Abstract
Diabetes mellitus is increasingly prevalent worldwide. Diabetic individuals are at markedly increased risk for premature death due to cardiovascular disease. Furthermore, substantial morbidity results from microvascular complications which include retinopathy, nephropathy, and neuropathy. Clinical studies involving diabetic patients have suggested that degree of diabetic hyperglycemia correlates with risk of complications. Recent evidence implicates a central role for oxidative stress and vascular inflammation in all forms of insulin resistance, obesity, diabetes and its complications. Although, glucose promotes glycoxidation reactions in vitro and products of glycoxidation and lipoxidation are elevated in plasma and tissue in diabetics, the exact relationships among hyperglycemia, the diabetic state, and oxidative stress are not well-understood. Using a combination of in vitro and in vivo experiments, we have identified amino acid oxidation markers that serve as molecular fingerprints of specific oxidative pathways. Quantification of these products utilizing highly sensitive and specific gas chromatography/mass spectrometry in animal models of diabetic complications and in humans has provided insights in oxidative pathways that result in diabetic complications. Our studies strongly support the hypothesis that unique oxidants are generated in the microenvironment of tissues vulnerable to diabetic damage. Potential therapies interrupting these reactive pathways in target tissue are likely to be beneficial in preventing diabetic complications.
Keywords: Oxidative stress, Oxidized amino acids, Low-density lipoprotein, High-density lipoprotein, Cardiovascular disease, Endothelial dysfunction, Gas chromatography mass spectrometry, Diabetic complications
1 Introduction
Diabetes has reached epidemic proportions worldwide. According to the American Diabetes Association, roughly 21 million people in the United States—more than 7% of the population—have the disorder [1]. A recent World Health Organization report states that the worldwide prevalence of diabetes is expected to reach 366 million by 2030 [2]. While the United States, China, and India, are expected to continue to have the highest prevalence of diabetes in 2030, the greatest relative increase is expected to be seen in the Middle East and sub-Saharan African regions. Adaptation of a high fat and high calorie “Western” diet and a more sedentary lifestyle in developing countries has resulted in increased obesity and metabolic syndrome which likely explains the continued rise of diabetes.
Complex alterations of glucose and lipid metabolism are the primary factors governing both type 1 and type 2 diabetes. Type 1 diabetes observed in approximately 10% of cases develops due to autoimmune destruction of the pancreatic β-cells, resulting in the failure of insulin secretion. Type 2 diabetes accounts for the remaining 90% cases and results from insulin resistance in skeletal muscle, liver, and adipose tissue and impaired insulin secretion from the pancreatic β-cell due to islet cell dysfunction.
Diabetics are at risk for both microvascular and macrovascular complications which can lead to significant morbidity and mortality. Macrovascular disease involves large vessel atherosclerosis which results in coronary artery disease (CAD), stroke and peripheral vascular disease. Indeed, atherosclerotic vascular disease remains the leading cause of death in diabetic patients. Diabetic atherogenesis associates with impaired endothelium-dependent vasomotion [3, 4] and is seen in subjects with established cardiovascular disease (CVD) in the coronary circulation or the peripheral arterial vasculature. Myocardial infarction, stroke, and peripheral vascular disease are 2- to 4-times more prevalent in diabetic patients [5]. Moreover, vascular disease occurs earlier, and follows a more aggressive course [6]. Thus, the cardiovascular event rate in diabetic patients without documented CAD is equivalent to that of nondiabetic patients with CAD [6, 7]. Women with diabetes lose their premenopausal cardioprotection, and are vulnerable to CAD at the same rate as men [8]. Microvascular complications include retinopathy, nephropathy, and neuropathy that eventually affect nearly all patients with diabetes. Diabetic nephropathy is the leading cause of end stage renal disease (ESRD) in the United States [5]. Diabetic retinopathy is the major cause of adult blindness in the diabetic population. Diabetic neuropathy, which affects roughly half of all type 1 and type 2 diabetic patients is the major cause of non-traumatic lower extremity amputation [9]. In addition, an autonomic neuropathy can develop which can lead to a myriad of disabling disorders including gastroparesis, urinary retention due to detrusor paresis, orthostatic hypotension, impaired sensation of angina due to defective cardiac innervation, and alteration in bowel habits due to disruption in intestinal innervation.
In this review, we will discuss how the diabetic state induces oxidative stress which may lead to diabetic complications. We will also discuss how oxidized amino acids can serve as molecular fingerprints of various oxidation pathways. Finally, we will describe how mass spectrometric quantification of these markers in animal models of diabetic complications and in humans can provide insights into tissue-specific oxidant generation in diabetes. Identifying specific pathways of reactive oxidant generation in diabetes will ultimately lead to rational design of drugs to interrupt this process and prevent diabetic complications.
2 Mechanisms of diabetic complications
2.1 Hyperglycemia and diabetic complications
Both the degree of glycemic control and the duration of diabetes predict the risk of diabetic complications [10, 11]. Over the last 15 years, many studies have shown an independent relationship between diabetic complications and glycemic control [12]. These observations have given rise to the “glucose hypothesis,” which suggests that glucose mediates many of the deleterious effects of the disease. Glucose as well as some of the proposed factors like abnormalities in lipoproteins, metabolic derangements (insulin resistance, elevated free fatty acid levels, etc.), and variations in genes controlling lipid metabolism, are important in microvascular as well as macrovascular disease [13].
The Diabetes Control and Complications Trial (DCCT) found that patients with type 1 diabetes who were treated with intensive insulin therapy to achieve a mean hemoglobin A1c of 7.0% had a markedly lowered incidence of retinopathy, neuropathy, and nephropathy as compared to subjects treated with conventional insulin therapy [14]. Similar results were demonstrated for type 2 diabetes in the United Kingdom Prospective Diabetes Study (UKPDS) [10, 15, 16].
Although it has been well-established by studies such as the DCCT and UKPDS that tight glycemic control reduces microvascular complications, whether aggressive glucose-lowering reduces macrovascular complications remains controversial. The initial DCCT did not show an improvement of macrovascular events with intensive glucose-lowering therapy. The majority of patients involved in the DCCT were subsequently followed up in the Epidemiology of Diabetes Interventions and Complications (EDIC) study. During a mean 17 years of follow-up, intensive glucose-lowering treatment reduced the risk of any CVD event by 42% suggesting that tight glycemic control lowers macrovascular disease end-points [17, 18]. In contrast, patients with type 2 diabetes whose glycated hemoglobin levels were reduced from 8% to 7% in the UKPDS did not exhibit a reduction in CV events. This observation was corroborated by the recent Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trials which found that lowering blood sugar to near normal levels (median hemoglobin A1c of 6.4%) did not lead to a reduction in macrovascular events [19–21]. In fact, in the ACCORD study aggressive glucose-lowering was associated with increased all cause mortality.
