Abstract
Rad/Rem/Gem/Kir (RGK) GTPases potently inhibit CaV1 and CaV2 (CaV1-2) channels, a paradigm of ion channel regulation by monomeric G-proteins with significant physiological ramifications and potential biotechnology applications. The mechanism(s) underlying how RGK proteins inhibit ICa is unknown, and it is unclear how key structural and regulatory properties of these GTPases (such as the role of GTP binding to the nucleotide binding domain (NBD), and the C-terminus which contains a membrane-targeting motif) feature in this effect. Here, we show that Rem inhibits CaV1.2 channels by three independent mechanisms that rely on distinct configurations of the GTPase: (1) a reduction in surface density of channels is accomplished by enhancing dynamin-dependent endocytosis, (2) a diminution of channel open probability (Po) that occurs without impacting on voltage sensor movement, and (3) an immobilization of CaV channel voltage sensors. The presence of both the Rem NBD and C-terminus (whether membrane-targeted or not) in one molecule is sufficient to reconstitute all three mechanisms. However, membrane localization of the NBD by a generic membrane-targeting module reconstitutes only the decreased Po function (mechanism 2). A point mutation that prevents GTP binding to the NBD selectively eliminates the capacity to immobilize voltage sensors (mechanism 3). The results reveal an uncommon multiplicity in the mechanisms Rem uses to inhibit ICa, predict new physiological dimensions of the RGK GTPase–CaV channel crosstalk, and suggest original approaches for developing novel CaV channel blockers.
Introduction
High-voltage-activated calcium (CaV1-2) channels convert electrical signals into biological responses in excitable cells. Action potential-evoked Ca2+ influx via CaV1-2 channels drives: synaptic transmission and regulation of gene expression in neurons (Catterall & Few, 2008); contraction and excitability in muscle (Bers, 2008); and hormone release in endocrine cells (Yang & Berggren, 2006). Accordingly, modulating CaV1-2 channel activity is exploited both for physiological regulation and as a therapy for cardiovascular and neurological diseases (Valentino et al. 1993; Kochegarov, 2003; Triggle, 2003).
Rad/Rem/Rem2/Gem/Kir (RGK) proteins belong to the Ras superfamily of monomeric GTPases (Colicelli, 2004) which function as GTP-regulated switches, cycling between inactive GDP-bound and active GTP-bound forms. These proteins are essential for a broad spectrum of cell biological functions including protein transport, cytoskeletal dynamics and mitogenic responses. Structurally, RGK GTPases have unique features compared to Ras including large N- and C-termini extensions, and non-conservative substitutions in the nucleotide-binding domain (NBD) of residues critical for GTP binding and hydrolysis (Reynet & Kahn, 1993; Maguire et al. 1994; Finlin & Andres, 1997; Finlin et al. 2000). The C-termini of RGK proteins lack prenylation motifs but, nevertheless, target the GTPases to the plasma membrane using electrostatic and hydrophobic interactions (Heo et al. 2006). Functionally, RGK GTPases have been linked to important biological roles including regulating cytoskeletal dynamics via actions on Rho kinases (Correll et al. 2008). Moreover, siRNA knockdown experiments indicate that Rem2 is necessary for synapse development (Paradis et al. 2007), and Rad knockout mice develop cardiac hypertrophy (Chang et al. 2007). Despite the biological importance of these RGK GTPases, little is known about their modus operandi and structure–function relationships at a mechanistic level.
All four RGK GTPases potently inhibit CaV1.2 channels by interacting with auxiliary CaVβ subunits (Beguin et al. 2001; Finlin et al. 2003). This RGK GTPase–CaV1.2 channel crosstalk is of interest for several reasons. First, the phenomenon is poised to have profound physiological significance. RGK GTPases are prevalent in many excitable and non-excitable cells, and their expression level is regulated under distinct (patho)physiological conditions (Reynet & Kahn, 1993; Maguire et al. 1994; Finlin & Andres, 1997; Finlin et al. 2000; Tan et al. 2002; Hawke et al. 2006). Their modulation of CaV1-2 channels positions them as potentially influential determinants of Ca2+ signalling profiles in excitable cells. Second, these proteins are prototype CaV channel inhibitors that act via CaVβ subunits. Blocking CaV1-2 channels is an important therapy for diseases including hypertension, stroke and neuropathic pain. Developing novel CaV1-2 channel blockers that act by targeting CaVβs has been a long sought after goal, but the approach has achieved only limited success (Young et al. 1998). Understanding how RGK GTPases potently inhibit ICa could provide clues for developing novel CaVβ-dependent CaV channel blockers (Xu & Colecraft, 2009). Third, the crosstalk provides unique opportunities to gain fundamental mechanistic insights into structure–function relationships of RGK GTPases.
How do RGK GTPases inhibit ICa, and can their crosstalk with CaV channels reveal new insights into the structure–function of this protein family? Though these questions have been intensely studied, several ambiguities persist. One debate has focused on whether RGK GTPases inhibit ICa exclusively by either reducing the surface density of channels, or by blocking the activity of channels in the membrane. Studies on recombinant CaV1.2 (L-type) channels reconstituted in HEK 293 cells suggest that RGK proteins inhibit ICa by reducing the surface density of CaV channels (Beguin et al. 2001, 2005b, 2006). By contrast, experiments on endogenous CaV channels in either superior cervical ganglion neurons (Chen et al. 2005) or mouse insulinoma MIN6 cells (Finlin et al. 2005) suggest that RGK proteins inhibit ICa without affecting the number of channels in the membrane. It is unclear whether these two putative mechanisms are mutually exclusive, or whether they can co-exist within the same preparation. Beyond the ambiguity surrounding their relative prevalence, it is unknown precisely how either candidate mechanism is accomplished. A reduction in channel surface density could be achieved either by diminishing forward trafficking of CaV channels to the membrane, or by an unrecognized ability of RGK GTPases to enhance CaV channel endocytosis. Regarding the putative silencing of channels at the membrane, it is unknown which step(s) of the channel activation pathway is/are affected.
Beyond the questions regarding mechanisms of inhibition, uncertainty surrounds the role that distinct structural determinants and regulatory features of RGK GTPases play in blocking ICa. Using point mutations predicted to lock RGK proteins in the GDP-bound state, some studies find that GTP binding is important for their ability to inhibit ICa, while others do not (Zhu et al. 1995; Ward et al. 2004; Chen et al. 2005; Yada et al. 2007). Further, several studies suggest that membrane targeting of RGK proteins, mediated through their C-termini, is essential for their ability to block ICa (Chen et al. 2005; Correll et al. 2007; Yang et al. 2007). However, it has also been proposed that RGK protein-mediated sequestration of CaVβ subunits in the nucleus represents another way to block ICa (Beguin et al. 2006). In short, there are significant gaps in current understanding of the mechanisms and structural determinants underlying how RGK GTPases inhibit CaV channels.
Here, using a transient transfection experimental protocol, we find that Rem potently inhibits recombinant CaV1.2 channels by three distinct mechanisms that require different functional conformations of the GTPase. The results reveal that Rem (and probably other RGK GTPases) poses a ‘triple threat’ to CaV channels, utilizing a versatile mix of mechanisms and structural determinants to inhibit ICa.
Methods
cDNA cloning
To generate fluorescent protein (XFP)-tagged Rem constructs, we PCR amplified and cloned XFP into pcDNA4.1 (Invitrogen) using KpnI and BamHI sites. Subsequently, Rem constructs were amplified by PCR and cloned downstream of the XFP molecule using BamHI and EcoRI sites. To generate cyan fluorescent protein (CFP)–Rem265–C1PKCγ, we used overlap extension PCR to fuse residues 26–89 of mouse protein kinase Cγ (PKCγ) (Oancea et al. 1998) to the C-terminus of Rem265. The fusion product was subsequently cloned downstream of CFP using BamHI and EcoRI sites. Point mutations in Rem were generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). The 13-residue bungarotoxin binding site (BBS) (Sekine-Aizawa & Huganir, 2004) was engineered into the domain II S5–S6 extracellular loop of α1C at residue 713 using unique restriction enzyme sites, StuI and BbrPI. Primers that extended from the unique restriction sites were used together with primers containing the BBS sequence in an overlap extension PCR reaction. The overlap extension product was directly ligated into α1C–yellow fluorescent protein (YFP) to generate α1C[BBS]–YFP. All PCR products were verified by sequencing.
