those of stainless steel pins. Finally, we examined the biocompatibility, degradation, and possible osteostimulatory properties of these implants. Based on our clinical experience, we hypothesized that the tested bioabsorbable materials would not differ in their effects on tissue restoration in the intramedullary cavity or on healing of a cortical bone defect.
Materials and Methods
Implants
Commercially available bioabsorbable PGA and PLLA pins (Bionx Implants, Tampere, Finland) and poly-p-dioxanone pins (OrthoSorb; Johnson and Johnson Orthopedics, New Brunswick, NJ) were used. According to the manufacturer, the molecular weight of the PGA was between 50,000 and 200,000 daltons. For PLLA, the viscometric mean molecular weight of the raw material was 700,000 daltons. After processing, the molecular weight of the PLLA was 50,000 daltons and the degree of crystallinity was 50%. The manufacturers sterilized the self-reinforced PGA pins and PDS pins using ethylene oxide and the self-reinforced PLLA pins using gamma radiation at doses of 25 kGy. Commercially available metallic, stainless steel Kirschner wires (Synthes, Solothurn, Switzerland) were used in the control group and to secure the fixation in the experimental animal groups. Based on the manufacturer’s information, the K-wires were “implant quality 316 L stainless steel” in the United States and “implant quality DIN 1.4441 stainless steel” in Europe. All bioabsorbable pins were 2.0 mm in diameter and 40 mm in length. All stainless steel Kirschner wires were also 2.0 mm in diameter, and their length was determined by the length of the intramedullary cavity of the femur.
Surgical Procedures
Surgery was performed in 80 skeletally mature New Zealand rabbits of both sexes with a mean weight of 3.5 kg (range 2.6–4.4). The rabbits were anesthetized with subcutaneous injections of medetomidine (300 μg/kg Domitor; Orion Pharma, Turku, Finland) and ketamine (25 mg/kg Ketalar; Parke-Davis, Barcelona, Spain) [25]. Under standard aseptic conditions, a lateral longitudinal incision was made in the right distal thigh and knee, the patella was dislocated medially, and the distal portion of the femur was exposed. On the distal third of the femur, a standardized, anterolateral, semicircular cortical bone defect was created using an oscillating saw (Fig. 1). The length of the semicircular defect was 10 mm and its depth was half of the lateromedial and anteroposterior thickness of the femur. The distal margin of the defect was located 30 mm from the articular surface of the distal lateral condyle of the rabbit knee. The loose cortical bone quadrant was removed, and the semicircular defect on the distal third of the femoral diaphysis was left open. A longitudinal channel, 4.0 mm in diameter, was drilled centrally to extend through the intercondylar portion into the intramedullary canal. Two implants were driven into the drill channel and the intramedullary canal (Fig. 1). To prevent the defect from advancing to a fracture, one of the implants in every operated knee was a metallic Kirschner wire reaching approximately to the level of the lesser trochanter of the femur. Previous reports indicate that without external support a bioabsorbable implant alone is not sufficient for fixation of a femoral shaft osteotomy in rabbits [26]. The 80 left femurs served as intact controls and were not operated on. The incision was closed in layers with 3-0 PGA sutures (Dexon; Davis & Geck, Gosport, UK), and the rabbits were returned to their cages. Postoperatively, all rabbits were fed ad libitum and allowed to use their limbs freely.
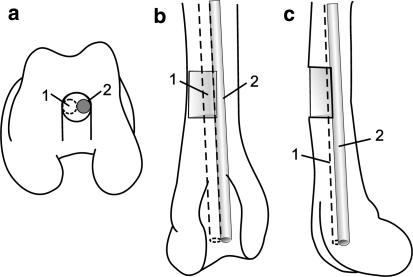
Fig. 1.
Schematic drawing of the operated femur. The distal end of the femur (a) shows the polymer implant (1) and metallic implant (2) in the drill hole, and the distal half of the femur shows the bioabsorbable (1) and metallic pin (2) placement in the intramedullary canal and their relation to the semicircular cortical defect (shaded rectangle) in anteroposterior (b) and lateral (c) views
The follow-up periods were 3, 6, 12, 24, and 52 weeks. For each time point, there were four groups of rabbits (n = 4 femurs/group), including those with a PGA pin, a PDS pin, a PLLA pin, and two metallic Kirschner wires. The 16 intact femurs constituted the controls. After the animals were killed with an overdose of sodium pentobarbital (Mebunat, Orion Pharma), both femurs were disarticulated and dissected free of soft tissue. The area of the cortical bone defect was handled cautiously, and care was taken not to damage the callus area. The metallic wires were removed. Throughout the study, the rabbits were handled according to Finnish laws and regulations on animal experimentation.
Radiographic, Histologic, and Histomorphometric Analyses
The disarticulated and dissected femora were radiographed in the anteroposterior and lateral views (target-tube distance, 100 cm; exposure factors, 40 kV, 5 mA, and 0.03 seconds) before removing the metallic Kirschner wires (Fig. 2). The distal third of each femur was fixed in 50% alcohol and dehydrated in increasing concentrations of alcohol. After dehydration was complete, the specimens were embedded in methylmethacrylate. For microradiography, 80-μm-thick longitudinal sections were cut using a Leitz 1600 saw microtome (Ernst Leitz, Wetzlar, Germany). The microradiographs were made using a Faxitron 43855A cabinet X-ray system (Hewlett-Packard, McMinnville, OR) and Kodak professional plates (type 649-0, CAT 1690718; Eastman Kodak, Rochester, NY). The exposure conditions were 50 kV, 9 mA, with a 12-min exposure and direct specimen-film contact. The radiographs and microradiographic specimens were scanned into digital format for further analysis.
