Abstract
Lysophosphatidic acid (LPA) G-protein-coupled receptors (GPCRs) play important roles in a variety of physiological and pathophysiological processes, including cell proliferation, angiogenesis, central nervous system development and carcinogenesis. Whilst many ion channels and transporters are recognized to be controlled by a change in cell membrane potential, little is known about the voltage dependence of other proteins involved in cell signalling. Here, we show that the InsP3-mediated Ca2+ response stimulated by the endogenous LPA GPCR in Xenopus oocytes is potentiated by membrane depolarization. Depolarization was able to repetitively stimulate transient [Ca2+]i increases after the initial agonist-evoked response. In addition, the initial rate and amplitude of the LPA-dependent Ca2+ response were significantly modulated by the steady holding potential over the physiological range, such that the response to LPA was potentiated at depolarized potentials and inhibited at hyperpolarized potentials. Enhancement of LPA receptor-evoked Ca2+ mobilization by membrane depolarization was observed over a wide range of agonist concentrations. Importantly, the amplitude of the depolarization-evoked intracellular Ca2+ increase displayed an inverse relationship with agonist concentration such that the greatest effect of voltage was observed at near-threshold levels of agonist. Voltage-dependent Ca2+ release was not induced by direct elevation of InsP3 or by activation of heterotrimeric G-proteins in the absence of agonist, indicating that the LPA GPCR itself represents the primary site of action of membrane voltage. This novel modulation of LPA signalling by membrane potential may have important consequences for control of Ca2+ signals both in excitable and non-excitable tissues.
Introduction
G-protein-coupled receptors (GPCRs) are the largest family of cell surface proteins and play key roles in the activation of virtually all cell types (Pierce et al. 2002). Although GPCRs are not normally considered to be sensitive to changes in the cell membrane potential, evidence is emerging to support this concept in both non-excitable and excitable tissues (Marty & Tan, 1989; Ganitkevich & Isenberg, 1993; Mahaut-Smith et al. 1999; Ben Chaim et al. 2003; Martinez-Pinna et al. 2005; Billups et al. 2006; Liu et al. 2009; reviewed in Mahaut-Smith et al. 2008). The most extensively studied example of this phenomenon is the bipolar voltage control of P2Y1 receptor-evoked Ca2+ release in the rat megakaryocyte, a non-excitable cell type lacking voltage-operated Ca2+ channels or ryanodine receptors (Mahaut-Smith et al. 2008). Evidence suggests that voltage is able to modulate InsP3-dependent Ca2+ release primarily by acting at the level of the P2Y receptor rather than directly regulating either associated G-proteins or downstream signals (Martinez-Pinna et al. 2005). This conclusion is also supported by observations of voltage-dependent charge movements, analogous to the gating currents of ion channels, in muscarinic (m2 and m1) GPCRs, expressed in Xenopus oocytes (Ben Chaim et al. 2006). Voltage control of GPCR signalling represents an important mechanism whereby physiological voltage waveforms (Martinez-Pinna et al. 2004) can modulate cellular signals initiated by chemical stimuli. Given the ubiquitous role of GPCRs in cellular physiology, an important question is the extent to which other receptors within this family of surface proteins are also sensitive to changes in the transmembrane potential. In the present study we have addressed this question for GPCRs stimulated by the agonist lysophosphatidic acid (LPA), which play important roles in a variety of physiological processes such as embryogenesis, vascular development and neurogenesis and pathophysiological processes such inflammation, pain, neurodegenerative diseases and cancer (Moolenaar et al. 2004; Ishii et al. 2004; Noguchi et al. 2009). LPA receptors are expressed in major excitable tissues such as the heart and the brain where they will be exposed to rapid changes in membrane potential during synaptic inputs and action potential firing and thus where voltage dependence may significantly influence receptor function.
Although classically used for studying heterologously expressed proteins (Miledi et al. 1989), Xenopus laevis oocytes possess a plethora of native membrane proteins, including LPA GPCRs, that can be easily studied (Kusano et al. 1977). In particular, many properties of the LPA GPCRs and characteristics of their signalling pathways were first derived from studies using oocytes (Fernhout et al. 1992; Tigyi & Miledi, 1992). Furthermore, LPA receptors in Xenopus oocytes show significant homology (∼89% identity) to mammalian LPA1 receptors (Kimura et al. 2001). Therefore, we consider the Xenopus oocyte as a good model to study the voltage dependence of LPA signals in a native environment. Activation of LPA GPCRs triggers several intracellular signalling pathways, leading to a variety of biologically important cellular responses. Many of these actions of lpa are mediated through InsP3-dependent Ca2+ release from the intracellular stores, which induces an [Ca2+]i increase. We now report that, during stimulation of native oocyte GPCRs by a wide range of LPA concentrations, membrane depolarization directly enhances the activity of these receptors, resulting in increased receptor-evoked [Ca2+]i, due to InsP3-dependent Ca2+ release from intracellular stores.