Cell culture and transfection
Low-passage-number HEK 293 cells were maintained in DMEM supplemented with 10% FBS and 100 μg ml−1 penicillin–streptomycin. For electrophysiology and flow cytometry experiments, HEK 293 cells cultured in 6 cm tissue culture dishes were transiently transfected with CaV1.2α1C (8 μg), β2a (6 μg), T antigen (2 μg) and the appropriate Rem construct (6 μg), using the calcium phosphate precipitation method. Cells were washed with PBS 5–8 h after transfection and maintained in supplemented DMEM. For confocal microscopy experiments, transfected HEK 293 cells were replated onto fibronectin-coated culture dishes with No. 0 glass coverslip bottoms (MaTek). For electrophysiology experiments, cells were replated onto fibronectin-coated glass coverslips 24 h after transfection.
Electrophysiology
Whole-cell recordings were conducted 48–72 h after transfection using an EPC-8 or EPC-10 patch clamp amplifier (HEKA Electronics) controlled by PULSE software (HEKA). Micropipettes fashioned from 1.5 mm thin-walled glass with filament (WPI Instruments) were filled with internal solution containing (in mm): 135 caesium methanesulphonate (MeSO3), 5 CsCl, 5 EGTA, 1 MgCl2, 4 MgATP (added fresh) and 10 Hepes (pH 7.3). Series resistance was typically 1.5–2 MΩ. External solution contained (in mm): 140 tetraethylammonium-MeSO3, 5 BaCl2 and 10 Hepes (pH 7.3). Whole-cell I–V curves were generated from a family of step depolarizations (−40 to +100 mV from a holding potential of −90 mV). Currents were sampled at 25 kHz and filtered at 5 or 10 kHz. Traces were acquired at a repetition interval of 6 s. Leak and capacitive currents were subtracted using a P/8 protocol.
Labelling of cell surface CaV1.2 channels with QD655
Transfected cells were washed twice with PBS containing calcium and magnesium (pH 7.4, 0.9 mm CaCl2 and 0.49 mm MgCl2), and incubated with 1 μm biotinylated α-bungarotoxin in DMEM/3% BSA in the dark for 1 h at room temperature. Cells were washed twice with DMEM/3% BSA, and incubated with 10 nm streptavidin-conjugated quantum dot (QD655) for 1 h at 4°C in the dark. For confocal microscopy, cells were washed with PBS, and imaged in the same buffer. For flow cytometry, cells were harvested with trypsin, washed with PBS and assayed in the same buffer.
Confocal microscopy
Static images of α1C[BBS]–YFP, XFP–Rem constructs and quantum dots signals were observed using a Zeiss LSM 510 META scanning confocal microscope. HEK 293 cells expressing CFP/YFP fusion proteins were imaged using a 458/514 nm argon laser line for excitation, and red signals were imaged using a 633 nm helium–neon laser line for excitation.
Flow cytometry
Cells were counted using a BD LSRII Cell Analyzer. HEK 293 cells expressing CFP/YFP fusion proteins were excited at 407 and 488 nm, respectively, and red signal was excited at 633 nm. For each group of experiments, we used isochronal untransfected and single colour controls to manually set the appropriate gain settings for each fluorophore to ensure signals remained in the linear range and to set threshold values. The same gain settings were then used for assaying all isochronal transfection samples.
Data and statistical analyses
Data were analysed off-line using PulseFit (HEKA), Microsoft Excel and Origin software. Statistical analyses were performed in Origin using built-in functions. Statistically significant differences between means (P < 0.05) were determined using Student's t test for comparisons between two groups, or one-way ANOVA followed by Bonferroni post hoc analyses for comparisons involving more than two groups. Data are presented as means ±s.e.m.
Online Supplemental material
Figure S1 shows exemplar electrophysiological recordings and population I–V curves from untransfected cells or cells transfected with either CaV1.2α1C or β2a cDNA alone. Figure S2 shows YFP- and QD655-fluorescence images of suspensions of cells expressing α1C[BBS]–YFP alone or α1C[BBS]–YFP +β2a which demonstrate the viability of a new assay to detect surface CaV1.2 channels. Figure S3 shows evidence from pull-down competition assays that Rem does not disrupt the CaV channel α1–β subunit interaction.
Results
Functional impact of Rem on recombinant CaV1.2 channel currents
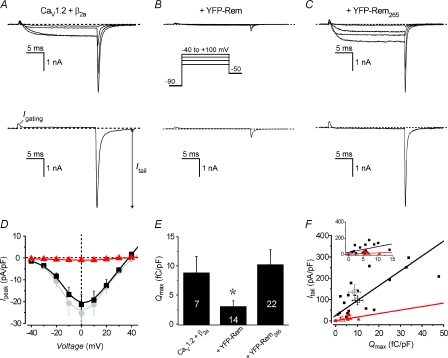
We assessed the impact of Rem on recombinant CaV1.2 channels reconstituted in HEK 293 cells (Fig. 1). Cells expressing CaV1.2 channel subunits (α1C/β2a) yielded robust ICa,L as indicated in exemplar traces (Fig. 1A) and population current density vs. voltage (Ipeak–V) plots (Fig. 1D). Co-expressing YFP–Rem dramatically depressed ICa,L amplitude at all voltages (Fig. 1B and D). By contrast, cells co-expressing YFP–Rem265, a truncation mutant lacking the last 32 amino acid residues of Rem, displayed currents indistinguishable in amplitude and voltage-dependence from those recorded in cells expressing CaV1.2 channels alone (Fig. 1C and D). These results reproduce studies documenting the severe impact of RGK GTPases on CaV1.2 channel currents, and the loss of ICa blocking activity incurred upon deleting the distal C-terminus extension of these proteins (Beguin et al. 2001; Finlin et al. 2003; Chen et al. 2005; Yang et al. 2007). Given its inertness in inhibiting ICa,L, we used cells co-expressing Rem265 as controls in subsequent experiments to negate potential confounding artifacts arising from differences in protein co-expression due to transfection of unequal numbers of plasmids (Supplemental material).
Figure 1. Rem inhibits ICa by reducing both channel Po and Qmax.
A, top, exemplar whole-cell currents in a HEK 293 cell expressing recombinant CaV1.2 channel subunits (α1C/β2a). Bottom, exemplar current showing simultaneous isolation of tail (Itail) and gating (Igating) currents using a test pulse depolarization to the reversal potential (+40 mV). B, exemplar currents from cells co-expressing recombinant CaV1.2 channels and YFP–Rem. Same format as for A. Top inset, voltage protocol. C, exemplar currents from cells co-expressing recombinant CaV1.2 channels and YFP–Rem265. Same format as for A. D, population peak current density (Ipeak) versus voltage (V) relationship for cells expressing recombinant CaV1.2 channels (α1C/β2a) alone (grey circles, n= 7 for each point) or co-expressed with either YFP–Rem (triangles, n= 15 for each point) or YFP–Rem265 (squares, n= 22 for each point). Data are means ±s.e.m.E, bar chart showing impact of Rem and Rem265 on Qmax, *P < 0.05 when compared to Rem265 using two-tailed unpaired t test. F, Itail–Qmax scatter plot and regression line for CaV1.2 channels co-expressed with either YFP–Rem265 (squares, slope = 6.98 ± 0.94 pA fC−1, R2= 0.67) or YFP–Rem (triangles, slope = 1.6 ± 0.6 pA fC−1, R2= 0.41). Open symbols are mean data for CaV1.2 +β2a channels alone (circles) and in the presence of either YFP–Rem (triangles) or YFP–Rem265 (squares). Inset, detail of scatter plots and linear fits near the origin.