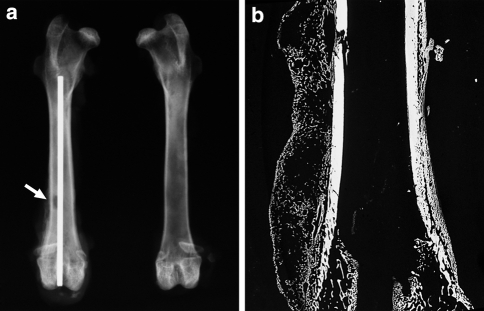
Fig. 2.
Rabbit femur with PLLA implant at 3 weeks. a Radiograph shows the operated right femur and the intact left femur. The PLLA implant is radiolucent. Metallic Kirschner wire, cortical bone defect (arrow), and callus can be seen in the operated femur. b Microradiograph of the same operated femur shows a calcified callus over the cortical defect
For histologic and histomorphometric analysis, 5-μm-thick sections were cut with a Polycut-S-microtome (Reichert-Jung, Nussloch, Germany) in the oblique sagittal plane, parallel to the long axis of the pin through the anterolateral midpoint of the cortical defect area, and stained using the Masson-Goldner trichrome method.
For quantitative histomorphometric analysis of the tissue–implant interface, three microscopic fields measuring 0.9 × 1.2 mm within 10 mm from the center of the cortical bone defect (Fig. 3) were digitally microphotographed (Nikon Eclipse 80i microscope, Nikon DS-Fi1 digital camera; Nikon, Tokyo, Japan). The microscopic fields were standardized so that the implant-occupied area or implant channel comprised 50% of the field. Sample fields located at the corresponding area of the intramedullary canal from the intact control femora were also microphotographed. The digital microphotographs, radiographs, and microradiographs were morphometrically analyzed using IP Lab software (Scanalytics, Fairfax, VA). Fractions of the following components were measured in the microphotographs: trabecular (cancellous) bone, implant channel, bone marrow fat, fibrous connective tissue, and hematopoietic tissue. Trabecular bone was defined as a meshwork of interconnecting rods and plates of calcified bone matrix, which was stained green with Masson-Goldner staining. The total area of the trabecular bone included both calcified trabeculae and osteoid lining of trabeculae, which were stained red with Masson-Goldner. Fat cells were defined as large unilocular cells (diameter 50–60 μm) with unstained cytoplasm. Connective tissue was defined as fibers, and the bundles of collagen were stained red or orange with Masson-Goldner. Hematopoietic tissue was defined as dense infiltrates of mature blood cells, megakaryocytes, and their precursors.
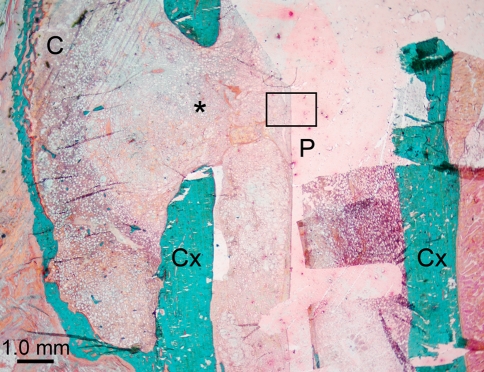
Fig. 3.
Photomicrograph of an operated femur with a PLLA implant at 3 weeks. Rectangle indicates one of the microscopic fields of histomorphometric analysis of the tissue–implant interface. * Cortical bone defect; C callus; P implant channel; Cx cortical bone
The projection area of the external callus at the anterolateral bone defect was measured from the radiographs. The callus area was defined by following the contours of the callus on the outer surface and the original periosteum in the inner surface. The callus area was normalized and reported as a percentage of the total radiographic area of the femoral bone in the anteroposterior view. Maturation and calcification of the callus were studied quantitatively on bone microradiographs. The trabecular bone area fraction of the callus was measured and reported as a percentage of the whole callus area. Polarizing microscopy was used to study degradation of the bioabsorbable implants. The presence or absence of birefringent polymeric material was examined in the histologic specimens.
Statistical Analysis
Histomorphometric measurements at each time point were pooled and reported as means for each animal. A nonparametric Kruskal–Wallis test was used to compare differences between the implant groups at each time point and within the implant groups over time. The difference was considered significant at a probability level of less than 0.05. Statistical analyses were performed with StatView 5.0 software (SAS Institute, Cary, NC).
Results
Postoperatively, the functional recovery of all rabbits was uneventful. Bacterial deep wound infections did not occur in any of the rabbits, nor were there macroscopically manifest signs of other inflammatory reactions, i.e., erythema, fluctuant swelling, or sinus formation at the implantation site, in any of the involved limbs. Two rabbits died due to illnesses not related to the internal fixation or postoperative complications before the end of the planned follow-up, leaving 78 rabbits for detailed analysis.
Tissue Restoration in the Intramedullary Canal
In the intact control specimens, the intramedullary canal consisted of fat (56%) and hematopoietic tissue (44%) (Table 1). Neither connective tissue nor trabecular bone was present in any of the control specimens.
Table 1.