2.2 Endothelial dysfunction and diabetes
Endothelium forms the physical and biological barrier between the vascular wall and circulating blood. Endothelial dysfunction is a key early feature of diabetic complications including atherogenesis and is also involved in plaque progression and its complications [3, 22]. It is characterized by a reduction in the bioavailability of vasodilators such as endothelium-derived nitric oxide (NO•) and a relative or absolute abundance of vasoconstrictors. This imbalance impairs endothelium-dependent vasodilation, the functional hallmark of endothelial function [3, 22]. Impaired endothelial function has been demonstrated in both type 1 and type 2 diabetes and in obese, insulin-resistant subjects [23]. It predates hyperglycemia in subjects who develop type 2 diabetes, suggesting that factors such as insulin resistance and increased concentrations of metabolites such as free fatty acids initiate endothelial dysfunction in this setting. In established diabetes, hypercholesterolemia, hypertension, and other factors can contribute to endothelial dysfunction.
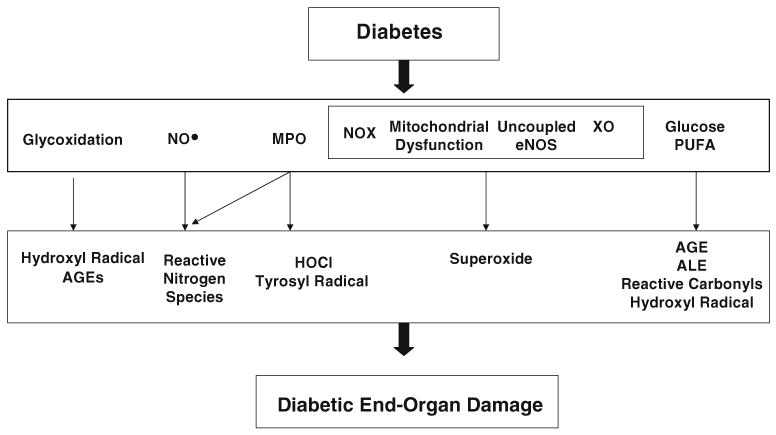
Hyperglycemia and elevated levels of free fatty acids promote oxidative phosphorylation in mitochondria and also promote the production of reactive intermediates such as superoxide that accelerate NO• degradation [24]. Additional pathways (discussed below) that can potentially result in vascular disease in the diabetic state include glycoxidation pathway which results in formation of advanced glycosylation end-products (AGEs) and the carbonyl-polyunsaturated fatty acid (PUFA) pathway which produces a hydroxyl radical-like oxidant (Fig. 1; ref [24–29]). In vascular smooth muscle cells, oxidants have also been implicated in changes to signaling pathways downstream of cyclic guanosine monophosphate (cGMP), the second-messenger of NO• [23]. Thus, increased oxidant generation in diabetes might contribute to endothelial dysfunction by several distinct mechanisms.
Fig. 1.
Oxidative stress pathways in diabetic complications. AGE advanced glycosylation end-products, ALE advanced lipoxidation end-products, eNOS endothelial nitric oxide synthase, HOCl hypochlorous acid, MPO myeloperoxidase, NO• nitric oxide, NOX NAD(P)H oxidase, PUFA polyunsaturated fatty acid, XO xanthine oxidase. Modified from [26]
2.3 Oxidative stress results in diabetic microvascular and macrovascular complications
Although the clinical sequelae of diabetic complications are well described, the pathophysiologic mechanisms underlying complications remain poorly understood. As discussed above, it appears that hyperglycemia promotes microvascular and probably macrovascular complications. However, glycemic control alone does not prevent diabetic complications, suggesting that additional factors must be involved. During the past two decades, considerable evidence has implicated oxidative stress in several distinct conditions, including aging, atherosclerosis, neurodegenerative diseases, and ESRD (reviewed in [25–27, 30–33]). Oxidative stress occurs when there is an imbalance in the relative rates of reactive oxidant generation and scavenging, with a subsequent increase in the level of oxidized biomolecules and associated tissue damage. Accumulating evidence suggests that oxidative stress has a central role in diabetes and vascular disease, and it might play this role by promoting endothelial dysfunction [24, 34]. It is clinically important to distinguish if oxidative stress is a primary event that occurs early in the disease or if it occurs as a secondary phenomenon merely reflecting end-stage tissue damage [35]. If oxidative stress simply reflects tissue damage, interventions that reduce it may fail to affect the disease process. If oxidative stress promotes tissue injury, therapies that interrupt oxidative pathways early in the disease may prevent complications, and those that act later may slow disease progression.
2.4 Pathways for generating oxidants in the microenvironment
Proteins, lipids, and nucleic acids are important targets for oxidative stress in vivo. However, the specific pathways that promote oxidative stress in diabetes have not been conclusively identified. One reason is that oxidizing intermediates are difficult to detect in vivo because they are short-lived and generated at low levels. Proposed pathways for increased oxidant generation and oxidative stress in diabetes are outlined in Fig. 1.
2.4.1 The glycoxidation pathway
Glucose-mediated oxidative reactions are collectively called glycoxidation pathways. The open-chain form of glucose has a carbonyl group that can be involved in oxidative chemistry. In the presence of oxygen, glucose can auto-oxidize to reactive oxygen species, such as hydroxyl radical, which cross-links proteins [36]. Glucose also reacts nonenzymatically with proteins to form the reversible Schiff-base adduct, which subsequently can rearrange itself into the irreversible Amadori product and AGE. An increase in the rate of glycation, decrease in renal clearance of AGE's and increase in the expression of AGE receptors occurs in diabetes [37]. This may be the cause of AGE-related basement membrane thickening and AGE-mediated cell activation. In addition to glucose, other sugars that accumulate in the diabetic state can induce protein glycation. It has been proposed that the ability of sugars to glycate substrates involves the following rank-order: glucose<fructose<ribose, and phosphorylated sugars being more potent than their unphosphorylated counterparts. In vitro, free metal ions catalyze steps in a nonenzymatic glycoxidation pathway that generates AGE products. One important intermediate is hydroxyl radical, which can peroxidize lipids and convert phenylalanine residues of proteins into two unnatural isomers of tyrosine, ortho-tyrosine and meta-tyrosine [38–40]. Reduced, redox-active metal ions such as copper or iron generate hydroxyl radical (HO•) when they react with hydrogen peroxide (H2O2). Once generated, AGEs can damage tissues through a number of mechanisms, including generation of oxidizing intermediates, formation of immune complexes, interaction with a cellular receptor called RAGE (Receptor for AGE), and promotion of cytokine release [25, 41]. Although RAGE binds to AGE-modified proteins in vitro with high affinity, its ligands in vivo are unclear. High levels of AGEs accumulate in renal failure, even in nondiabetic patients, and this process reverses after renal transplantation, implicating the kidneys in AGE production and/or clearance [35, 42–44]. Many studies have shown that age-adjusted levels of pentosidine and Nε-(carboxymethyl) lysine (CML), two widely investigated AGE products, correlate with the development of diabetic complications [39, 45–48]. Thus, glycoxidation reactions can be one mechanism for diabetic complications.