Two competing hypotheses have been proposed to explain the dramatic inhibition of ICa by RGK GTPases: (1) a reduction in the number of surface channels, and (2) diminishing the activity of surface channels. To gain first-order insights into the potential contribution of the two putative mechanisms to Rem-induced inhibition of ICa, we analysed current waveforms measured at the reversal potential. These records permit simultaneous isolation of gating (Igating) and tail (Itail) currents (Fig. 1A–C, bottom row). The time integral of Igating yields the maximal gating charge (Qmax), a metric for the number of channels with moveable voltage sensors in the membrane (Wei et al. 1994; Takahashi et al. 2004). Moreover, the Itail/Qmax ratio reports on the relative effective open probability (Po), thus providing a useful measure of the electrical activity of surface CaV1.2 channels. Cells expressing Rem displayed a significantly diminished Qmax compared to isochronal control cells (Fig. 1E: for Rem265 control cells, Qmax= 10.1 ± 2.5 fC pF−1, n= 22; for Rem-expressing cells, Qmax= 2.9 ± 1.0 fC pF−1, n= 14, P < 0.05). Linear regression analyses on Itailvs. Qmax scatter plots indicated a shallower slope (suggesting a lower Po) for Rem-expressing cells compared to control (Fig. 1F; slope = 6.98 ± 0.94 pA fC−1 for Rem265 control cells compared to slope = 1.6 ± 0.6 pA fC−1 for Rem-expressing cells). It should be noted that under conditions where the ionic current is markedly inhibited, such as occurs with Rem, Itail measurements are probably contaminated with OFF Igating. When this is taken into account, this would have the effect of depressing the slope of the Itail–Qmax relationship even further. Cells expressing α1C alone, β2a alone or untransfected cells do not display measurable Igating or Itail, ruling out contamination from either endogenous (calcium or non-calcium) channels or capacitive transients as sources of potentially confounding artifacts in our measurements (Supplemental Fig. S1).
The finding that Rem reduces channel Po provides direct electrophysiological evidence for the notion that RGK GTPases reduce the electrical activity of CaV1.2 channels in the plasma membrane. However, the finding that Qmax is also appreciably decreased hinted that Rem may also either reduce the surface density of CaV1.2 channels or immobilize voltage sensors (or a combination of both). To gain further insights into this issue we directly measured the impact of Rem on CaV1.2 channel surface density.
Rem reduces cell surface density of CaV1.2 channels
Several approaches have been used to track plasma membrane targeting of CaV1.2 channels. One method visualizes the sub-cellular localization of GFP-tagged CaV channel α1 subunits by fluorescence microscopy (Harry et al. 2004; Viard et al. 2004). In our hands, this method proved unreliable for identifying surface CaV1.2 channels in HEK 293 cells because α1C–YFP fluorescence was typically present throughout the cell even under conditions where electrical recordings proved the presence of channels in the plasma membrane. Another approach utilizes biotinylation of surface proteins followed by pull-down and Western blot detection of CaV channel α1 subunits (Finlin et al. 2005). Disadvantages of this approach include its low sensitivity and the relative difficulty in obtaining a rigorous quantitative assessment of channel trafficking.
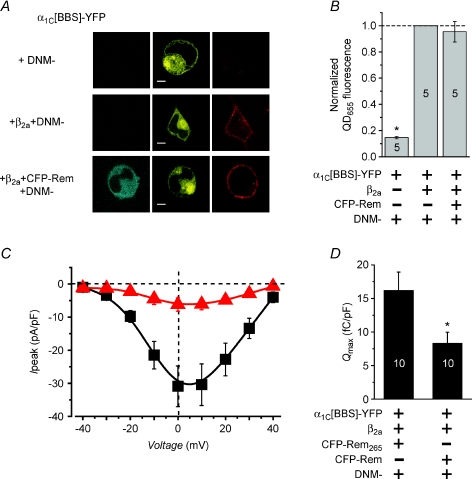
To overcome these limitations, we developed a method similar in principle to assays where surface CaV channels are detected by antibodies against extracellular epitope tags (Altier et al. 2002; Beguin et al. 2005b; Kanevsky & Dascal, 2006). To implement our approach, we introduced a 13-residue high-affinity bungarotoxin (BTX) binding site (BBS) into the extracellular domain II S5–S6 loop in α1C–YFP (Sekine-Aizawa & Huganir, 2004; Green et al. 2007). Surface α1C[BBS]–YFP was detected in non-permeabilized live cells using sequential exposure of cells to biotinylated BTX followed by streptavidin-conjugated quantum dot (QD655) (Fig. 2A). In cells expressing α1C[BBS]–YFP alone, we detected minimal QD655 fluorescence in the plasma membrane (Fig. 2B, top row; Supplemental Fig. S2). By contrast, cells co-expressing α1C[BBS]–YFP + CaVβ2a displayed a robust plasma membrane-localized QD655 fluorescence signal (Fig. 2B, middle row; Supplemental Fig. S2). This recapitulates the well-known role of CaVβs in trafficking CaV1.2 channels to the plasma membrane (Dolphin, 2003). To quantify surface CaV1.2 channels we used flow cytometry which permitted high throughput evaluation of fluorescence signals. The raw data from flow cytometry experiments confirmed a substantial deficit in QD655 fluorescence signal in cells expressing α1C[BBS]–YFP alone (Fig. 2C) compared to those co-expressing α1C[BBS]–YFP + CaVβ2a (Fig. 2D).
Figure 2. Rem reduces surface density of CaV1.2 channels.
A, cartoon showing strategy for detecting cell surface CaV1.2 channels with streptavidin-conjugated quantum dot (QD655). B, top, confocal images of a HEK 293 cell expressing α1C[BBS]–YFP alone. Middle, confocal images of a cell expressing α1C[BBS]–YFP +β2a. Bottom, images of a cell expressing α1C[BBS]–YFP +β2a+ CFP–Rem. The three channels detect CFP, YFP and QD655 fluorescence signals, respectively. Scale bar is 2 μm, here and throughout. C–E, raw data from flow cytometry experiment showing the intensity of QD655versus YFP signals for cells expressing α1C[BBS]–YFP alone (C), α1C[BBS]–YFP +β2a (D) and α1C[BBS]–YFP +β2a+ CFP–Rem (E). 50,000 cells were counted for each condition. The vertical and horizontal lines represent threshold values arbitrarily set based on isochronal experiments using untransfected and single-colour control cells. Each dot represents a single cell. Dots have been arbitrarily colour-coded to facilitate visualization of distinct populations. Loosely, green dots represent α1C[BBS]–YFP-positive cells that lack appreciable trafficking to the membrane (low QD655 signal), while red dots represent α1C[BBS]–YFP-positive cells that display robust CaV1.2 channel trafficking to the surface (high QD655 signal). Grey dots in the bottom left quadrant correspond to untransfected cells. F, bar chart showing normalized mean QD655 fluorescence signals across separate flow cytometry experiments, n= 5 for each condition. Data were normalized to mean QD655 signals from cells expressing α1C[BBS]–YFP +β2a+ CFP–Rem265 in isochronal experiments. *Significantly different (P < 0.05) from α1C[BBS]–YFP +β2a using one-way ANOVA and Bonferroni post hoc means comparisons. G, normalized α1C[BBS]–YFP fluorescence signals in the same analyses window used to compute QD655 signals in F. Data were normalized to mean YFP fluorescence signals from cells expressing α1C[BBS]–YFP +β2a+ CFP–Rem265. H, population Ipeak–V relationship for channels reconstituted with α1C[BBS]–YFP +β2a in the presence of either CFP–Rem265 (black squares, n= 5 for each point) or CFP–Rem (red triangles, n= 5 for each point).