Area fraction of the tissue components at the tissue–implant interface
| Follow-up time (weeks) | n | Fata | SD | Range | Hematopoietic tissuea | SD | Range | Connective tissuea | SD | Range | Bonea | SD | Range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intact specimen | 10 | 0.56 | 0.23 | 0.14–0.82 | 0.44 | 0.23 | 0.18–0.86 | 0.00 | 0.00 | 0.00–0.00 | 0.00 | 0.00 | 0.00–0.00 |
| PLLA | |||||||||||||
| 3 | 4 | 0.78 | 0.11 | 0.67–0.93 | 0.11 | 0.15 | 0.00–0.33 | 0.10 | 0.08 | 0.00–0.19 | 0.01 | 0.03 | 0.00–0.05 |
| 6 | 4 | 0.79 | 0.21 | 0.48–0.92 | 0.02 | 0.03 | 0.00–0.06 | 0.14 | 0.22 | 0.00–0.46 | 0.06 | 0.03 | 0.03–0.10 |
| 12 | 4 | 0.88 | 0.04 | 0.84–0.91 | 0.00 | 0.00 | 0.00–0.00 | 0.08 | 0.02 | 0.05–0.11 | 0.04 | 0.03 | 0.00–0.06 |
| 24 | 4 | 0.83 | 0.13 | 0.65–0.95 | 0.00 | 0.00 | 0.00–0.00 | 0.06 | 0.05 | 0.00–0.12 | 0.11 | 0.09 | 0.00–0.23 |
| 52 | 4 | 0.77 | 0.09 | 0.69–0.88 | 0.09 | 0.04 | 0.04–0.14 | 0.10 | 0.07 | 0.05–0.20 | 0.04 | 0.03 | 0.01–0.09 |
| PGA | |||||||||||||
| 3 | 4 | 0.66 | 0.24 | 0.43–0.92 | 0.01 | 0.02 | 0.00–0.04 | 0.32 | 0.21 | 0.08–0.50 | 0.02 | 0.04 | 0.00–0.07 |
| 6 | 4 | 0.74 | 0.27 | 0.34–0.92 | 0.05 | 0.05 | 0.00–0.10 | 0.17 | 0.19 | 0.01–0.45 | 0.04 | 0.05 | 0.00–0.10 |
| 12 | 4 | 0.61 | 0.10 | 0.49–0.74 | 0.00 | 0.01 | 0.00–0.01 | 0.12 | 0.10 | 0.01–0.25 | 0.26 | 0.18 | 0.00–0.38 |
| 24 | 4 | 0.63 | 0.32 | 0.22–0.90 | 0.00 | 0.00 | 0.00–0.00 | 0.34 | 0.27 | 0.10–0.64 | 0.04 | 0.06 | 0.00–0.13 |
| 52 | 3 | 0.87 | 0.14 | 0.02–0.12 | 0.01 | 0.01 | 0.00–0.02 | 0.11 | 0.11 | 0.05–0.24 | 0.01 | 0.02 | 0.00–0.04 |
| PDS | |||||||||||||
| 3 | 4 | 0.52 | 0.23 | 0.20–0.75 | 0.00 | 0.00 | 0.00–0.00 | 0.43 | 0.22 | 0.17–0.71 | 0.05 | 0.04 | 0.00–0.09 |
| 6 | 4 | 0.80 | 0.09 | 0.69–0.89 | 0.00 | 0.00 | 0.00–0.00 | 0.18 | 0.07 | 0.10–0.26 | 0.02 | 0.02 | 0.00–0.05 |
| 12 | 4 | 0.77 | 0.22 | 0.45–0.90 | 0.00 | 0.00 | 0.00–0.00 | 0.21 | 0.21 | 0.09–0.52 | 0.02 | 0.01 | 0.00–0.03 |
| 24 | 4 | 0.67 | 0.33 | 0.29–0.95 | 0.06 | 0.11 | 0.00–0.23 | 0.15 | 0.10 | 0.05–0.26 | 0.13 | 0.22 | 0.00–0.45 |
| 52 | 3 | 0.81 | 0.11 | 0.68–0.89 | 0.03 | 0.05 | 0.00–0.08 | 0.10 | 0.03 | 0.08–0.14 | 0.06 | 0.07 | 0.01–0.15 |
| Metal | |||||||||||||
| 3 | 4 | 0.42 | 0.12 | 0.29–0.55 | 0.09 | 0.17 | 0.00–0.34 | 0.42 | 0.13 | 0.29–0.58 | 0.08 | 0.06 | 0.00–0.13 |
| 6 | 4 | 0.62 | 0.25 | 0.38–0.91 | 0.00 | 0.00 | 0.00–0.00 | 0.26 | 0.25 | 0.07–0.62 | 0.12 | 0.22 | 0.00–0.45 |
| 12 | 4 | 0.68 | 0.35 | 0.16–0.89 | 0.01 | 0.02 | 0.00–0.04 | 0.19 | 0.14 | 0.07–0.38 | 0.12 | 0.23 | 0.00–0.46 |
| 24 | 4 | 0.72 | 0.19 | 0.50–0.92 | 0.00 | 0.00 | 0.00–0.00 | 0.24 | 0.14 | 0.08–0.41 | 0.04 | 0.04 | 0.00–0.09 |
| 52 | 4 | 0.81 | 0.16 | 0.57–0.89 | 0.01 | 0.02 | 0.00–0.05 | 0.17 | 0.13 | 0.08–0.36 | 0.02 | 0.03 | 0.00–0.06 |
SD standard deviation
aFraction of tissue component
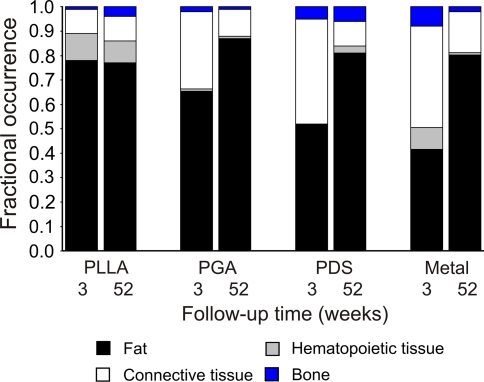
In all implanted femora, a zone of collagenous connective tissue surrounded the intramedullary implants at 3 weeks (Table 1, Figs. 4 and 5). Formation of bone trabeculae at the tissue–implant interface was also observed in all implant groups.