Methods
Oocyte preparation and microinjection
Animal handling was carried out in accordance with the guidelines for care and use of laboratory animals adopted by the EU and with the standards of The Journal of Physiology (Drummond, 2009). Twenty adult female Xenopus laevis (purchased from Biological Blades, Edenbridge, Kent, UK) frogs were fully anaesthetized by immersion in cold 0.17% MS-222 for 15 min and a piece of ovary was aseptically removed. Fully grown immature oocytes were isolated from the ovary and their surrounding layers removed manually, as previously described (Ivorra & Morales, 1997). Cells were kept at 15–16°C in a modified Barth's solution (mm): 88 NaCl, 1 KCl, 2.40 NaHCO3, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4, 10 Hepes, pH 7.4, supplemented with penicillin (100 units ml−1) and streptomycin (0.1 mg ml−1) until used for recordings. Oocytes were intracellularly microinjected with 50 nl of the fluorescent Ca2+ indicator K5-Fluo5 (1 mm, final oocyte concentration 50 μm) at least 1 h before the recording, using a nanolitre injector (Nanoliter 2000, WPI, Stevenage, UK). InsP3 was injected into the oocyte from a micropipette containing 1 mm InsP3 using a pneumatic pressure ejection system (Picospritzer, General Valve, Fairfield, NJ, USA) with pulses of 35 p.s.i. and 30 ms duration. The volume of InsP3 injected was ∼500, estimated from the diameter of the droplet expelled with the pipette tip in air, and would result in an intracellular concentration of ∼500 nm, assuming even distribution throughout the oocyte.
Solutions
Normal frog Ringer solution contained (mm): 115 NaCl, 2 KCl, 1.8 CaCl2, 5 Hepes, pH 7.0. A few minutes after the beginning of the experiment the oocytes were usually superfused with Ca2+-free external solution (mm): 115 NaCl, 2 KCl, 1.8 MgCl2, 5 Hepes, 0.5 EGTA, pH 7.0 (see Results). Drugs were diluted in the appropriate external solution and applied by superfusing the oocyte at a flow rate of 7–10 ml min−1. Cells were exposed to AlF4− by the addition of 10 mm NaF and 200 μm AlCl3 to the external saline. K5-Fluo5 was purchased from Invitrogen (Eugene, OR, USA), InsP3, stocked in aqueous solution including 50 μm EDTA and 5 mm Hepes at pH 7.0, from Calbiochem (San Diego, CA, USA) and the rest of reagents from either Sigma (St Louis, MO, USA) or Sharlau Chemie SA (Barcelona, Spain). Oocytes were kept in the dark during incubation with U-73122 or U-73343 for the periods shown in Fig. 4B.
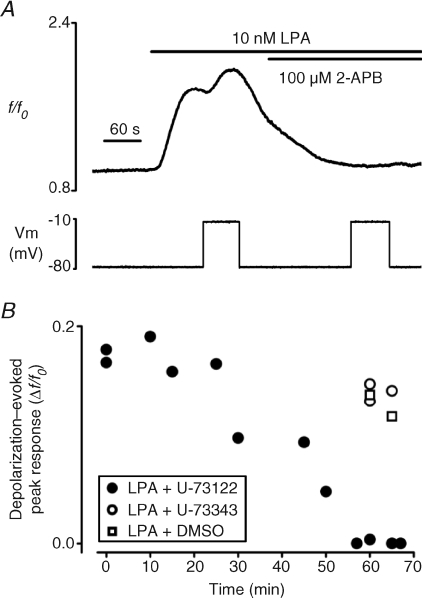
Figure 4. Functional InsP3 receptors and phospholipase-C activation are required for the voltage control of Ca2+ release during LPA receptor stimulation.