With this method in place, we determined the impact of Rem on surface density of CaV1.2 channels. We used CFP-tagged Rem to permit unambiguous three-colour assessment of channel trafficking in cells co-expressing Rem and α1C[BBS]–YFP + CaVβ2a. HEK 293 cells expressing wild-type Rem have a typical rounded appearance (Fig. 2B, bottom row) as previously observed (Yang et al. 2007). Visual inspection of confocal images hinted that CFP–Rem decreased QD655 staining compared to cells expressing α1C[BBS]–YFP +β2a alone (Fig. 2B, bottom row), an impression confirmed by flow cytometry analyses (Fig. 2E). In five independent experiments, cells co-expressing CFP–Rem displayed a 60% reduction in mean QD655 fluorescence compared to those expressing either α1C[BBS]–YFP + CaVβ2a alone, or α1C[BBS]–YFP + CaVβ2a+ CFP–Rem265 (Fig. 2F; normalized mean QD655 fluorescence = 0.41 ± 0.04, n= 5, for cells expressing Rem compared to those expressing Rem265, P < 0.05). Nevertheless, mean QD655 fluorescence intensity in Rem-expressing cells was still 5-fold greater than in cells expressing α1C[BBS]–YFP alone (Fig. 2F; normalized mean QD655 fluorescence = 0.08 ± 0.004, n= 5, for cells expressing α1C[BBS]–YFP alone compared to those expressing Rem265, P < 0.05) indicating a substantial number of channels at the membrane compared to the no-β condition. These results were not due to differences in α1C[BBS]–YFP transfection efficiency and expression level among the distinct experimental groups since the analyses (here and throughout) were carried out over a range in which α1C[BBS]–YFP fluorescence was similar among the various conditions (Fig. 2G). Channels reconstituted with α1C[BBS]–YFP + CaVβ2a generated currents that were ablated by Rem (Fig. 2H), ruling out any potential confounding artifacts introduced by the extracellular epitope tag.
Dominant negative dynamin reverses Rem-induced decrease in CaV1.2 channel surface density
Rem could decrease CaV1.2 channels surface density by either reducing forward trafficking or enhancing channel retrieval from the plasma membrane. To investigate this, we examined the impact of dominant negative dynamin 1 (DNM–, which features a K44A mutation) on the Rem-mediated decrease in channel surface density. DNM– has been previously shown to block dynamin-dependent endocytosis of CaV1.2 channels (Green et al. 2007). If Rem solely inhibits forward trafficking, then DNM– would not be expected to rescue the surface density of channels since they would never arrive at the plasma membrane in the first place. By contrast, if Rem solely enhances the rate of CaV1.2 channel endocytosis, then DNM– might be expected to restore channel surface density. In cells expressing α1C[BBS]–YFP alone or α1C[BBS]–YFP + CaVβ2a, DNM– had minimal impact on QD655 fluorescence signal (Fig. 3A and B). However, DNM– completely rescued surface expression of CaV1.2 channels in cells expressing CFP–Rem (Fig. 3B), suggesting that Rem reduces surface CaV1.2 channels by enhancing dynamin-dependent endocytosis. Importantly, DNM– did not rescue either ICa,L (Fig. 3C) or Qmax (Fig. 3D) in CFP–Rem-expressing cells despite the complete recovery of channel surface density. The inability of DNM– to fully rescue Qmax suggests that the Rem-mediated decrease in this parameter is mainly accomplished through immobilizing voltage sensors. Furthermore, the failure of DNM– to recover ICa,L suggested that Rem utilizes multiple, functionally redundant mechanisms to block CaV1.2 channels, and raised the question of how the GTPase inhibits channels present at the cell surface. Insights into this question were provided by experiments examining the role of GTP binding and the Rem C-terminus in the mechanism of ICa,L inhibition.
Figure 3. Dominant negative dynamin reverses Rem-induced decrease in CaV1.2 channel surface density.
A, confocal images of cells expressing α1C[BBS]–YFP + DNM– (top row), α1C[BBS]–YFP +β2a+DNM– (middle), and α1C[BBS]–YFP +β2a+ CFP–Rem +DNM– (bottom). B, normalized mean QD655 fluorescence. Data were normalized to mean QD655 signals from α1C[BBS]–YFP +β2a+ DNM– cells in isochronal experiments. *P < 0.05 compared to α1C[BBS]–YFP +β2a+ DNM– using two-tailed unpaired t test. C, population Ipeak–V relationship for channels reconstituted with α1C[BBS]–YFP +β2a+ Rem265+ DNM– (squares, n= 5 for each point) or α1C[BBS]–YFP +β2a+ Rem + DNM– (triangles, n= 5 for each point). D, impact of DNM– on Qmax from cells expressing CaV1.2 in the presence of either Rem265 or Rem, *P < 0.05 when compared to Rem265+ DNM– using two-tailed unpaired t test.
Functional role of the Rem GTP–GDP switch activity in ICa inhibition
Canonically, Ras-like G-proteins function as nucleotide-regulated molecular switches, cycling between inactive GDP-bound and active GTP-bound conformations (Fig. 4A). To investigate whether GTP binding is necessary for Rem regulation of CaV1.2 channels we introduced a point mutation (T94N), analogous to one which locks Ras (S17N) in an inactive GDP-bound state. A caveat of this approach is that the functional impact of the Ras[S17N] mutation may not always extrapolate to other related family members (Feig, 1999). In this regard, it is reassuring that the analogous mutation in the RGK GTPase, Rad, conforms to the data on Ras[S17N] with respect to deficits in GTP binding (Zhu et al. 1995, 1999). YFP–Rem[T94N] markedly inhibited ICa at all voltages compared to isochronal controls (Fig. 4B and C), suggesting that GTP binding is not necessary for this function of Rem. Slope analyses (Itail/Qmax) indicated that Rem[T94N] profoundly decreased channel Po (Fig. 4D). Intriguingly, the whole-cell current waveforms revealed a fundamental qualitative difference between Rem[T94N] and wild-type Rem with respect to the amplitude of Igating and Qmax (Fig. 4B and E). Whereas wild-type Rem markedly decreases Igating and Qmax (Fig. 1), Rem[T94N] diminished ICa without significantly affecting Qmax (Fig. 4E). Flow cytometry analyses indicated that CFP–Rem[T94N] reduced surface density of CaV1.2 channels by 40% compared to CFP–Rem265, and this effect was reversed by DNM– (Fig. 4F).
Figure 4. Role of Rem GTP binding in ICa inhibition.
A, cartoon showing canonical regulation of monomeric GTPases. Proteins cycle between an inactive GDP-bound and an active GTP-bound state. GTP–GDP exchange and GTPase reactions are catalysed by guanine nucleotide exchange factors (GEF) and GTPase activating proteins (GAP), respectively. B, left, exemplar current in control (YFP–Rem265) cells showing simultaneous isolation of Itail and Igating using a test pulse depolarization to Vrev. Right, exemplar Itail and Igating in cells co-expressing recombinant CaV1.2 channels and YFP–Rem[T94N]. C, population Ipeak–V relationships for isochronal cells expressing CaV1.2 channels together with either YFP–Rem265 (squares, n= 8 for each point) or YFP–Rem[T94N] (triangles, n= 10 for each point). Data are means ±s.e.m. D, Itail–Qmax scatter plot for CaV1.2 channels in the presence of either YFP–Rem265 (squares, slope = 8.9 ± 1.9 pA fC−1, R2= 0.65) or YFP–Rem[T94N] (triangles, slope = 1.15 ± 0.53 pA fC−1, R2= 0.23). E, bar chart showing lack of effect of YFP–Rem[T94N] on Qmax. F, normalized mean QD655 fluorescence, *P < 0.05 compared to Rem[T94N]+ DNM– using two-tailed unpaired t test.
The dissonance between the impact of CFP–Rem[T94N] on the surface density of CaV1.2 channels and Qmax was initially surprising and appeared paradoxical. However, examination of the flow cytometry data between cells expressing CFP–Rem versus CFP–Rem265 (Fig. 2D and E) reveals a great deal of overlap and a large variance in the measurements, despite the approximately 2-fold difference in mean QD655 fluorescent intensities. This situation suggested the simple explanation that Qmax measurements may not be sensitive enough to distinguish 2-fold changes in channel surface density because of under-sampling due to the low-throughput nature of the electrophysiological method. To investigate this possibility, we calculated the sample size needed to distinguish mean QD655 values between cells expressing CFP–Rem265 and CFP–Rem[T94N]. In one typical set of experiments, QD655= 3031 ± 5363 (s.d.) for CFP–Rem265, and QD655= 1860 ± 8383 for CFP–Rem[T94N]. The calculated sample size (Origin) required to distinguish these means (95% confidence level; 50%β error level) is 195 for both samples, a number that may be impractical for electrophysiological experiments. Taken together with the finding that wild-type Rem reduces Qmax even when there is a full complement of CaV1.2 channels at the surface (Fig. 3), the results suggest that measured reductions in Qmax (Figs 1E and 3D) principally reflect a capacity of wild-type Rem to immobilize CaV1.2 channel voltage sensors, rather than decrease the number of channels. Importantly, under the assumption that Rem[T94N] has a compromised ability to bind GTP, these results also suggest that the ability to immobilize voltage sensors is a separable function of Rem that requires GTP binding to the NBD.