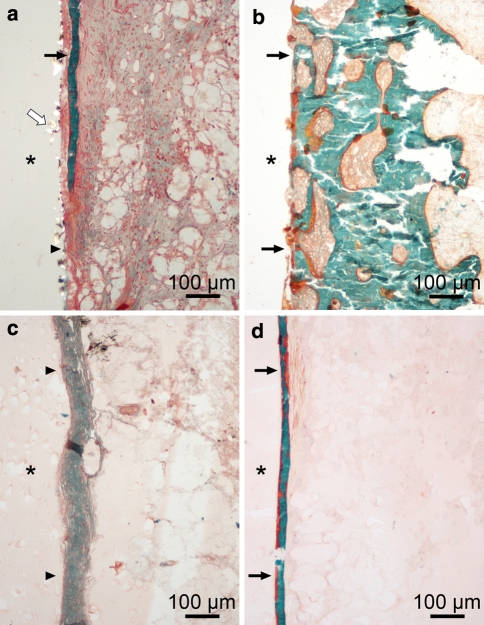
Fig. 4.
In the histologic specimens, a walling-off response was observed for both bioabsorbable and metallic pins. a Fibrous tissue and bone trabecula outlining the PDS pin at 3 weeks. b A bone rim is seen outlining the metallic Kirschner wire at 12 weeks. c A fibrous tissue zone surrounds the PGA pin at 24 weeks. d Thin bone trabecula outlining the PLLA pin at 52 weeks. * Implant channel; black arrows, bone; arrowheads, fibrous tissue; white arrow, PDS particles. Masson-Goldner trichrome staining
Fig. 5.
Bar chart showing the occurrence of the tissue elements after implantation of polymeric and metallic pins in 3-week and 52-week specimens. PLLA polylevolactide; PGA polyglycolide; PDS polydioxanone
At the 6-week follow-up, collagenous connective tissue surrounded (walled off) the implant at the tissue–implant interface (Table 1). There was no significant difference in trabecular bone area between the groups at the 3- and 6-week follow-ups.
At 12 weeks, the PGA group had formed the most intramedullary bone compared to the other implant groups (Table 1). The differences between groups were not statistically significant.
At 24 weeks, bone and connective tissue formation began to decline in the PDS and metallic implant groups (Table 1). The differences between the groups were not statistically significant. At the end of the follow-up, 52 weeks after surgery, bone and connective tissue had formed in the intramedullary canal in all implant groups, in contrast to the intact femora (Table 1, Fig. 5). Hematopoietic cells were observed sporadically within the bone marrow fat in all implant specimens, and their proportion was not correlated with the implant material.
Healing of the Cortical Bone Defect
Three weeks after surgery, the callus area of the cortical bone defect was largest in the PDS implant group (Table 2, Fig. 2). Callus formation tended to increase for up to 6 weeks in the PLLA and metallic implant groups and for up to 24 weeks in the PGA group. The callus area was not statistically different between the groups. Maturation of the callus was assessed microradiographically by studying the calcified bone trabeculae (Fig. 2b). The area fraction of the mineralized trabeculae ranged from 12% to 51%. There was no statistically significant difference in the trabecular bone area fraction of the callus between the implant groups.
Table 2.
Area of the callus in the cortical bone defect
| Follow-up time (weeks) | n | Meana | SD | Range |
|---|---|---|---|---|
| PLLA | ||||
| 3 | 4 | 6.6 | 3.1 | 2.0–9.2 |
| 6 | 4 | 10.7 | 5.1 | 5.2–17.5 |
| 12 | 4 | 5.0 | 0.6 | 4.5–5.7 |
| 24 | 4 | 4.5 | 0.7 | 3.8–5.4 |
| 52 | 4 | 6.7 | 5.4 | 2.0–12.4 |
| PGA | ||||
| 3 | 4 | 5.2 | 5.3 | 2.1–12.3 |
| 6 | 4 | 3.6 | 3.6 | 1.0–7.6 |
| 12 | 4 | 8.2 | 3.7 | 4.2–12.4 |
| 24 | 4 | 6.4 | 0.9 | 5.4–7.2 |
| 52 | 3 | 6.3 | 2.3 | 4.0–8.6 |
| PDS | ||||
| 3 | 4 | 10.9 | 3.8 | 7.7–16.3 |
| 6 | 4 | 6.3 | 3.0 | 3.6–10.6 |
| 12 | 4 | 5.5 | 2.0 | 2.8–7.3 |
| 24 | 4 | 4.4 | 1.8 | 4.0–6.7 |
| 52 | 3 | 4.6 | 0.8 | 4.0–5.5 |
| Metal | ||||
| 3 | 4 | 8.7 | 2.4 | 6.3–12.1 |
| 6 | 4 | 10.0 | 6.0 | 6.8–19.0 |
| 12 | 4 | 5.9 | 0.3 | 5.6–6.1 |
| 24 | 4 | 6.3 | 1.0 | 5.2–7.6 |
| 52 | 4 | 6.6 | 4.7 | 3.0–13.4 |
aArea of callus over total femur projection area (%). SD, standard deviation
Degradation and Biocompatibility of Bioabsorbable Pins
The shape of the implant was histologically unchanged in the PGA, PDS, and PLLA samples at 3–6 weeks. At 12 weeks, phagocytosing macrophages and birefringent intracellular fragments were observed at the tissue–implant interface in the PGA and PDS samples. At 24 weeks, no PGA material was detected in any of the samples. The shape of the PDS pin could not be identified, but single implant fragments and macrophages were observed at the implant area. At 52 weeks, no birefringent material could be detected in any of the PDS specimens. The PLLA implants were histologically unchanged at all follow-up points for up to 52 weeks. Inflammation, as indicated by accumulation of lymphocytes or polymorphonuclear cells, was not observed in any femur.