2.4.2 The reactive nitrogen pathway
Endothelial cells line the blood vessels produce NO• via endothelial nitric oxide synthase (eNOS) and during inflammation by macrophages, which are early components of atherosclerotic lesions. NO• reacts with to generate peroxynitrite (ONOO−), a potent oxidant that converts tyrosine residues to 3-nitrotyrosine. Thus, 3-nitrotyrosine is a marker for the reactive nitrogen pathway. Nitrotyrosine has been detected in low-density and high-density lipoproteins (LDL and HDL) isolated from human diabetic atherosclerotic lesions [49, 50], and plasma nitrotyrosine levels are elevated in patients with CAD [51, 52]. Because acute hyperglycemia promotes vasodilation in humans, glucose might directly or indirectly enhance NO• release and oxidant generation [13]. Myeloperoxidase (MPO) present in neutrophils and macrophages can utilize nitrite as substrate and generate reactive nitrogen species [53].
2.4.3 The myeloperoxidase pathway
H2O2 can be used by the phagocyte enzyme, MPO [54, 55], to convert chloride ion to hypochlorous acid (HOCl) Oxidation of NO• with oxygen yields nitrite (NO2−), which MPO converts to nitrogen dioxide radical , a potent nitrating intermediate [53, 56]. MPO levels are elevated in persons with angiographically documented cardiovascular disease and within culprit lesions prone to rupture [57, 58]. Indeed, plasma levels of MPO in patients presenting with chest pain predicted their CVD outcomes [59]. Lipoproteins oxidized by MPO have been detected in human atherosclerotic lesions [49, 50, 60, 61]. Thus, this pathway may be relevant in diabetic macrovascular disease. The role of this pathway in diabetic microvascular disease remains to be determined.
2.4.4 Pathways that generate superoxide
At neutral pH, is a reducing agent rather than an oxidant. However, it dismutates enzymatically or nonenzymatically into H2O2, which can oxidize thiol residues and act as an oxidizing substrate for heme proteins such as MPO. also reacts at a diffusion-controlled rate with NO• to form ONOO−, a powerful reactive nitrogen species that nitrates tyrosine residues and damages a wide range of biomolecules.
a) NAD(P)H oxidases
A family of NAD(P)H oxidases, also known as the NOX enzymes, are major producers of in the vasculature [62]. Potential factors like the angiotensin II, endothelin-1, hypercholesterolemia, shear stress, non-esterified fatty acids (NEFA), hyperglycemia, and growth factors upregulate several NOX isoforms present in the endothelium and smooth muscle cells. Angiotensin II may represent a pathophysiologically relevant pathway for stimulating the production of reactive intermediates by artery wall cells because inhibitors of this pathway lower the risk for cardiovascular events [63]. In humans, NOX activity correlates inversely with endothelial function, even after other major risk factors for atherosclerosis, including diabetes and hypercholesterolemia, are taken into account [64]. The recent finding that Nox4 NAD(P)H oxidase can mediate hypertrophy in the diabetic kidney [65] suggests that this pathway could also be involved in diabetic nephropathy.
b) The mitochondrial electron transport pathway
Mitochondrial overproduction of and the subsequent dismutation to H2O2 in diabetic tissues can lead to oxidant injury. Both hyperglycemia and excess free fatty acids can induce mitochondrial dysfunction and lead to excess production [24]. Moreover, inhibits glyceraldehyde phosphate dehydrogenase, a key glycolytic enzyme whose inactivity could make upstream metabolites accumulate. Such inhibition of glycolysis might promote end-organ damage by diverting metabolites into the hexosamine pathway or stimulating the polyol and diacylglycerol-protein kinase C pathways. Exposing endothelial cells to exogenous oxidants leads to mitochondrial damage and can augment production, a mechanism whereby oxidative stress perpetuates oxidative stress [62]. Benfotiamine, a lipid-soluble thiamine analog that inhibits these pathways by activating transketolase, an enzyme in the pentose pathway shunt, can prevent complications from experimental diabetes in animal models [41].
c) Uncoupled eNOS
NO synthesized by eNOS in endothelial cells and its uncoupling plays a major role in diabetes, hypertension, and hypercholesterolemia. Oxidation of its cofactor tetrahydrobiopterin (BH4; ref [66]) uncouples eNOS which then transfers electrons to molecular oxygen, generating [67]. An alternative mechanism for uncoupling eNOS involves overproduction of angiotensin II, which can induce dihydrofolate reductase deficiency. Dihydrofolate reductase maintains BH4 in its reduced form, and therefore its deficiency uncouples eNOS. Administering BH4 improves endothelium-dependent vasodilation in experimental animals and humans with those conditions [68]. Atherosclerotic plaque formation has been shown to increase in Apo E/eNOS knockout mice models [69–71]. Recently, studies of eNOS deficient mice reported from two separate groups show dramatic histopathology and decline in glomerular filtration rate in settings of hyperglycemia attesting to the importance of this pathway in diabetic nephropathy [72, 73].
d) Xanthine oxidase
Xanthine oxidase (XO) is a major endothelial source of and H2O2 in mammalian cells. It converts hypoxanthine and xanthine to uric acid while reducing molecular oxygen. H2O2 by itself can increase levels of XO, further accentuating production. Inflammatory cytokines, such as tumor necrosis factor-alpha, and oxidation of cysteine residues by oxidants such as ONOO− can result in the conversion of xanthine dehydrogenase to XO [68]. Endothelial levels of XO and activity are elevated in humans with heart failure and subjects with CAD, and they correlate with degree of impairment in endothelium-dependent vasodilation [68]. In a recent study Landmesser et al. [74] have shown that angiotensin II is a major stimulus for the XO production in endothelial cells.
2.4.5 The glucose–PUFA pathway
In previous experiments, Pennathur et al. have demonstrated that glucose can generate reactive intermediates by interacting with PUFA [28]. Glucose was incubated with LDL or a model protein, ribonuclease (RNase). The buffer had been treated with Chelex resin and supplemented with a metal chelator to get rid of redox active metal ions. Glucose at pathophysiologically relevant concentrations modified LDL as evidenced by the formation of oxidized amino acids. In striking contrast, glucose exposure did not increase levels of oxidized amino acids in RNase. The observation that levels of lipid oxidation products rise when LDL is exposed to glucose is consistent with this proposal. To test the hypothesis that lipid can enhance protein oxidation, RNase was incubated with saturated, monounsaturated, or PUFA. Glucose stimulated protein oxidation only in the presence of a PUFA. Thus, glucose appears to promote protein oxidation by a pathway involving peroxidation of PUFAs as this reaction is inhibited by lipid-soluble antioxidants [28]. To determine whether glucose's carbonyl group promotes LDL oxidation, glucose was replaced with a variety of short-chain and phosphorylated sugars that have highly reactive carbonyl groups [28]. All of the carbonyl compounds promoted oxidation of LDL (but not RNAse) protein more effectively than did glucose. In contrast, LDL oxidation was not enhanced by sorbitol, the reduced form of glucose that lacks a carbonyl moiety. Collectively, these observations indicate that glucose can generate a species resembling the hydroxyl radical by a carbonyl/PUFA pathway. This pathway might therefore promote localized oxidative stress in tissues vulnerable to diabetic damage [28].