Functional role of the Rem C-terminus extension
The distal C-terminus of RGK GTPases is a critical determinant of the ability of these proteins to block ICa (Fig. 1) (Finlin et al. 2003; Chen et al. 2005; Correll et al. 2007; Yang et al. 2007). The distal Rem C-terminus contains polybasic and aliphatic residues that target the GTPase to the plasma membrane using electrostatic and hydrophobic interactions (Heo et al. 2006), and it is believed that the importance of the C-terminus to ICa inhibition derives solely from its ability to localize Rem to the plasma membrane (Finlin et al. 2003; Yang et al. 2007).
We tested the functional contribution of the C-terminus to the various effects of Rem by replacing the last 32 residues with PKCγ1 C1 domain. We previously showed that placing the PKCγ1 C1 domain on the N-terminus of YFP–Rem265 generates a molecule, C1PKCγ–YFP–Rem265, which acutely translocates from the cytosol to the plasma membrane in response to phorbol ester, and concomitantly inhibits ICa (Yang et al. 2007). An ambiguity associated with C1PKCγ–YFP–Rem265 is the placement of the membrane targeting module on the N- rather than the C-terminus. In Rem2, the polarity of the membrane targeting module is crucial, and it is, therefore, possible that C1PKCγ–YFP–Rem265 may not accurately reflect how Rem inhibits ICa (Chen et al. 2005). Here, to more closely mimic the native geometry of wild-type Rem, we placed PKCγ C1 on the C-terminus of CFP–Rem265, generating CFP–Rem265–C1PKCγ. When expressed in HEK 293 cells, CFP–Rem265–C1PKCγ was primarily present in the cytosol and nucleus (Fig. 5A, inset). PdBu (1 μm) rapidly translocated CFP–Rem265–C1PKCγ to the plasma membrane (as well as the nucleus membrane) (Fig. 5A) and caused a concomitant rapid inhibition of ICa at all test voltages (Fig. 5A–C). We have previously shown that 1 μm PdBu has no direct impact on recombinant CaV1.2 channels expressed in HEK 293 (Yang et al. 2007). Current waveforms at the Vrev revealed that PdBu significantly inhibited Itail while Qmax was unchanged, consistent with a selective reduction in channel Po with no effect on voltage sensors (Fig. 5A–D). These results agree with our previous findings (Yang et al. 2007). Moreover, membrane translocation of CFP–Rem265–C1PKCγ had no impact on channel surface density at a time when ICa was significantly diminished, consolidating the idea of a sole effect on Po (Fig. 5E and F). Overall, these results establish that membrane targeting of the Rem NBD is sufficient specifically for the capacity to reduce channel Po, and suggest the distal C-terminus may play an active role in the functions of decreasing channel surface density and Qmax.
Figure 5. Role of the Rem C-terminus extension in ICa inhibition.
A, exemplar currents from a cell expressing recombinant CaV1.2 channels and CFP–Rem265–C1PKCγ in the absence (left) or presence (right) of 1 μm PdBu. Inset, confocal images showing that PdBu translocates CFP–Rem265–C1PKCγ from the cytosol to plasma and nuclear membranes. B, diary plot showing time course and relative effects of PdBu (red symbols) on normalized Itail (black squares) and Qmax (black triangles). C, population Ipeak–V plot in cells co-expressing recombinant CaV1.2 channels and CFP–Rem265–C1PKCγ in the absence (black squares, n= 6 for each point) or presence (red circles, n= 6) of 1 μm PdBu. D, bar charts showing the relative effects of PdBu on Itail (left) and Qmax (right). Data are means ±s.e.m. *P < 0.05 compared to control (no PdBu) using two-tailed paired t test. E, confocal images of HEK 293 cells expressing recombinant CaV1.2 channels with either CFP–Rem265 (left) or CFP–Rem265–C1PKCγ (right) in the absence and presence of PdBu. F, normalized mean QD655 fluorescence.
Inhibition of ICa by non-membrane targeted Rem
Though Rem is typically membrane-enriched when expressed in cells, the localization can be highly variable, and a significant fraction of the protein often remains in the cytosol. Does non-membrane-localized Rem inhibit ICa, and if so what mechanisms are used to achieve this effect? A key tool to probe this question is a point mutation in the C-termini of RGK GTPases (corresponding to L271G in Rem), discovered to eliminate autonomous membrane targeting, and instead results in their localization to the nucleus and cytosol (Beguin et al. 2001, 2006). Intriguingly, Rem[L271G] retains the capacity to inhibit ICa (Beguin et al. 2006), providing a unique opportunity to define the mechanism of action of non-membrane targeted Rem species.
We confirmed that when expressed alone, YFP–Rem[L271G] was not detectably membrane-targeted, but instead accumulated in the nucleus and diffusely throughout the cytosol (Fig. 6A). Co-expression of YFP–Rem[L271G] with CaV1.2 channel subunits resulted in a significant suppression of current compared to isochronal controls (Fig. 6B and C). Slope analyses suggested that the remnant currents had a Po similar to that of control cells (Fig. 6D), suggesting that these emanated from unmodified channels on the membrane. It has been previously suggested that the ability of Rem[L271G] to inhibit ICa is due to its ability to sequester CaVβ in the nucleus, thereby rendering the auxiliary subunit unavailable to chaperone α1 subunits to the plasma membrane (Beguin et al. 2006). However, the presence of a significant amount of Rem[L271G] in the cytosol (Fig. 6A) leaves open the possibility that this pool actually represents the active component that inhibits ICa. To resolve this ambiguity we first appended a nuclear localization signal (NLS) (Pusl et al. 2002) to Rem[L271G] to distribute it exclusively in the nucleus. NLS–YFP–Rem[L271G] expressed in HEK 293 cells was localized predominantly in the nucleus, and appears to drag a fraction of co-expressed CFP–β3 into the nuclear compartment (Fig. 6E). Surprisingly, NLS–CFP–Rem[L271G] was completely ineffective in blocking ICa (Fig. 6F–H). This result contradicts the idea that Rem[L271G] inhibits ICa by sequestering CaVβ in the nucleus, and instead suggests the cytosolic pool of the GTPase as the active component. We tested this notion by using a nucleus export signal (NES) (Pusl et al. 2002) to localize Rem[L271G] exclusively in the cytosol. NES–YFP–Rem[L271G] was predominantly localized to the cytosol (Fig. 6I), and essentially ablated ICa when co-expressed with CaV1.2 channels (Fig. 6J–L). Similar to wild-type Rem, NES–YFP–Rem[L271G] significantly suppressed Qmax (Fig. 6M; one-way ANOVA, P < 0.05), Po (Fig. 6L) and CaV1.2 channel surface density (Fig. 6N). With the caveat that these data are derived from a point mutant, these results indicate that when the Rem NBD is paired with its own C-terminus, it does not require membrane targeting to recapitulate any of the three mechanisms. This suggests that the Rem C-terminus module has a bioactive role in CaV1.2 channel inhibition that goes beyond merely targeting the GTPase to the membrane.
Figure 6. Non-membrane targeted Rem derivatives that inhibit ICa.