Discussion
There were two principal findings in the present study. First, restoration of the tissue in the intramedullary canal to its original composition did not completely occur within the follow-up times of the study. Due to the walling-off response, connective tissue and bone partially replaced the bone marrow fat and hematopoietic tissue. Second, based on the callus formation, the bioabsorbable pins and metallic Kirschner wires did not differ in their effect on the healing of the cortical bone defect. Consequently, these polymers do not possess any specific osteostimulatory or osteoinhibitory properties compared with stainless steel.
Within the follow-up times of the present study, PGA, PDS, PLLA, and metallic implants induced a bony and fibrous walling-off response after intramedullary implantation in the rabbit cortical bone. Interestingly, with the PGA and PDS implants, the response remained manifest even after the pins had totally degraded. Previous studies on the implantation of bioabsorbable devices into cancellous bone reported signs of a walling-off response to the implants [27, 28]. The walling-off response in cancellous bone may lengthen the degradation and absorption process by interfering with the centripetal tissue replacement within the implant cavity [27]. To our knowledge, there are no previous studies on the tissue response to bioabsorbable pins after intramedullary implantation in cortical bone. The walling-off response seems to accompany implantation of bioabsorbable devices regardless of the bone implantation site.
Complete tissue restoration of the intramedullary canal did not occur within the follow-up times of the present study, even after complete degradation of the PGA and PDS implants with degradation times that clearly differed from that of PLLA. To our knowledge, there are no previous studies of tissue restoration after implantation of bioabsorbable implants in cortical bone. The few studies in the literature reporting implantation of bioabsorbable devices into cortical bone investigated the internal fixation properties [20, 22–24]. Previous studies describing a tissue response to bioabsorbable implants examined only cancellous bone [18, 19, 29–33]. The anatomic structure of cortical bone differs from that of cancellous bone, however, so these results are not directly comparable.
With regard to callus formation, bioabsorbable pins and metallic Kirschner wires did not differ in their effect on the healing of the cortical bone defect. Our results indicate that none of these polymers possesses specific osteostimulatory or osteoinhibitory properties in cortical bone compared with stainless steel. The few previous studies reporting cortical bone defects have used different study designs, with membranous or bone chamber implants instead of internal fixation devices [34–36]. In cancellous bone, no differences in the osseous response were observed among the PGA, PDS, PLLA implant, and metallic Kirschner wire groups [28].
In the present study, two of the implants, PGA and PDS, became partially bioabsorbed at 24 weeks and fully degraded at 52 weeks in cortical bone. Previously, the degradation time of these two polymers was demonstrated mainly in cancellous bone. In rabbits, PGA screws and rods degrade in 24–36 weeks in cancellous bone [29, 33, 37], whereas in growing dogs PGA rods (4.5 mm in diameter) implanted in the femoral intramedullary cavity degrade completely by 24 weeks [38]. PDS pins (1.3 mm in diameter) biodegrade almost fully in the juxtaepiphyseal area of the proximal tibial metaphysis of skeletally immature rabbits at 4 months [39] and in cancellous bone between 24 and 52 weeks [28]. The degradation time of PLLA, on the other hand, is much longer, based on both experimental and clinical studies. Remnants of PLLA have been detected within bone tissue over 4–5 years [30, 40–43] following implantation. The exact degradation time of PLLA is so far unknown.
Assessment of the biocompatibility of the bioabsorbable polymers was not the main objective of our study, but within the follow-up times there was good biocompatibility of all the bioabsorbable implants and the metallic Kirschner wires. Based on the bone- or connective tissue–evoking potential and effects on hematopoiesis, there were no significant differences in biocompatibility between the implants. No adverse inflammatory foreign-body reactions or osteolytic lesions occurred in the present study. Foreign-body reactions to PGA implants have not been reported. PGA is reported to be an immunologically inert implant material in patients with effusion around PGA implants in the medial malleolus [44]. In several clinical studies of PGA devices, however, transient inflammatory foreign-body reactions have unexpectedly occurred [45–47]. Clinical case studies have reported inflammatory tissue reactions and swelling in response to PDS sheets and plates used in orbital floor reconstructions [48, 49]. In one experimental study on PLLA, a late foreign-body tissue reaction was reported in a single animal after 143 weeks of follow-up [31]. According to two clinical follow-up studies, a clinically manifest foreign-body reaction to polylactide devices is very rare when the fixation devices are implanted intraosseously [50, 51]; but late foreign-body reactions have been reported in some cases when PLLA plates have been used on the bone [40, 52]. Intra-articular inflammatory reactions after the use of PLLA implants in reconstruction of the anterior cruciate ligament of the knee have been reported [53, 54].
In conclusion, bioabsorbable PGA, PDS, PLLA, and metallic pins evoke a bony and fibrous walling-off response when implanted in the intramedullary cavity of a rabbit femur. Complete tissue restoration of the intramedullary canal did not occur within the follow-up times of the present study, even after complete degradation of PGA and PDS, with degradation times clearly shorter than that of PLLA. With regard to callus formation, bioabsorbable pins and metallic Kirschner wires did not differ in their effect on healing of the cortical bone defect. Consequently, these polymers do not possess any specific osteostimulatory or osteoinhibitory properties compared to stainless steel. Within the follow-up period, no significant differences in biocompatibility between the implants were observed and there were no adverse inflammatory foreign-body reactions.