2.5 Lipoxidation in diabetes mellitus
Several observations suggest a complex interplay among protein oxidation, lipid peroxidation, and AGE formation. When LDL is incubated with glucose or glycated proteins, lipoprotein oxidation increases [75–78]. Moreover, CML, an extensively studied AGE, forms from PUFAs during lipid peroxidation [79] and is a product of both glycoxidation and lipoxidation. CML and related glycoxidation products can form not only from early intermediates in the Maillard reaction but also directly from glyoxal or methylglyoxal, which may in turn be derived from either carbohydrates or lipids [35]. PUFAs are oxidized much more readily than is glucose, and dyslipidemia is common in diabetic patients. Thus, elevated levels of plasma lipoproteins might contribute to lipoxidation in vivo. AGE lipids have also been found in diabetic plasma lipoproteins [75, 76, 80–82] and have been termed as advanced lipoxidation end products (ALE). Moreover, systemic levels of isoprostanes—specific markers of nonenzymatic lipoxidation—are elevated in diabetic patients [83, 84]. CML and other AGE compounds could thus originate in vivo when carbohydrates or lipids react with proteins. Aminoguanidine and pyridoxamine comprise of a new class of agents termed “amadorins” that prevent the Amadori product from forming AGEs. Both inhibit AGE formation from carbohydrates, and pyridoxamine also inhibits [85, 86] ALE formation. Aminoguanidine prevents diabetic complications in animal models, which may reflect its ability to inhibit AGE formation and inducible nitric oxide synthase [87–91]. Pyridoxamine is effective in preventing retinopathy and nephropathy in diabetic rats [92, 93], but its effects on humans remain to be determined.
2.6 Carbonyl stress in diabetes
Glucose is a reactive carbonyl that can generate additional carbonyls and other reactive compounds through both enzymatic and nonenzymatic reactions. Baynes and Thorpe proposed that diabetes is a characterized by carbonyl stress that in turn may account for the increased protein and lipid modifications that typify diabetes and uremia [35]. In this model, reactive carbonyl production rises due to increased substrate stress (elevated levels of glucose and its derivatives), and is compounded by deficiency or overload of pathways that detoxify carbonyl compounds. The abundance of reactive carbonyls enhances protein and lipid modification. Oxidative stress is thus a primary event that increases carbonyl production, but is also a secondary event due to elevated carbonyl levels [35].
3 Mass spectrometry is a highly sensitive and specific approach for detecting oxidized biomolecules in vivo
Immunohistochemistry and dihydroethidium fluorescence have been extensively used to study oxidation-specific epitopes and oxidant production in targets of diabetic damage and atherosclerosis. These techniques are highly sensitive, and their ability to provide structural data can localize oxidative events. However, they are nonspecific and, at best, only semiquantitative. In contrast, mass spectrometry (MS) offers a powerful set of analytical tools for quantifying and identifying biomolecules. Isotope dilution gas-chromatography/mass spectrometry (GC/MS) is a highly sensitive and specific method that we and others have used to quantify oxidation of specific markers.
Biomolecules derived from plasma or tissue are separated by GC, derivatized and ionized. The mass-to-charge ratios of ions derived by fragmenting the ionized, derivatized parent compound are determined by MS. A full scan mass spectrum obtained for a target analyte can unequivocally identify a target biomolecule because each compound has a unique fragmentation pattern. The analyte is quantified by adding a stable, isotopically labeled internal standard, which is identical to the target analyte except for the heavy isotope. With certain ionization processes, such as electron capture negative-ion chemical ionization, it is possible to detect and quantify sub-femtomole levels of biomolecules.
3.1 Patterns of oxidized amino acids can reliably detect activated pathways of oxidation
To understand the molecular mechanisms that promote oxidative stress in vivo, we first used a chemical approach to define the patterns of oxidation products that are formed by well-characterized oxidant-generating systems in vitro. We then looked for similar patterns in tissue and plasma. We focused on proteins because aromatic amino acids retain the initial footprint of the reactive intermediate that initiates oxidative damage. In contrast, lipid peroxidation products readily undergo subsequent chain-propagating reactions, which mask the products formed early in the pathway. Moreover, many different oxidizing intermediates give the same spectrum of oxidized lipids, making it difficult to identify specific pathways that trigger lipid oxidation. Using a combination of free radical generating systems in vitro and studying tissue and plasma samples in animal models of disease and humans, we and others have defined patterns of these oxidative markers that accurately indicate pathways of oxidation that are activated [26, 28, 29, 33, 49–52, 60, 94–104]. We have utilized this molecular fingerprinting strategy to identify tissue-specific pathways of oxidation in diabetic complications.
3.2 MS quantification of oxidized amino acids reveals pathways of oxidative stress activated in diabetic macrovascular disease
To investigate the potential role of localized oxidative stress in diabetic macrovascular disease, we used a primate model of diabetic atherosclerosis. These studies involved Cynomolgus monkeys that had been hyperglycemic for 6 months due to streptozotocin-(STZ) induced diabetes [29]. We analyzed samples from seven controls and eight diabetic Cynomolgus monkeys, sampling three different areas from the thoracic aorta of each animal. Compared with the control samples, the aorta wall proteins from the diabetic animals showed a significant 40% increase in ortho-tyrosine (Fig. 2a) and a similar (60%) increase in meta-tyrosine (Fig. 2b). The concentration of o,o′-dityrosine, a marker of tyrosyl radical that is produced in lower yield by hydroxyl radical (Fig. 2c), was fourfold greater in the diabetic samples than in the control samples. Thus, ortho-tyrosine, meta-tyrosine, and o,o′-dityrosine levels were higher in aortic proteins from diabetic monkeys than in those from control animals. However, we found no significant difference in 3-nitrotyrosine levels in proteins isolated from aortic tissue of control and diabetic Cynomolgus monkeys (Fig. 2d). Collectively, these results indicate that ortho-tyrosine, meta-tyrosine, and o,o′-dityrosine levels are selectively elevated in aortic proteins of diabetic monkeys, whereas 3-nitrotyrosine levels remain unchanged. This pattern of oxidized amino acids suggests that a hydroxyl radical-like oxidant promotes aortic damage in this animal model of diabetic macrovascular disease.
Fig. 2.