A, confocal images showing subcellular localization of YFP–Rem[L271G] and CFP–β3. B, exemplar whole-cell currents in a HEK 293 cell expressing recombinant CaV1.2 channels and YFP–Rem[L271G]. C, population Ipeak–V plots for cells expressing recombinant CaV1.2 channels and either YFP–Rem265 (black squares, n= 15 for each point) or YFP–Rem[L271G] (red triangles, n= 9 for each point). Data are means ±s.e.m. D, Itail–Qmax scatter plots and regression lines for CaV1.2 channels co-expressed with either YFP–Rem265 (black squares, slope = 5.73 ± 1.05 pA pF−1, R2= 0.73) or YFP–Rem[L271G] (red triangles, slope = 4.31 ± 1.49 pA fC−1, R2= 0.38). E–H and I–L, data for NLS–YFP–Rem[L271G] (n= 15 for each point) and NES–YFP–Rem[L271G] (n= 15 for each point), respectively. Inset, detail of scatter plots and linear fits near the origin. E–H and I–L, same format as for A–D. For NLS–YFP–Rem[L271G] (slope = 5.48 ± 1.48 pA fC−1, R2= 0.56), and for NES–YFP–Rem[L271G] (slope = 0.08 ± 0.37 pA fC−1). Data for control cell expressing YFP–Rem265 (black squares) is identical to those in C and D. M, bar chart showing the impact of various Rem derivatives on Qmax. *Significantly different (P < 0.05) from Rem265 using one-way ANOVA and Bonferroni post hoc analyses. N, Left, confocal images of a cell expressing α1C[BBS]–YFP +β2a+ NES–CFP–Rem[L271G]. Right, normalized mean QD655 fluorescence. *P < 0.05 compared to control cells expressing α1C[BBS]–YFP +β2a using two-tailed unpaired t test.
Discussion
RGK GTPases are the most potent intracellular inhibitors of CaV1-2 channels discovered to date. We show that one RGK protein, Rem, utilizes at least three distinct mechanisms to powerfully block recombinant ICa,L (Fig. 7). Moreover, the three mechanisms depend on distinct conformations of Rem. Given that Rem does not disrupt the CaV channel α1–β subunit interaction (Supplemental Fig. S3) (Finlin et al. 2006; Beguin et al. 2007), all three mechanisms are probably mediated by a ternary α1–β–Rem complex (Fig. 7). Overall, the results reveal new mechanistic insights into the RGK GTPase–CaV channel crosstalk, and predict new physiological dimensions of this interaction.
Figure 7. Mechanisms underlying Rem inhibition of ICa.
In control, unmodified channels, channels respond to depolarization by undergoing voltage-dependent closed–closed (C–C) transitions during which the voltage sensors move, followed by a voltage-independent conformational change that opens the channel (C–O transition). Rem constitutes a ‘triple threat’ to CaV1.2 channels, inhibiting ICa by three distinct, functionally redundant mechanisms that rely on different configurations of the GTPase. All three mechanisms are assumed to rely on the formation of a ternary α1–β–Rem complex since Rem does not disrupt the CaV channel α1–β subunit interaction. (I) Rem reduces surface density of CaV1.2 channels by enhancing dynamin-dependent endocytosis. This mechanism requires the Rem C-terminus and nucleotide-binding domain, but is GTP-independent; (II) Rem diminishes Po without affecting voltage sensor movement (C–C transitions are unaffected, while forward equilibrium for the C–O transition is reduced). Membrane targeting of the NBD by either the C-terminus or a generic membrane-targeting module is sufficient for this effect, whereas GTP binding to the NBD is not necessary (represented as a red hexagon). (III) Rem immobilizes CaV1.2 channel voltage sensors (forward equilibria for C–C transitions are reduced). This mechanism requires the C-terminus and GTP bound to the nucleotide-binding domain of Rem (represented by green hexagon).
Multiple mechanisms underlie Rem inhibition of recombinant CaV1.2 channels
The mechanism by which RGK proteins inhibit ICa has been intensely studied by several groups. One debate has focused on whether inhibition occurs exclusively via either reducing CaV channel surface density or by silencing channels at the plasma membrane. In their seminal work, Beguin et al. (2001) suggested from immuno-fluorescence experiments that Gem inhibited ICa by interfering with the trafficking of recombinant CaV1.2 channels to the cell surface. Later, this work was refined by using extracellular epitope-tagged CaV1.2 channels, and extended to all RGK proteins (Beguin et al. 2005a,b, 2006). A limitation of these previous reports concerns the non-quantitative nature of the analyses of channel surface density. Specifically, the data were presented as exemplar confocal images that showed either robust or no cell surface CaV1.2 channels in the absence and presence of RGK GTPases, respectively. This binary yes–no presentation of the data suggested that RGK GTPase-mediated inhibition of ICa was wholly accounted for by eliminating surface channels. Our results make three important contributions to this debate. First, we provide critical support to the notion that Rem reduces surface expression of CaV1.2 channels in HEK 293 cells. However, quantitative analysis indicates that a significant number of channels remain on the surface when compared to cells expressing CaV1.2 α1C alone (no CaVβ). Second, we show that Rem decreases surface CaV1.2 channels by enhancing dynamin-dependent endocytosis rather than by reducing forward trafficking of α1C to the membrane. Third, we find that even when Rem-induced decrease in channel surface density is completely reversed by DNM–, this does not result in rescue of ICa,L.
In contrast to our observations, Ikeda and colleagues found that Rem2 inhibits CaV2.2 channels in tsA201 cells without decreasing channel surface density assessed by radio-labelled ω-conotoxin GVIA binding assays (Chen et al. 2005). Similarly, Rem2 inhibited ICa,L in mouse insulinoma MIN6 cells without reducing endogenous CaV1.2 channels at the membrane, as measured in surface biotinylation/Western blot experiments (Finlin et al. 2005). Several possibilities could underlie the observed differences. First, the ability to promote endocytosis could be a selective function of distinct members of the RGK GTPase family. Second, important determinants for endocytosis could reside in the CaVα1 subunit, thus rendering the phenomenon specific for particular CaV channel subtypes. Finally, there could be genuine differences among distinct cell types in their capacity to support this mode of CaV channel regulation by RGK GTPases.
It has been inferred that RGK proteins inhibit ICa by decreasing electrical activity of surface CaV1.2 channels (Chen et al. 2005; Finlin et al. 2005). However, the mechanism(s) by which this occurs is unknown. Our results indicate that Rem can reduce the Po of surface CaV1.2 channels in two ways: (1) by interfering with channel opening without any impact on voltage sensors, and (2) by immobilizing CaV1.2 channel voltage sensors. How does Rem inhibit channel opening without affecting voltage sensors? While our experiments do not directly resolve this question, an important clue is provided by the finding that membrane targeting of the Rem NBD is sufficient for this effect. One possibility is that membrane-targeted Rem, via its interaction with CaVβ, tugs on the intracellular CaV1.2 I–II loop in a manner that favours closing of the channel pore. The finding that Rem can inhibit CaV1.2 channel voltage sensors is novel. Previously, Gem and Rem were found to significantly reduce gating currents in heart and skeletal myocytes, respectively (Murata et al. 2004; Bannister et al. 2008). However, it was unresolved whether RGK GTPase-mediated reduction in gating current was due to a loss of surface membrane channels or to immobilization of voltage sensors. Here, we show that in the presence of DNM– Rem can inhibit Qmax without reducing CaV1.2 channel surface density, explicitly demonstrating the capacity to immobilize voltage sensors. As each CaV channel has four voltage sensors that need to move in order for the channel to open, Rem need not block all the gating charge per channel in order to effectively block current. This may explain the observation that the impact of Rem on Qmax is typically partial (50–60% inhibition) rather than complete. It will be important to determine in future experiments how Rem immobilizes CaV1.2 channel voltage sensors.
Overall, these results are the first to demonstrate multiple mechanisms contributing to RGK GTPase inhibition of ICa co-existing within a single experimental system. This perspective helps reconcile the previous disparate views and suggests a unifying framework for understanding Rem action in different experimental settings: Rem can inhibit CaV1.2 channels in at least three distinct ways, and different cell types may utilize various combinations of the mechanisms to achieve effective ICa block.
Structure–function of RGK GTPases
The discovery that the three distinct mechanisms of ICa inhibition rely on different configurations of Rem provides new insights into the structure–function of RGK GTPases that congregate around two areas: (1) the role of GTP binding, and (2) the role of the Rem C-terminus.