Acknowledgement
The study was supported by grants from The Scientific Advisory Board for Defense in Finland. The authors will receive no benefit of any kind, either directly or indirectly.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The authors have stated that they have no conflict of interest.
References
- 1.Weckström M, Parviainen M, Kiuru MJ, Mattila VM, Pihlajamäki HK. Comparison of bioabsorbable pins and nails in the fixation of adult osteochondritis dissecans fragments of the knee: an outcome of 30 knees. Am J Sports Med. 2007;35:1467–1476. doi: 10.1177/0363546507300692. [DOI] [PubMed] [Google Scholar]
- 2.Pihlajamäki H, Böstman O, Hirvensalo E, Törmälä P, Rokkanen P. Absorbable pins of self-reinforced poly-l-lactic acid for fixation of fractures and osteotomies. J Bone Joint Surg Br. 1992;74:853–857. doi: 10.1302/0301-620X.74B6.1447246. [DOI] [PubMed] [Google Scholar]
- 3.Rokkanen P, Böstman O, Vainionpää S, Vihtonen K, Törmälä P, Laiho J, Kilpikari J, Tamminmäki M. Biodegradable implants in fracture fixation: early results of treatment of fractures of the ankle. Lancet. 1985;1:1422–1424. doi: 10.1016/S0140-6736(85)91847-1. [DOI] [PubMed] [Google Scholar]
- 4.Matsusue Y, Nakamura T, Iida H, Shimizu K. A long-term clinical study on drawn poly-l-lactide implants in orthopaedic surgery. J Long Term Eff Med Implants. 1997;7:119–137. [PubMed] [Google Scholar]
- 5.Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14:726–737. doi: 10.1016/s0749-8063(98)70099-4. [DOI] [PubMed] [Google Scholar]
- 6.Böstman OM. Absorbable implants for the fixation of fractures. J Bone Joint Surg Am. 1991;73:148–153. [PubMed] [Google Scholar]
- 7.Hofmann GO. Biodegradable implants in traumatology: a review on the state-of-the-art. Arch Orthop Trauma Surg. 1995;114:123–132. doi: 10.1007/BF00443385. [DOI] [PubMed] [Google Scholar]
- 8.Pihlajamäki H, Böstman O, Rokkanen P. Absorbable polyglycolide and polylactide devices for fracture fixation. Surg Technol Int. 1998;7:395–401. [PubMed] [Google Scholar]
- 9.Rokkanen PU, Böstman O, Hirvensalo E, Mäkelä EA, Partio EK, Pätiälä H, Vainionpää SI, Vihtonen K, Törmälä P. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21:2607–2613. doi: 10.1016/S0142-9612(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 10.Suuronen R, Kallela I, Lindqvist C. Bioabsorbable plates and screws: current state of the art in facial fracture repair. J Craniomaxillofac Trauma. 2000;6:19–30. [PubMed] [Google Scholar]
- 11.Yamamuro T, Matsusue Y, Uchida A, Shimada K, Shimozaki E, Kitaoka K. Bioabsorbable osteosynthetic implants of ultra high strength poly-l-lactide. A clinical study. Int Orthop. 1994;18:332–340. doi: 10.1007/BF00187076. [DOI] [PubMed] [Google Scholar]
- 12.Weiler A, Hoffmann RF, Stähelin AC, Helling HJ, Südkamp NP. Biodegradable implants in sports medicine: the biological base. Arthroscopy. 2000;16:305–321. doi: 10.1016/s0749-8063(00)90055-0. [DOI] [PubMed] [Google Scholar]
- 13.Ray JA, Doddi N, Regula D, Williams JA, Melveger A. Polydioxanone (PDS), a novel monofilament synthetic absorbable suture. Surg Gynecol Obstet. 1981;153:497–507. [PubMed] [Google Scholar]
- 14.Hetherington VJ, Shields SL, Wilhelm KR, Laporta DM, Nicklas BJ. Absorbable fixation of first ray osteotomies. J Foot Ankle Surg. 1994;33:290–294. [PubMed] [Google Scholar]
- 15.Gill LH, Martin DF, Coumas JM, Kiebzak GM. Fixation with bioabsorbable pins in chevron bunionectomy. J Bone Joint Surg Am. 1997;79:1510–1518. doi: 10.2106/00004623-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 16.DeOrio JK, Ware AW. Single absorbable polydioxanone pin fixation for distal chevron bunion osteotomies. Foot Ankle Int. 2001;22:832–835. doi: 10.1177/107110070102201010. [DOI] [PubMed] [Google Scholar]
- 17.Nordström P, Pihlajamäki H, Toivonen T, Törmälä P, Rokkanen P. Tissue response to polyglycolide and polylactide pins in cancellous bone. Arch Orthop Trauma Surg. 1998;117:197–204. doi: 10.1007/s004020050229. [DOI] [PubMed] [Google Scholar]
- 18.Pihlajamäki H, Böstman O, Manninen M, Päivärinta U, Rokkanen P. Tissue-implant interface at an absorbable fracture fixation plug made of polylactide in cancellous bone of distal rabbit femur. Arch Orthop Trauma Surg. 1994;113:101–105. doi: 10.1007/BF00572915. [DOI] [PubMed] [Google Scholar]
- 19.Viljanen JT, Pihlajamäki HK, Törmälä PO, Rokkanen PU. Comparison of the tissue response to absorbable self-reinforced polylactide screws and metallic screws in the fixation of cancellous bone osteotomies: an experimental study on the rabbit distal femur. J Orthop Res. 1997;15:398–407. doi: 10.1002/jor.1100150312. [DOI] [PubMed] [Google Scholar]