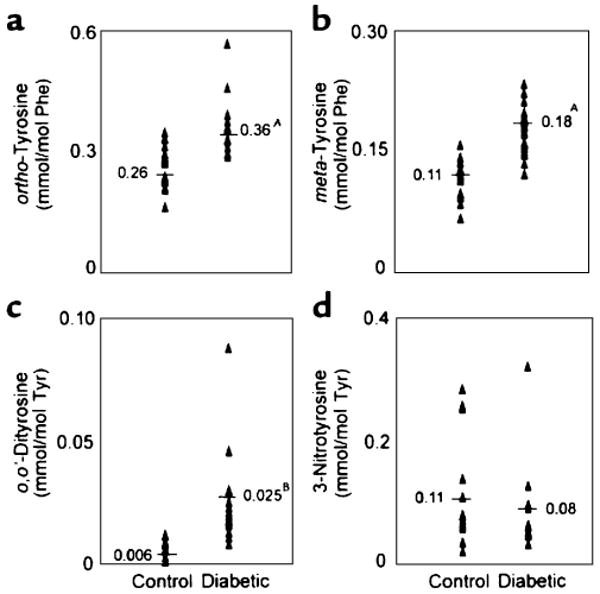
Quantification of ortho-tyrosine (a), meta-tyrosine (b), o,o′-dityrosine (c), and 3-nitrotyrosine (d) in aortic proteins isolated from control and diabetic Cynomolgus monkeys. Aortic tissue was harvested from control and diabetic animals at the end of the 6-month study. Tissue was delipidated, hydrolyzed, and amino acids were quantified by GC/MS. *p<0.01 by Student's t-test. Reproduced from [29]
3.3 Correlations between aortic tissue levels of the oxidized amino acids and serum levels of glycated hemoglobin
To determine whether glucose promotes protein oxidation in vivo, we assessed the relationship between level of glycemic control (measured as serum glycated hemoglobin) and levels of amino acid oxidation products in aortic tissue in control and diabetic Cynomolgus monkeys. Linear regression analysis demonstrated a strong correlation between levels of both ortho-tyrosine and meta-tyrosine and glycated hemoglobin (r2=0.9 and 0.8, respectively; both p<0.001). Levels of o,o′-dityrosine and glycated hemoglobin correlated less strongly (r2=0.3; p=0.07). In contrast, there was no correlation between levels of 3-nitrotyrosine and glycated hemoglobin. These observations support the hypothesis that glucose promotes the formation of ortho-tyrosine and meta-tyrosine in the artery wall and suggest that both glucose and other pathways contribute to the generation of o,o′-dityrosine [29].
3.4 Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions
Macrophage proliferation has been implicated in the progression of atherosclerosis. Recent studies have investigated the effects of hyperglycemia and hyperlipidemia on macrophage proliferation in murine atherosclerotic lesions and isolated primary macrophages [97]. Glucose promoted lipid and protein oxidation of LDL in vitro. Oxidation of LDL with glucose resulted in a selective increase in protein-bound ortho-tyrosine and meta-tyrosine. Moreover, glucose-oxidized LDL—but not elevated levels of glucose alone—stimulated proliferation of isolated macrophages. These observations may be pertinent to diabetic vascular disease because macrophage proliferation in atherosclerotic lesions was observed in LDL receptor-deficient mice that were both hypercholesterolemic and hyperglycemic but in not mice that were only hyperglycemic [97].
3.5 MS quantification of oxidized amino acids reveals a novel pathway of glucose induced protein and lipid oxidation in an animal model of diabetic microvascular disease
To begin to explore the pathophysiological relevance of the carbonyl-PUFA pathway, we quantified levels of protein and lipid oxidation products in the retina of hyperglycemic Sprague–Dawley rats, a well-characterized model of diabetic retinopathy [28]. Abnormalities such as increased vascular permeability became well established in retinal tissue as early as 4 weeks after STZ treatment [105, 106]. After 6 weeks, retinal tissue contained markedly elevated levels of ortho-tyrosine and meta-tyrosine and high levels of hydroxyoctadecadienoic acid (HODE), a major product of lipid oxidation. Treatment with the carbonyl scavenger aminoguanidine blocked these changes but failed to affect levels of ortho- and meta-tyrosine in control animals (Fig. 3). We observed no differences in levels of D-glucose or hemoglobin A1c in the rats on the two different diets. Thus, glucose or other reactive carbonyls that abound in the diabetic milieu promote lipid and protein oxidation in vivo by a pathway requiring PUFAs. These observations suggest that glucose might operate locally by a variety of mechanisms, damaging artery wall proteins even in the absence of generalized oxidative stress. It will be important to determine whether the pattern of oxidation products in diabetic humans resembles the ones we observed in the monkey and rat studies.
Fig. 3.
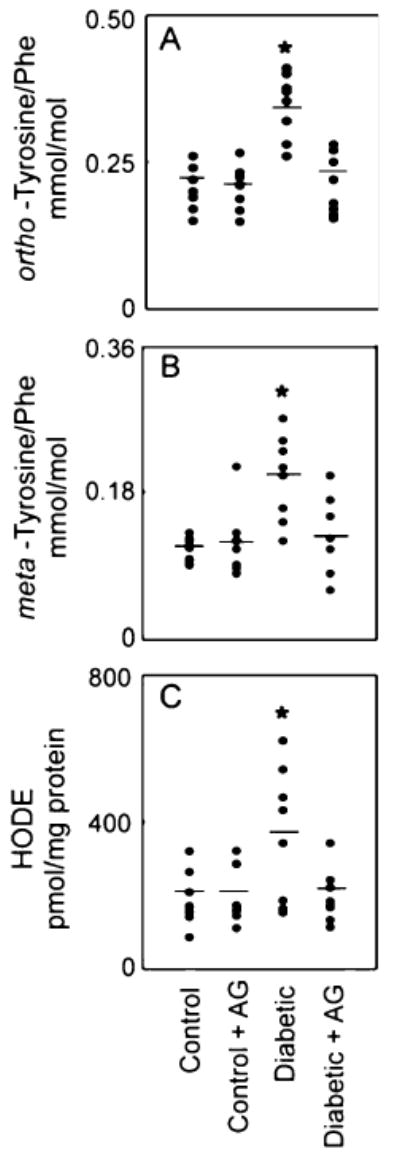
Quantification of oxidized amino acids and lipids in retinal tissue isolated from control and hyperglycemic Sprague–Dawley rats. Rats were rendered hyperglycemic with STZ. At the end of the 6-week study, retinal tissue was harvested from the animals. The isolated amino acids were derivatized and analyzed by GC/MS with selected ion monitoring (a and b). HODEs (hydroxyoctadecadienoic acid) were quantified by reversed-phase HPLC (c). *p<0.05 by analysis of variance. Reproduced from [28]
To investigate the potential relationship between lipid peroxidation and protein oxidation in vivo, we assessed the relationship between HODE levels and oxidized amino acid levels in the retinal tissue of control and STZ-treated rats (Fig. 4). Linear regression analysis demonstrated a strong correlation between levels of ortho-tyrosine and HODEs (r2=0.59; p<0.05). Levels of meta-tyrosine also correlated highly with HODE (r2=0.50; p<0.05). In contrast, there was no correlation between HODEs and nitrotyrosine and dityrosine (Fig. 4). These observations suggest that protein oxidation and lipid oxidation increase in parallel in the retina of diabetic rats and that hyperglycemia may provide the link.
Fig. 4.