There has been considerable ambiguity in defining whether GTP binding is important for RGK GTPase inhibition of ICa. The typical approach has been to investigate the functional impact of mutations predicted to lock RGK proteins in a GDP-bound state. In superior cervical ganglion neurons, Gem[S89N] was ineffective in blocking ICa (Ward et al. 2004). However, in the same preparation, the equivalent Rem2 mutant (S129N) potently inhibited ICa (Chen et al. 2005). Moreover, Rad[S105N] has been reported to be ineffective in inhibiting recombinant CaV1.2 channels expressed in HEK 293 cells, and in fact acts as a dominant negative suppressor of wild-type Rad when expressed in heart cells (Yada et al. 2007). Hence, RGK GTPases may not be equivalent in the requirement for GTP binding to inhibit ICa. Here, we demonstrate that for Rem, GTP binding is specifically required for the ability to immobilize voltage sensors. Nevertheless, Rem[T94N] still potently inhibited ICa because of the redundancy in mechanisms available to block CaV1.2 channels. A caveat for these experiments and our interpretation is they are based on the assumption that Rem[T94N] is deficient in binding GTP. However, this has not been definitively shown to date, and will be an important goal for future experiments.
A surprising result from our experiments concerned discovery of a possible bioactive role for the Rem distal C-terminus. Previous experiments firmly established that deleting the distal C-terminus extension in RGK GTPases disrupts their ability to inhibit ICa (Finlin et al. 2003; Chen et al. 2005; Yang et al. 2007). This effect has been ascribed specifically to the role of the distal C-terminus as a plasma membrane-targeting module (Chen et al. 2005; Correll et al. 2007; Yang et al. 2007). However, we find that replacing the Rem C-terminus with an inducible membrane-targeting module selectively recapitulates only the reduced Po function. Moreover, we explicitly show that NES–Rem[L271G] effectively blocks ICa even though it does not autonomously target to the plasma membrane. Overall, the results show that the requirement for membrane targeting of Rem to inhibit ICa is context-dependent, depending on the presence or absence of the Rem C-terminus. Therefore, the Rem C-terminus plays an active role in the ability of the GTPase to inhibit ICa that goes beyond its task as a membrane targeting motif.
Physiological implications
The finding that Rem uses multiple mechanisms to inhibit CaV1.2 channels significantly enriches the potential physiological dimensions of this crosstalk. Under conditions in which the Rem-mediated endocytosis is prevalent, the GTPase would be expected to exert a chronic influence on Ca2+ signalling by constitutively down-regulating the number of cell surface channels. By contrast, in circumstances where decreased channel Po is predominant, the number of channels would not change while Ca2+ influx would be severely compromised. Nevertheless, surface channels may gate normally with respect to voltage-dependent movement of the voltage sensors. An interesting consequence of this is that in this regime, Rem would be expected to discriminate between physiological responses that relied on Ca2+ influx via CaV1 channels (such as cardiac EC coupling) versus those that relied on voltage-dependent CaV1 channel gating (skeletal EC coupling). It will be interesting to determine whether this prediction is met in conditions where RGK GTPase expression is elevated in skeletal muscle such as occurs in some cases of diabetes (Reynet & Kahn, 1993). The finding that the distinct actions of Rem on ICa depend on different GTPase conformations raises the possibility that the relative prevalence of the different inhibition modes may be regulated by GTPase activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs) in different cells. There is a paucity of data on GAPs and GEFs for RGK GTPases, although the tumour suppressor nm23 has been shown to be a specific GAP for Rad, and a GEF for Rad and Gem (Zhu et al. 1996). Our work motivates the search for new GAPs and GEFs for the RGK GTPase protein family.
Perspectives for developing novel CaV channel blockers
Blocking CaV1-2 channels is a vital or potential therapy for hypertension, angina, neuropathic pain, arrhythmias and stroke (Valentino et al. 1993; Kochegarov, 2003; Triggle, 2003; Murata et al. 2004; Staats et al. 2004). Presently, most small molecule modulators of CaV channels act by interacting with pore-forming α1 subunits. However, auxiliary CaV channel subunits potentially represent a rich target for developing novel therapeutics. As CaVβs are obligatory for the trafficking and functional maturation of CaV channels, they have long been considered as a potentially effective target site for developing novel small molecule modulators of ICa (Catterall, 1998; Young et al. 1998). The potent inhibition of ICa by RGK proteins offers a resounding validation of this idea. Interestingly, a chemically synthesized derivative of rapamycin was recently found to bind CaVβ subunits and inhibit CaV channels (Ruan et al. 2008). The mechanisms by which Rem inhibits CaV1.2 channels suggest that such small molecules need not act by simply disrupting the CaV channel α1–β subunit interaction, as previously envisioned (Catterall, 1998; Young et al. 1998). A potential limitation with the strategy of inhibiting CaV channels by targeting auxiliary β subunits is the issue of whether such agents can be designed to display selectivity for distinct CaV channel types. Recently, we showed that an engineered Rem derivative, C1PKCγ–YFP–Rem265, that inhibits ICa when inducibly translocated to the plasma membrane was selective for CaV2.2 and CaV1.2, but not CaV2.1 or CaV2.3 channels (Yang et al. 2007). Hence, it may be possible to engineer selectivity for distinct CaV channels into agents that inhibit ICa by targeting CaVβs. Discovering the design principles that will endow selectivity to these agents is an important challenge for the future.
Acknowledgments
The authors thank Eun Sook Park for technical assistance; Dr Doug Andres (University of Kentucky) for wild-type Rem cDNA; Dr Ricardo Dolmetsch (University of Stanford) for DNM– cDNA; Hsiang-Jer Tseng, Hojjat Bazzazi and Akil Puckerin for their contributions to the development of the QD655 labelling technique. This work was supported by grants from the National Institutes of Health (RO1 HL069911 and RO1 HL084332) to H.M.C. H.M.C. is an Established Investigator of the American Heart Association.
Glossary
Abbreviations
- BTX
bungarotoxin
- BBS
bungarotoxin binding site
- CaV channel
voltage-dependent calcium channel
- DNM–
dominant negative dynamin 1
- CFP
cyan fluorescent protein
- GFP
green fluorescent protein
- ICa,L
voltage-dependent L-type calcium current
- Qmax
maximum gating charge
- RGK GTPase
Rad/Rem/Rem2/Gem/Kir subfamily of Ras-like GTPases
- YFP
yellow fluorescent protein
- NBD
nucleotide-binding domain
- NES
nuclear export signal
- NLS
nuclear localization signal
Author contributions
T.Y. designed and conducted experiments in all areas of the work, analysed results, and helped write the paper; X.X. conducted electrophysiological experiments on Rem[T94N]; T.K. made cDNA constructs and conducted flow cytometry experiments; V.W. generated and conducted electrophysiological experiments on CFP–Rem265–C1PKCγ; H.M.C. conceived of and designed experiments, analysed results, and wrote the paper. All authors approved the final version.