- 20.Manninen MJ, Pohjonen T. Intramedullary nailing of the cortical bone osteotomies in rabbits with self-reinforced poly-l-lactide rods manufactured by the fibrillation method. Biomaterials. 1993;14:305–312. doi: 10.1016/0142-9612(93)90123-J. [DOI] [PubMed] [Google Scholar]
- 21.Hara Y, Tagawa M, Ejima H, Orima H, Sugiyama M, Shikinami Y, Hyon SH, Ikada Y. Clinical evaluation of uniaxially oriented poly-l-lactide rod for fixation of experimental femoral diaphyseal fracture in immature cats. J Vet Med Sci. 1994;56:1041–1045. doi: 10.1292/jvms.56.1041. [DOI] [PubMed] [Google Scholar]
- 22.Viljanen J, Pihlajamäki H, Kinnunen J, Bondestam S, Rokkanen P. Comparison of absorbable poly-l-lactide and metallic intramedullary rods in the fixation of femoral shaft osteotomies: an experimental study in rabbits. J Orthop Sci. 2001;6:160–166. doi: 10.1007/s007760100065. [DOI] [PubMed] [Google Scholar]
- 23.Saikku-Bäckström A, Tulamo RM, Räihä JE, Kellomäki M, Toivonen T, Törmälä P, Rokkanen P. Intramedullary fixation of cortical bone osteotomies with absorbable self-reinforced fibrillated poly-96L/4D-lactide (SR-PLA96) rods in rabbits. Biomaterials. 2001;22:33–43. doi: 10.1016/S0142-9612(00)00142-3. [DOI] [PubMed] [Google Scholar]
- 24.Saikku-Bäckström A, Tulamo RM, Räihä JE, Pohjonen T, Toivonen T, Törmälä P, Rokkanen P. Intramedullary fixation of femoral cortical osteotomies with interlocked biodegradable self-reinforced poly-96L/4D-lactide (SR-PLA96) nails. Biomaterials. 2004;25:2669–2677. doi: 10.1016/j.biomaterials.2003.09.096. [DOI] [PubMed] [Google Scholar]
- 25.Mero M, Vainionpää S, Vasenius J, Vihtonen K, Rokkanen P. Medetomidine–ketamine–diazepam anesthesia in the rabbit. Acta Vet Scand Suppl. 1989;85:135–137. [PubMed] [Google Scholar]
- 26.Miettinen H, Mäkelä EA, Vainio J, Rokkanen P, Törmälä P. The effect of an intramedullary self-reinforced poly-l-lactide (SR-PLLA) implant on growing bone with special reference to fixation properties. An experimental study on growing rabbits. J Biomater Sci Polym Ed. 1992;3:443–450. doi: 10.1163/156856292X00420. [DOI] [PubMed] [Google Scholar]
- 27.Böstman OM, Laitinen OM, Tynninen O, Salminen ST, Pihlajamäki HK. Tissue restoration after resorption of polyglycolide and poly-laevo-lactic acid screws. J Bone Joint Surg Br. 2005;87:1575–1580. doi: 10.1302/0301-620X.87B11.16520. [DOI] [PubMed] [Google Scholar]
- 28.Pihlajamäki H, Böstman O, Tynninen O, Laitinen O. Long-term tissue response to bioabsorbable poly-l-lactide and metallic screws: an experimental study. Bone. 2006;39:932–937. doi: 10.1016/j.bone.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Nordström P, Pihlajamäki H, Toivonen T, Törmälä P, Rokkanen P. Tissue response to polyglycolide and polylevolactide pins in osteotomized cancellous bone. Clin Orthop Relat Res. 2001;382:247–257. doi: 10.1097/00003086-200101000-00033. [DOI] [PubMed] [Google Scholar]
- 30.Laitinen O, Pihlajamäki H, Sukura A, Böstman O. Transmission electron microscopic visualization of the degradation and phagocytosis of a poly-l-lactide screw in cancellous bone: a long-term experimental study. J Biomed Mater Res. 2002;61:33–39. doi: 10.1002/jbm.10115. [DOI] [PubMed] [Google Scholar]
- 31.Bos RR, Rozema FR, Boering G, Nijenhuis AJ, Pennings AJ, Verwey AB, Nieuwenhuis P, Jansen HW. Degradation of and tissue reaction to biodegradable poly(l-lactide) for use as internal fixation of fractures: a study in rats. Biomaterials. 1991;12:32–36. doi: 10.1016/0142-9612(91)90128-W. [DOI] [PubMed] [Google Scholar]
- 32.Böstman O, Päivärinta U, Partio E, Manninen M, Majola A, Vasenius J, Rokkanen P. Absorbable polyglycolide screws in internal fixation of femoral osteotomies in rabbits. Acta Orthop Scand. 1991;62:587–591. doi: 10.3109/17453679108994502. [DOI] [PubMed] [Google Scholar]
- 33.Böstman O, Päivärinta U, Partio E, Vasenius J, Manninen M, Rokkanen P. Degradation and tissue replacement of an absorbable polyglycolide screw in the fixation of rabbit femoral osteotomies. J Bone Joint Surg Am. 1992;74:1021–1031. [PubMed] [Google Scholar]
- 34.Winet H. The role of microvasculature in normal and perturbed bone healing as revealed by intravital microscopy. Bone. 1996;19:39S–57S. doi: 10.1016/S8756-3282(96)00133-0. [DOI] [PubMed] [Google Scholar]
- 35.Winet H, Hollinger JO. Incorporation of polylactide-polyglycolide in a cortical defect: neoosteogenesis in a bone chamber. J Biomed Mater Res. 1993;27:667–676. doi: 10.1002/jbm.820270514. [DOI] [PubMed] [Google Scholar]