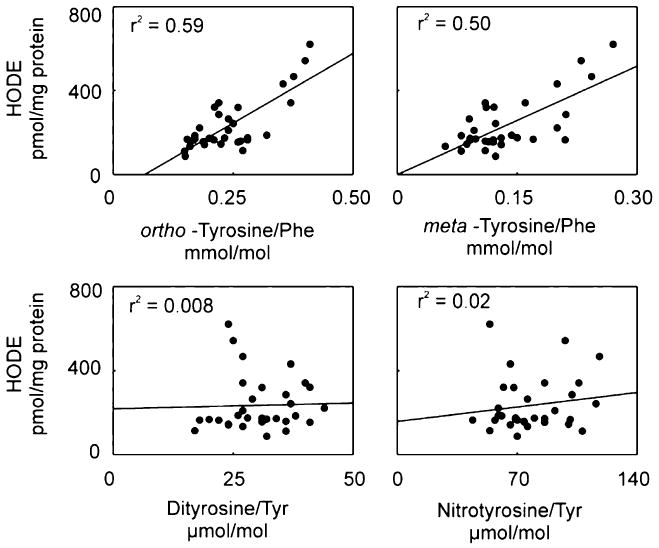
Correlation of ortho-tyrosine, meta-tyrosine o,o′-dityrosine, or 3-nitrotyrosine with HODEs in retinal tissue of control and diabetic Sprague–Dawley rats. At the end of the 6-week study, retinal tissue harvested from control animals, control animals treated with aminoguanidine, diabetic animals, and diabetic animals treated with aminoguanidine was analyzed for oxidation products. Lines represent the linear least-squares fit of the data. Reproduced from [28]
3.6 Oxidized amino acids are present in the plasma and urine of animals and humans: potential markers for assessment of in vivo oxidative stress
There is increasing evidence that oxidized amino acids in plasma and urine can serve as markers for non-invasively assessing oxidative stress in vivo [33, 50–52, 60, 94, 99, 104, 107]. In steady state, plasma and urine levels of these markers are proportional to their rate of generation and can serve as indices of chronic oxidative stress in vivo. A case–control study demonstrated that systemic levels of protein-bound nitrotyrosine were significantly higher among patients with CAD compared with those with healthy arteries and that statin therapy lowered levels of oxidation markers in plasma, raising the possibility that statins can potentially be antioxidants [51, 52, 107]. Therefore, these markers would serve not only to assess degree of oxidative stress but also to monitor efficacy of therapy. However, the role of these markers has not been determined systematically in response to antioxidant therapies in diabetic mouse models and humans.
3.7 MPO-oxidized HDL, a novel marker for CAD in humans
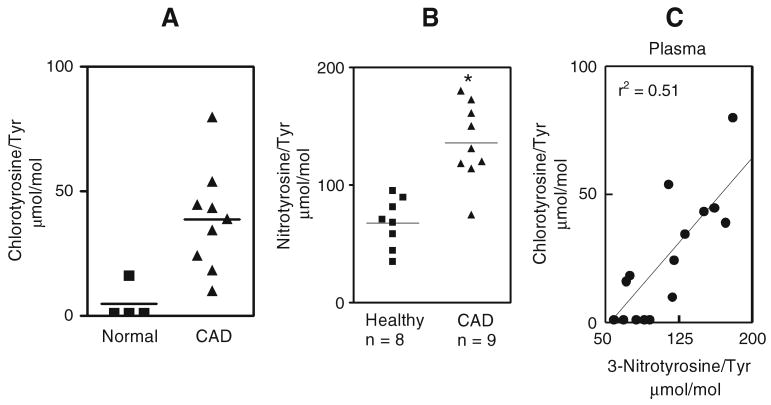
We employed our oxidized amino acid fingerprinting strategy to discover novel biomarkers in humans with CAD. Preliminary studies demonstrated that HDL, but not LDL, isolated from plasma of subjects with established CAD contained high levels of 3-chlorotyrosine, a highly specific marker for the myeloperoxidase pathway. The level of 3-chlorotyrosine was 13-fold higher in HDL isolated from plasma of subjects with established CAD (Fig. 5a) than in HDL from plasma of healthy subjects [60]. We also found that levels of 3-nitrotyrosine were twice as high in HDL from plasma of patients with established CAD (Fig. 5b) [50]. These observations raise the exciting possibility that oxidized HDL might be a novel marker for clinically significant CAD. It will clearly be important to determine whether levels of oxidized amino acids are elevated in HDL and LDL of diabetic humans.
Fig. 5.
MPO generates chlorinated and nitrated HDL in human plasma in subjects with CAD. Levels of MPO-oxidized amino acids were determined in HDL isolated from healthy subjects and subjects with CAD. a 3-chlorotyrosine and b 3-nitrotyrosine. Linear regression analysis demonstrated a strong correlation between levels of 3-chlorotyrosine and levels of 3-nitrotyrosine (c) in plasma HDL consistent with similar pathway of generation of both these markers. Reproduced from [50, 60]
To determine whether MPO might promote protein nitration in vivo, we assessed the relationship between 3-chlorotyrosine, a marker of protein oxidation that is generated only by MPO at plasma concentrations of halide ion, and levels of 3-nitrotyrosine in both circulating and lesion HDL (Fig. 5c). Linear regression analysis demonstrated a strong correlation between levels of 3-chlorotyrosine and levels of 3-nitrotyrosine (r2=0.51; p<0.01) in plasma HDL. These observations strongly support the hypothesis that MPO promotes the formation of 3-chlorotyrosine and 3-nitrotyrosine in circulating HDL.
4 Conclusion and perspectives
The ability to accurately quantify amino acid oxidation markers in tissue samples, plasma, and urine can provide invaluable mechanistic insights into disease pathogenesis. We have utilized the power of MS to profile oxidized amino acids to facilitate understanding of tissue specific oxidative mechanisms of diabetic complications. Further studies of the biochemical basis for oxidant generation might facilitate the development of specific antioxidant therapies designed to retard diabetic complications.
The potential role of antioxidant therapies in preventing microvascular and macrovascular disease in diabetic humans is another important issue. To examine the oxidative stress hypothesis in diabetes, suitable test subjects and optimal antioxidant regimens need to be identified. Such a trial should involve diabetic patients with evidence of increased oxidative stress (e.g. subjects with elevated levels of oxidized amino acids in their plasma or urine). These high-risk individuals are most likely to benefit from antioxidant therapy. The optimal regimen for inhibiting oxidative tissue injury in humans also needs to be determined so that test compounds can be administered in effective antioxidant regimens.
Acknowledgments
Dr. Pennathur's laboratory is supported by grants from the National Institutes of Health (R21HL092237, P50DK039255, P30AG024824), Career Development Award from the Juvenile Diabetes Research Foundation, the Doris Duke Clinical Scientist Development Award, the Hartford foundation, the Michigan Institute for Clinical and Health Research-CTSA pilot grant, the Michigan Metabolomics and Obesity Center and the Biomedical Mass Spectrometry Facility (University of Michigan).