Supplemental material
SUPPLEMENTAL MATERIAL – FIGURE 1
SUPPLEMENTAL MATERIAL – FIGURE 2
SUPPLEMENTAL MATERIAL – FIGURE 3
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Altier C, Dubel SJ, Barrere C, Jarvis SE, Stotz SC, Spaetgens RL, et al. Trafficking of L-type calcium channels mediated by the postsynaptic scaffolding protein AKAP79. J Biol Chem. 2002;277:33598–33603. doi: 10.1074/jbc.M202476200. [DOI] [PubMed] [Google Scholar]
- Bannister RA, Colecraft HM, Beam KG. Rem inhibits skeletal muscle EC coupling by reducing the number of functional L-type Ca2+ channels. Biophys J. 2008;94:2631–2638. doi: 10.1529/biophysj.107.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Ikeda H, Yamada Y, et al. Nuclear sequestration of β-subunits by Rad and Rem is controlled by 14-3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J Mol Biol. 2006;355:34–46. doi: 10.1016/j.jmb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Kuwamura N, Yamada Y, et al. Roles of 14-3-3 and calmodulin binding in subcellular localization and function of the small G-protein Rem2. Biochem J. 2005a;390:67–75. doi: 10.1042/BJ20050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Takahashi A, Yamada Y, et al. 14-3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J Cell Sci. 2005b;118:1923–1934. doi: 10.1242/jcs.02321. [DOI] [PubMed] [Google Scholar]
- Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, et al. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- Beguin P, Ng YJ, Krause C, Mahalakshmi RN, Ng MY, Hunziker W. RGK small GTP-binding proteins interact with the nucleotide kinase domain of Ca2+-channel β-subunits via an uncommon effector binding domain. J Biol Chem. 2007;282:11509–11520. doi: 10.1074/jbc.M606423200. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium cycling and signalling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Yeasty brew yields novel calcium channel inhibitor. Nat Biotechnol. 1998;16:906. doi: 10.1038/nbt1098-906. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Chang L, Zhang J, Tseng YH, Xie CQ, Ilany J, Bruning JC, et al. Rad GTPase deficiency leads to cardiac hypertrophy. Circulation. 2007;116:2976–2983. doi: 10.1161/CIRCULATIONAHA.107.707257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Puhl HL, 3rd, Niu SL, Mitchell DC, Ikeda SR. Expression of Rem2, an RGK family small GTPase, reduces N-type calcium current without affecting channel surface density. J Neurosci. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll RN, Pang C, Finlin BS, Dailey AM, Satin J, Andres DA. Plasma membrane targeting is essential for Rem-mediated Ca2+ channel inhibition. J Biol Chem. 2007;282:28431–28440. doi: 10.1074/jbc.M706176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA. The RGK family of GTP-binding proteins: regulators of voltage-dependent calcium channels and cytoskeleton remodelling. Cell Signal. 2008;20:292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. β Subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- Feig LA. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1:E25–E27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- Finlin BS, Andres DA. Rem is a new member of the Rad- and Gem/Kir Ras-related GTP-binding protein family repressed by lipopolysaccharide stimulation. J Biol Chem. 1997;272:21982–21988. doi: 10.1074/jbc.272.35.21982. [DOI] [PubMed] [Google Scholar]
- Finlin BS, Correll RN, Pang C, Crump SM, Satin J, Andres DA. Analysis of the complex between Ca2+ channel β-subunit and the Rem GTPase. J Biol Chem. 2006;281:23557–23566. doi: 10.1074/jbc.M604867200. [DOI] [PubMed] [Google Scholar]
- Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci U S A. 2003;100:14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlin BS, Mosley AL, Crump SM, Correll RN, Ozcan S, Satin J, Andres DA. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem. 2005;280:41864–41871. doi: 10.1074/jbc.M414261200. [DOI] [PubMed] [Google Scholar]
- Finlin BS, Shao H, Kadono-Okuda K, Guo N, Andres DA. Rem2, a new member of the Rem/Rad/Gem/Kir family of Ras-related GTPases. Biochem J. 2000;347:223–231. [PMC free article] [PubMed] [Google Scholar]
- Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE. The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel CaV1.2. Neuron. 2007;55:615–632. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry JB, Kobrinsky E, Abernethy DR, Soldatov NM. New short splice variants of the human cardiac Cavβ2 subunit: redefining the major functional motifs implemented in modulation of the Cav1.2 channel. J Biol Chem. 2004;279:46367–46372. doi: 10.1074/jbc.M409523200. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Kanatous SB, Martin CM, Goetsch SC, Garry DJ. Rad is temporally regulated within myogenic progenitor cells during skeletal muscle regeneration. Am J Physiol Cell Physiol. 2006;290:C379–C387. doi: 10.1152/ajpcell.00270.2005. [DOI] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanevsky N, Dascal N. Regulation of maximal open probability is a separable function of Cavβ subunit in L-type Ca2+ channel, dependent on NH2 terminus of alpha1C (Cav1.2α) J Gen Physiol. 2006;128:15–36. doi: 10.1085/jgp.200609485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochegarov AA. Pharmacological modulators of voltage-gated calcium channels and their therapeutical application. Cell Calcium. 2003;33:145–162. doi: 10.1016/s0143-4160(02)00239-7. [DOI] [PubMed] [Google Scholar]
- Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K. Gem: an induced, immediate early protein belonging to the Ras family. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- Murata M, Cingolani E, McDonald AD, Donahue JK, Marban E. Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circ Res. 2004;95:398–405. doi: 10.1161/01.RES.0000138449.85324.c5. [DOI] [PubMed] [Google Scholar]
- Oancea E, Teruel MN, Quest AF, Meyer T. Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signalling in living cells. J Cell Biol. 1998;140:485–498. doi: 10.1083/jcb.140.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, et al. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, et al. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002;277:27517–27527. doi: 10.1074/jbc.M203002200. [DOI] [PubMed] [Google Scholar]
- Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- Ruan B, Pong K, Jow F, Bowlby M, Crozier RA, et al. Binding of rapamycin analogs to calcium channels and FKBP52 contributes to their neuroprotective activities. Proc Natl Acad Sci U S A. 2008;105:33–38. doi: 10.1073/pnas.0710424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine-Aizawa Y, Huganir RL. Imaging of receptor trafficking by using α-bungarotoxin-binding-site-tagged receptors. Proc Natl Acad Sci U S A. 2004;101:17114–17119. doi: 10.1073/pnas.0407563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- Takahashi SX, Miriyala J, Colecraft HM. Membrane-associated guanylate kinase-like properties of β-subunits required for modulation of voltage-dependent Ca2+ channels. Proc Natl Acad Sci U S A. 2004;101:7193–7198. doi: 10.1073/pnas.0306665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan FL, Moravec CS, Li J, Apperson-Hansen C, McCarthy PM, Young JB, Bond M. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci U S A. 2002;99:11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle DJ. Drug targets in the voltage-gated calcium channel family: why some are and some are not. Assay Drug Dev Technol. 2003;1:719–733. doi: 10.1089/154065803770381075. [DOI] [PubMed] [Google Scholar]
- Valentino K, Newcomb R, Gadbois T, Singh T, Bowersox S, Bitner S, et al. A selective N-type calcium channel antagonist protects against neuronal loss after global cerebral ischemia. Proc Natl Acad Sci U S A. 1993;90:7894–7897. doi: 10.1073/pnas.90.16.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- Ward Y, Spinelli B, Quon MJ, Chen H, Ikeda SR, Kelly K. Phosphorylation of critical serine residues in Gem separates cytoskeletal reorganization from down-regulation of calcium channel activity. Mol Cell Biol. 2004;24:651–661. doi: 10.1128/MCB.24.2.651-661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Neely A, Lacerda AE, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J Biol Chem. 1994;269:1635–1640. [PubMed] [Google Scholar]
- Xu X, Colecraft HM. Engineering proteins for custom inhibition of CaV channels. Physiology (Bethesda) 2009;24:210–218. doi: 10.1152/physiol.00010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada H, Murata M, Shimoda K, Yuasa S, Kawaguchi H, Ieda M, et al. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res. 2007;101:69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Endocr Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- Yang T, Suhail Y, Dalton S, Kernan T, Colecraft HM. Genetically encoded molecules for inducibly inactivating CaV channels. Nat Chem Biol. 2007;3:795–804. doi: 10.1038/nchembio.2007.42. [DOI] [PubMed] [Google Scholar]
- Young K, Lin S, Sun L, Lee E, Modi M, Hellings S, et al. Identification of a calcium channel modulator using a high throughput yeast two-hybrid screen. Nat Biotechnol. 1998;16:946–950. doi: 10.1038/nbt1098-946. [DOI] [PubMed] [Google Scholar]
- Zhu J, Bilan PJ, Moyers JS, Antonetti DA, Kahn CR. Rad, a novel Ras-related GTPase, interacts with skeletal muscle β-tropomyosin. J Biol Chem. 1996;271:768–773. doi: 10.1074/jbc.271.2.768. [DOI] [PubMed] [Google Scholar]
- Zhu J, Reynet C, Caldwell JS, Kahn CR. Characterization of Rad, a new member of Ras/GTPase superfamily, and its regulation by a unique GTPase-activating protein (GAP)-like activity. J Biol Chem. 1995;270:4805–4812. doi: 10.1074/jbc.270.9.4805. [DOI] [PubMed] [Google Scholar]
- Zhu J, Tseng YH, Kantor JD, Rhodes CJ, Zetter BR, Moyers JS, Kahn CR. Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci U S A. 1999;96:14911–14918. doi: 10.1073/pnas.96.26.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.