TRPM2 (transient receptor potential melastatin 2) is a non-selective cation channel expressed in the brain and in immune cells (Nagamine et al. 1998) which contributes to immunocyte activation and postischaemic neuronal death. It is activated under conditions of oxidative stress, which causes intracellular accumulation of its primary activators ADP-ribose (ADPR) and Ca2+. ADPR binds to an enzymatic domain formed by the intracellular C-terminus of the channel (Perraud et al. 2001; Sano et al. 2001), while Ca2+ binds in a protected crevice on the intracellular side of the gate but very near the pore (Csanády & Torocsik, 2009). Ca2+ permeation through the pore provides a strong positive feedback which helps maintain channel activity in the presence of ADPR.
Two recent studies, Du et al. (2009) and in a recent issue of The Journal of PhysiologyStarkus et al. (2010), have revealed strong inhibition of TRPM2 currents by extra- or intracellular acidification. The significance of this finding is that TRPM2 activity is frequently associated which conditions that lower extra- or intracellular pH, such as inflammation or ischaemia, and that this phenomenon offers a rapid and effective way to limit intracellular Ca2+ load resulting from the activity of the channel. Although both studies found that extracellular acidity reduces single-channel conductance and that intracellular acidity suppresses channel gating, very different conclusions were reached about the molecular mechanism of these effects.
Du and colleagues concluded that protons do not premeate through the TRPM2 pore, and therefore had to postulate both intra- and extracellular proton interaction sites to explain inhibition by protons from either side. This conclusion was based on the following experimental evidence: (i) inability to demonstrate measurable inward proton currents when no other permeant ions were present on the extracellular side, (ii) apparent linearity of whole-cell and single-channel current–voltage relationships under conditions of an inward-directed proton gradient, (iii) lack of effect of membrane potential on the sensitivity to inhibition by extracellular protons, (iv) inhibition by extracellular protons being outcompeted by extracellular, but not by intracellular, Ca2+, and (v) an increase in sensitivity to extracellular protons caused by removal of negatively charged amino acid side chains in the channel's outer vestibule.
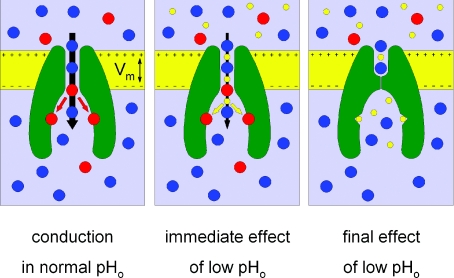
By carefully reexamining the above experimental paradigms the work by Starkus and colleagues, published in a recent issue of the Journal of Physiology, challenges the conclusions of the previous study and provides convincing support for a simple mechanism whereby extracellular protons permeate through the TRPM2 pore to exert their inhibitory effect at an intracellular interaction site, likely to be identical to that to which activating Ca2+ binds (Fig. 1).
Figure 1. Molecular mechanism of TRPM2 inhibition by external protons.
Normal Na+/Ca2+ influx through the open pore (left) is instantaneously slowed by co-permeating H+ (centre) at low extracellular pH (pHo). Eventually, H+ binding to the intracellular activating sites shuts the channel (right); intracellular acidification immediately leads to this scenario. Na+, blue; Ca2+, red; H+, yellow.
How can this picture be reconciled with the apparently consistent set of data enumerated above? First, Starkus and colleagues rightfully note that the micromolar concentration range of extracellular protons might in itself preclude observation of a measurable proton current. Second, the authors demonstrate a small but clear deviation from linearity of instantaneous whole-cell current–voltage relationships acquired at low extracellular pH. This slight outward rectification, previously overlooked, is consistent with a reduction of the single-channel conductance at negative voltages, signalling decreased Na+ flux through the open pore when protons co-permeate. Third, they extend the voltage range for studying inhibition by external protons to include voltages higher than the proton reversal potential (VH), and report not only the steady-state values, but also the kinetics, of inhibition. They find little inhibition at voltages higher than VH, and, although at voltages below VH external protons cause strong current suppression, the rate of inhibition is clearly voltage dependent, correlated with the proton driving force. This stresses the necessity of studying the kinetics in addition to the steady state – if the proton interaction site is indeed located in a restricted space, as suggested for activating Ca2+, then protons will eventually accumulate in this space even if the rate of entry is slowed. Fourth, increasing intracellular [Ca2+] similarly slows the kinetics of inhibition by extracellular protons. And how about the extracellular mutations which Du and colleagues have found to increase pH sensitivity? Those mutations were also shown to impair the apparent affinity for extracellular Ca2+, probably by reducing local [Ca2+] in the outer vestibule. Since extracellular Ca2+ and protons compete for permeation, a mutation-induced depression of Ca2+ influx might increase sensitivity to extracellular protons simply by facilitating proton entry through the pore. Finally, to cap it all, elegant demonstration of state dependence of the extracellular proton effect provides compelling evidence for proton permeation. While an extracellular pH of 4 irreversibly inhibits whole-cell TRPM2 currents activated by intracellular dialysis of ADPR from the pipette solution, a comparable lowering of external pH in the cell-attached stage, while the channels are still shut, does not prevent subsequent current activation upon whole-cell break-in. These experiments provide a satisfyingly simple, unifying explanation for the various effects of intra- and extracellular acidity, centred on competition for intracellular binding sites between activating Ca2+ and inhibitory protons, which arrive either from the cytosol or via permeation through the pore (Fig. 1).
References
- Csanády L, Torocsik B. J Gen Physiol. 2009;133:189–203. doi: 10.1085/jgp.200810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Xie J, Yue L. J Gen Physiol. 2009;134:471–488. doi: 10.1085/jgp.200910254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, Shimizu N. Genomics. 1998;54:124–131. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- Starkus JG, Fleig A, Penner R. J Physiol. 2010;588:1227–1240. doi: 10.1113/jphysiol.2010.187476. [DOI] [PMC free article] [PubMed] [Google Scholar]