Abbreviations
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- AGEs
advanced glycation end-products
- ALE
advanced lipoxidation end-products
- BHT
butylated hydroxytoluene
- BH4
tetrahydrobiopterin
- CAD
coronary artery disease
- CML
Nε-(carboxymethyl)lysine
- cGMP
cyclic guanosine monophosphate
- CVD
cardiovascular disease
- DCCT
Diabetes Control and Complications Trial
- DTPA
diethylenetriaminepentaacetic acid
- EDIC
Epidemiology of Diabetes Interventions and Complications
- eNOS
endothelial nitric oxide synthase
- ESRD
end stage renal disease
- GC/MS
gas chromatography/mass spectrometry
- HDL
high-density lipoprotein
- HO•
hydroxyl radical
- HODE
hydroxyoctadecadienoic acid
- H2O2
hydrogen peroxide
- HOCl
hypochlorous acid
- LDL
low density lipoprotein
- MPO
myeloperoxidase
- NEFA
non-esterified fatty acid
- NOX
NAD(P)H oxidase
- NO•
nitric oxide
- NO2−
nitrite
nitrogen dioxide
- ONOO−
peroxynitrite
- PUFA
polyunsaturated fatty acid
- RAGE
Receptor for AGE
- RNase
ribonuclease
- STZ
streptozotocin
superoxide anion
- UKPDS
United Kingdom Prospective Diabetes Study
- XO
xanthine oxidase
References
- 1.American Diabetes Association. Diabetes Statistics. http://www.diabetes.org/diabetes-statistics.jsp.
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. US Department of Health and Human Services. Centers for Disease Control and Prevention; Atlanta GA: 2004. [Google Scholar]
- 6.Haffner SM. Coronary heart disease in patients with diabetes. N Engl J Med. 2000;342:1040–2. doi: 10.1056/NEJM2000040 63421408. [DOI] [PubMed] [Google Scholar]
- 7.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 8.Hu FB, Stampfer MJ, Solomon CG, Liu S, Willett WC, Speizer FE, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med. 2001;161:1717–23. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 9.Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest. 2003;111:431–3. doi: 10.1172/JCI17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan DM. Some answers, more controversy, from UKPDS. United Kingdom Prospective Diabetes Study. Lancet. 1998;352:832–3. doi: 10.1016/S0140-6736(98)22937-0. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klien BE, Moss SE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: a review. Diabetes Metab Rev. 1989;5:559–70. doi: 10.1002/dmr.5610050703. [DOI] [PubMed] [Google Scholar]
- 12.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–42. doi: 10.2337/diabetes. 48.5.937. [DOI] [PubMed] [Google Scholar]
- 13.Semenkovich CF, Heinecke JW. The mystery of diabetes and atherosclerosis: time for a new plot. Diabetes. 1997;46:327–34. doi: 10.2337/diabetes.46.3.327. [DOI] [PubMed] [Google Scholar]
- 14.DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 15.UKPDS Research Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 16.UKPDS Research Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 17.DCCT/EDIC Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 21.Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–3. doi: 10.1056/NEJMe0804182. [DOI] [PubMed] [Google Scholar]
- 22.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 23.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 25.Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med. 2000;28:1708–16. doi: 10.1016/S0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 26.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–69. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- 27.Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7:257–64. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 28.Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, et al. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem. 2005;280:22706–14. doi: 10.1074/jbc.M500839200. [DOI] [PubMed] [Google Scholar]
- 29.Pennathur S, Wagner JD, Leeuwenburgh C, Litwak KN, Heinecke JW. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J Clin Invest. 2001;107:853–60. doi: 10.1172/JCI11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–34. doi: 10.1146/annurev. med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 32.Heinecke JW. Oxidative stress: new approaches to diagnosis and prognosis in atherosclerosis. Am J Cardiol. 2003;91:12A–6A. doi: 10.1016/S0002-9149(02)03145-4. [DOI] [PubMed] [Google Scholar]
- 33.Pennathur S, Heinecke JW. Mechanisms of oxidative stress in diabetes: implications for the pathogenesis of vascular disease and antioxidant therapy. Front Biosci. 2004;9:565–74. doi: 10.2741/1257. [DOI] [PubMed] [Google Scholar]
- 34.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 35.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988;256:205–12. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–21. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 38.Huggins TG, Wells-Knecht MC, Detorie NA, Baynes JW, Thorpe SR. Formation of o-tyrosine and dityrosine in proteins during radiolytic and metal-catalyzed oxidation. J Biol Chem. 1993;268:12341–7. [PubMed] [Google Scholar]
- 39.Huggins TG, Staton MW, Dyer DG, Detorie NJ, Walla MD, Baynes JW, et al. o-Tyrosine and dityrosine concentrations in oxidized proteins and lens proteins with age. Ann N Y Acad Sci. 1992;663:436–7. doi: 10.1111/j.1749-6632.1992.tb38692.x. [DOI] [PubMed] [Google Scholar]
- 40.Kaur H, Halliwell B. Detection of hydroxyl radicals by aromatic hydroxylation. Methods Enzymol. 1994;233:67–82. doi: 10.1016/S0076-6879(94)33009-3. [DOI] [PubMed] [Google Scholar]
- 41.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–9. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 42.Miyata T, Ueda Y, Yoshido A, Sugiyama S, Iida Y, Jadoul M, et al. Clearance of pentosidine, an advanced glycation end-product, by different modalities of renal replacement therapy. Kidney Int. 1997;51:880–7. doi: 10.1038/ki.1997.124. [DOI] [PubMed] [Google Scholar]
- 43.Miyata T, van Ypersele de Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999;55:389–99. doi: 10.1046/j.1523-1755.1999.00302.x. [DOI] [PubMed] [Google Scholar]
- 44.Miyata T, Fu MX, Kurokawa K, van Ypersele de Strihou C, Thorpe SR, Baynes JW. Autoxidation products of both carbohydrates and lipids are increased in uremic plasma: is there oxidative stress in uremia. Kidney Int. 1998;54:1290–5. doi: 10.1046/j.1523-1755.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 45.Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991;266:11654–60. [PubMed] [Google Scholar]
- 46.McCance DR, Dyer DG, Dunn JA, Bailie KE, Thorpe SR, Baynes JW, et al. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91:2470–8. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monnier VM. Transition metals redox: reviving an old plot for diabetic vascular disease. J Clin Invest. 2001;107:799–801. doi: 10.1172/JCI12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sell DR, Lapolla A, Odetti P, Fogarty J, Monnier VM. Pentosidine formation in skin correlates with the severity of complications in individuals with long-standing IDDM. Diabetes. 1992;41:1286–92. doi: 10.2337/diabetes.41.10.1286. [DOI] [PubMed] [Google Scholar]
- 49.Leeuwenburgh C, Hardy MM, Hazen SL, Wagner P, Oh-ishi S, Steinbrecher UP, et al. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem. 1997;272:1433–6. doi: 10.1074/jbc.272. 3.1433. [DOI] [PubMed] [Google Scholar]
- 50.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279:42977–83. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 51.Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–80. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 52.Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–31. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 53.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–7. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 54.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–9. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 55.Klebanoff SJ, Clark RA. The neutrophil: function and clinical disorders. Amsterdam: North Holland; 1978. pp. 447–51. [Google Scholar]