- 36.Meinig RP, Rahn B, Perren SM, Gogolewski S. Bone regeneration with resorbable polymeric membranes: treatment of diaphyseal bone defects in the rabbit radius with poly(l-lactide) membrane. A pilot study. J Orthop Trauma. 1996;10:178–190. doi: 10.1097/00005131-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Vasenius J, Vainionpää S, Vihtonen K, Mero M, Mäkelä A, Törmälä P, Rokkanen P. A histomorphological study on self-reinforced polyglycolide (SR-PGA) osteosynthesis implants coated with slowly absorbable polymers. J Biomed Mater Res. 1990;24:1615–1635. doi: 10.1002/jbm.820241206. [DOI] [PubMed] [Google Scholar]
- 38.Miettinen H, Mäkelä EA, Vainio J, Rokkanen P, Törmälä P. The effect of an intramedullary biodegradable self-reinforced polyglycolic acid implant on tubular bone. An experimental study on growing dogs. J Biomater Sci Polym Ed. 1992;3:435–442. doi: 10.1163/156856292X00411. [DOI] [PubMed] [Google Scholar]
- 39.Otsuka NY, Mah JY, Orr FW, Martin RF. Biodegradation of polydioxanone in bone tissue: effect on the epiphyseal plate in immature rabbits. J Pediatr Orthop. 1992;12:177–180. doi: 10.1097/01241398-199203000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Bergsma JE, de Bruijn WC, Rozema FR, Bos RR, Boering G. Late degradation tissue response to poly(l-lactide) bone plates and screws. Biomaterials. 1995;16:25–31. doi: 10.1016/0142-9612(95)91092-D. [DOI] [PubMed] [Google Scholar]
- 41.Böstman OM, Pihlajamäki HK. Late foreign-body reaction to an intraosseous bioabsorbable polylactic acid screw. A case report. J Bone Joint Surg Am. 1998;80:1791–1794. doi: 10.1302/0301-620X.80B2.8302. [DOI] [PubMed] [Google Scholar]
- 42.Suuronen R, Pohjonen T, Hietanen J, Lindqvist C. A 5-year in vitro and in vivo study of the biodegradation of polylactide plates. J Oral Maxillofac Surg. 1998;56:604–615. doi: 10.1016/S0278-2391(98)90461-X. [DOI] [PubMed] [Google Scholar]
- 43.Tams J, Rozema FR, Bos RR, Roodenburg JL, Nikkels PG, Vermey A. Poly(l-lactide) bone plates and screws for internal fixation of mandibular swing osteotomies. Int J Oral Maxillofac Surg. 1996;25:20–24. doi: 10.1016/S0901-5027(96)80006-3. [DOI] [PubMed] [Google Scholar]
- 44.Santavirta S, Konttinen YT, Saito T, Grönblad M, Partio E, Kemppinen P, Rokkanen P. Immune response to polyglycolic acid implants. J Bone Joint Surg Br. 1990;72:597–600. doi: 10.1302/0301-620X.72B4.2166048. [DOI] [PubMed] [Google Scholar]
- 45.Barfod G, Svendsen RN. Synovitis of the knee after intraarticular fracture fixation with Biofix. Report of two cases. Acta Orthop Scand. 1992;63:680–681. doi: 10.1080/17453679209169736. [DOI] [PubMed] [Google Scholar]
- 46.Böstman O, Hirvensalo E, Mäkinen J, Rokkanen P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J Bone Joint Surg Br. 1990;72:592–596. doi: 10.1302/0301-620X.72B4.2199452. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann R, Krettek C, Hetkamper A, Haas N, Tscherne H. Osteosynthesis of distal radius fractures with biodegradable fracture rods. Results of two years follow-up. Unfallchirurg. 1992;95:99–105. [PubMed] [Google Scholar]
- 48.Baumann A, Burggasser G, Gauss N, Ewers R. Orbital floor reconstruction with an alloplastic resorbable polydioxanone sheet. Int J Oral Maxillofac Surg. 2002;31:367–373. doi: 10.1054/ijom.2001.0219. [DOI] [PubMed] [Google Scholar]
- 49.Kontio R, Suuronen R, Salonen O, Paukku P, Konttinen YT, Lindqvist C. Effectiveness of operative treatment of internal orbital wall fracture with polydioxanone implant. Int J Oral Maxillofac Surg. 2001;30:278–285. doi: 10.1054/ijom.2001.0067. [DOI] [PubMed] [Google Scholar]
- 50.Böstman OM, Pihlajamäki HK, Partio EK, Rokkanen PU. Clinical biocompatibility and degradation of polylevolactide screws in the ankle. Clin Orthop Relat Res. 1995;320:101–109. [PubMed] [Google Scholar]
- 51.Bucholz RW, Henry S, Henley MB. Fixation with bioabsorbable screws for the treatment of fractures of the ankle. J Bone Joint Surg Am. 1994;76:319–324. doi: 10.2106/00004623-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Eitenmüller J, David A, Pommer A, Muhr G. Operative Behandlung von Sprunggelenksfrakturen mit biodegradablen Schrauben und Platten aus Poly-l-Lactide. Chirurg. 1996;67:413–418. [PubMed] [Google Scholar]
- 53.Konan S, Haddad FS. A clinical review of bioabsorbable interference screws and their adverse effects in anterior cruciate ligament reconstruction surgery. Knee. 2009;16:6–13. doi: 10.1016/j.knee.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Kwak JH, Sim JA, Kim SH, Lee KC, Lee BK. Delayed intra-articular inflammatory reaction due to poly-l-lactide bioabsorbable interference screw used in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:243–246. doi: 10.1016/j.arthro.2007.11.012. [DOI] [PubMed] [Google Scholar]