- 56.Gaut JP, Byun J, Tran HD, Lauber WM, Carroll JA, Hotchkiss RS, et al. Myeloperoxidase produces nitrating oxidants in vivo. J Clin Invest. 2002;109:1311–9. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–42. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 58.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–91. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 60.Bergt C, Pennathur S, Fu X, Byun J, O'Brien K, McDonald TO, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101:13032–7. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–81. doi: 10.1172/JCI 119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res. 2005;96:818–22. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 63.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–9. doi: 10.1016/S0140-6736(99)12323-7. [DOI] [PubMed] [Google Scholar]
- 64.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, et al. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–9. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 65.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–26. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 66.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–5. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today. 2006;11:524–33. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS(−/−)Apoe(−/−) mice are ameliorated by enalapril treatment. J Clin Invest. 2000;105:451–8. doi: 10.1172/JCI8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–54. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 71.Takaya T, Hirata K, Yamashita T, Shinohara M, Sasaki N, Inoue N, et al. A specific role for eNOS-derived reactive oxygen species in atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2007;27:1632–7. doi: 10.1161/ATVBAHA.107.142182. [DOI] [PubMed] [Google Scholar]
- 72.Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–50. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 73.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–9. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, et al. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol. 2007;27:943–8. doi: 10.1161/01.ATV.0000258415.32883.bf. [DOI] [PubMed] [Google Scholar]
- 75.Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA. 1993;90:6434–8. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 1994;91:9441–5. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunt JV, Smith CC, Wolf SP. Oxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39:1420–4. doi: 10.2337/diabetes.39.11.1420. [DOI] [PubMed] [Google Scholar]
- 78.Kawamura M, Heinecke JW, Chait A. Pathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxide-dependent pathway. J Clin Invest. 1994;94:771–8. doi: 10.1172/JCI117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–6. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 80.Requena JR, Ahmed MU, Fountain CW, Degenhardt TP, Reddy S, Perez C, et al. Carboxymethylethanolamine, a biomarker of phospholipid modification during the maillard reaction in vivo. J Biol Chem. 1997;272:17473–9. doi: 10.1074/jbc.272.28.17473. [DOI] [PubMed] [Google Scholar]
- 81.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Baynes JW, et al. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem J. 1997;322(Pt 1):317–25. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996;11(Suppl 5):48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- 83.Mezzetti A, Cipollone F, Cuccurullo F. Oxidative stress and cardiovascular complications of diabetes: isoprostanes as new markers on an old paradigm. Cardiovasc Res. 2000;47:475–88. doi: 10.1016/S0008-6363(00)00118-8. [DOI] [PubMed] [Google Scholar]
- 84.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–9. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 85.Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. Mechanism of action of pyridoxamine. J Biol Chem. 2000;275:21177–84. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- 86.Voziyan PA, Metz TO, Baynes JW, Hudson BG. A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J Biol Chem. 2002;277:3397–403. doi: 10.1074/jbc.M109935200. [DOI] [PubMed] [Google Scholar]
- 87.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–32. doi: 10.1126/science. 3487117. [DOI] [PubMed] [Google Scholar]
- 88.Edelstein D, Brownlee M. Aminoguanidine ameliorates albuminuria in diabetic hypertensive rats. Diabetologia. 1992;35:96–7. doi: 10.1007/BF00400859. [DOI] [PubMed] [Google Scholar]
- 89.Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Natl Acad Sci USA. 1991;88:11555–8. doi: 10.1073/pnas.88.24.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hammes HP, Brownlee M, Edelstein D, Saleck M, Martin S, Federlin K. Aminoguanidine inhibits the development of accelerated diabetic retinopathy in the spontaneous hypertensive rat. Diabetologia. 1994;37:32–5. doi: 10.1007/BF00428774. [DOI] [PubMed] [Google Scholar]
- 91.Ihm S, Hyung J, Park S, Ihm J. Effect of aminoguanidine on lipid-peroxidation in streptozotocin-induced diabetic rats. Metabolism. 1999;48:1141–5. doi: 10.1016/S0026-0495(99)90128-2. [DOI] [PubMed] [Google Scholar]
- 92.Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, et al. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–32. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 93.Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939–50. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 94.Bhattacharjee S, Pennathur S, Byun J, Crowley J, Mueller D, Gischler J, et al. NADPH oxidase of neutrophils elevates o,o′-dityrosine cross-links in proteins and urine during inflammation. Arch Biochem Biophys. 2001;395:69–77. doi: 10.1006/abbi.2001.2557. [DOI] [PubMed] [Google Scholar]
- 95.Brennan ML, Hazen SL. Amino acid and protein oxidation in cardiovascular disease. Amino Acids. 2003;25:365–74. doi: 10.1007/s00726-003-0023-y. [DOI] [PubMed] [Google Scholar]
- 96.Hazen SL, Crowley JR, Mueller DM, Heinecke JW. Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: a sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation. Free Radic Biol Med. 1997;23:909–16. doi: 10.1016/S0891-5849(97)00084-1. [DOI] [PubMed] [Google Scholar]
- 97.Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, et al. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes. 2004;53:3217–25. doi: 10.2337/diabetes.53.12.3217. [DOI] [PubMed] [Google Scholar]
- 98.Leeuwenburgh C, Hansen P, Shaish A, Holloszy JO, Heinecke JW. Markers of protein oxidation by hydroxyl radical and reactive nitrogen species in tissues of aging rats. Am J Physiol. 1998;274:R453–61. doi: 10.1152/ajpregu.1998.274.2.R453. [DOI] [PubMed] [Google Scholar]
- 99.Leeuwenburgh C, Hansen PA, Holloszy JO, Heinecke JW. Hydroxyl radical generation during exercise increases mitochondrial protein oxidation and levels of urinary dityrosine. Free Radic Biol Med. 1999;27:186–92. doi: 10.1016/S0891-5849(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 100.Leeuwenburgh C, Hansen PA, Holloszy JO, Heinecke JW. Oxidized amino acids in the urine of aging rats: potential markers for assessing oxidative stress in vivo. Am J Physiol. 1999;276:R128–35. doi: 10.1152/ajpregu.1999.276.1.R128. [DOI] [PubMed] [Google Scholar]
- 101.Leeuwenburgh C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem. 1997;272:3520–6. doi: 10.1074/jbc.272.6.3520.. [DOI] [PubMed] [Google Scholar]
- 102.Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346:74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- 103.Pennathur S, Jackson-Lewis V, Przedborski S, Heinecke JW. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o′-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson's disease. J Biol Chem. 1999;274:34621–8. doi: 10.1074/jbc.274.49.34621. [DOI] [PubMed] [Google Scholar]
- 104.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–41. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lorenzi M, Gerhardinger C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia. 2001;44:791–804. doi: 10.1007/s001250100544. [DOI] [PubMed] [Google Scholar]
- 106.Zhang SX, Sima J, Shao C, Fant J, Chen Y, Rohrer B, et al. Plasminogen kringle 5 reduces vascular leakage in the retina in rat models of oxygen-induced retinopathy and diabetes. Diabetologia. 2004;47:124–31. doi: 10.1007/s00125-003-1276-4. [DOI] [PubMed] [Google Scholar]
- 107.Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep. 2004;6:243–50. doi: 10.1007/s11883-004-0038-1. [DOI] [PubMed] [Google Scholar]