A, effect of depolarization (−80 to −10 mV) on [Ca2+]i in the presence of 10 nm LPA alone (first voltage step) or following co-application of 100 μm 2-APB, an inhibitor of InsP3 receptors (second voltage step). B, amplitude of the depolarization-evoked Ca2+ response (−80 to −10 mV, 60 s) in the presence of LPA after incubation with the phospholipase C inhibitor U-73122 (10 μm, filled circles), for different durations or following exposure to either its inactive analogue U-73343 (10 μm, open circles) or to DMSO at the same concentration used to dissolve these drugs (1/100 dilution, open triangles), for the duration required to observe the maximal effect with U-73122 (60–65 min). All experiments were performed in Ca2+-free external solution. Each point represents a different cell.
Electrophysiological recordings
Membrane current recordings were performed at 21–25°C, 1–10 h after K5-Fluo5 injection, using a high-compliance two-microelectrode voltage-clamp system (TurboTEC-10CD npi, Tamm, Germany). Intracellular electrodes (1–3 MΩ) were filled with 3 m KCl and 3 m potassium acetate for voltage recording and current injection, respectively. Oocytes were placed in a 150 μl recording chamber that was continuously superfused with the appropriate external solution. The membrane potential was held at −80 mV and voltage steps to −10 mV were applied for a duration of 60 s, unless otherwise stated. Membrane currents were low-pass filtered at 30–200 Hz and recorded on a PC, after sampling (Digidata 1200, Molecular Devices, Sunnyvale, CA, USA) at fivefold the filter frequency, using the WCP v.3.2.8 package developed by J. Dempster (Strathclyde Electrophysiology Software, University of Strathclyde, UK).
Fluorescence recordings
Fluorescence measurements were conducted using an epifluorescence system coupled to a stereomicroscope MZ12 (Leica Microsystems AG, Wetzlar, Germany). Light from a mercury arc lamp (LEJ, Jena, Germany) passed through a 470/40 nm bandpass filter to excite the fluorophore. Emitted light (>515 nm) was selected using a dichroic mirror and monitored without further filtering by a photomultiplier tube (Hamamatsu Photonics, Tokyo, Japan). Fluorescence signals were acquired using WCP v.3.2.8 software, sampled at 30–100 Hz and exported for analysis with Origin (OriginLab Corp., Northampton, MA, USA). For presentation, traces were filtered using 5–20 point averaging. LPA or voltage steps were considered to induce a response when the fluorescence changed by ≥3% from the prestimulus level. Fluo-5 fluorescence signals are expressed as ratios (f/f0), where f0 is the basal fluorescence level prior to the stimulus (agonist or depolarization). The amplitude of responses to different stimuli was measured as the peak change in the f/f0 response (Δf/f0). Experiments used oocytes with a similar baseline fluorescence level (f0), indicating a constant intracellular concentration of K5-Fluo5. To minimize variations due to hemispheric asymmetry in Ca2+ responses mediated through receptors coupled to InsP3-dependent Ca2+ release (Miledi & Parker, 1989) and also to avoid light absorption by pigment in the animal hemisphere, oocytes were positioned such that the vegetal (translucent) hemisphere faced the light source.
Data analysis
Averaged data are expressed as the mean ±s.e.m. and statistical difference was tested using Student's unpaired t test. A level of P < 0.05 was considered significant.
Results
Voltage-dependent [Ca2+]i modulation during LPA receptor stimulation
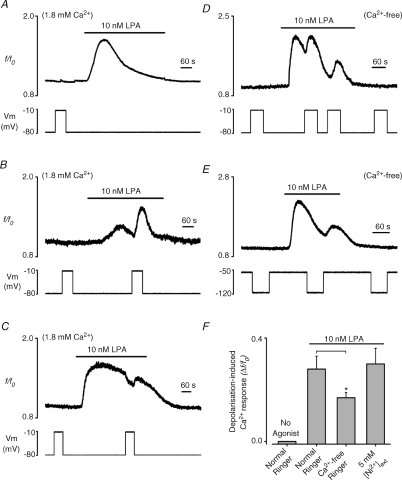
In oocytes perfused with normal Ringer solution, depolarization from a holding potential of −80 mV to −10 mV had no effect on [Ca2+]i (n= 20; Fig. 1A–C and F), consistent with a lack of voltage-dependent Ca2+ influx over this range of potentials. Thus, although voltage-gated Ca2+ channels are occasionally observed in Xenopus oocytes (Miledi, 1982), their contribution to the Ca2+ responses in the oocytes used in the present study was negligible. At a constant holding potential of −80 mV, application of 10 nm LPA evoked a transient increase in [Ca2+]i (peak = 0.66 ± 0.08 Δf/f0, n= 20; Fig. 1A), that slowly decayed to basal levels in the continued presence of agonist. As can be seen by comparing Fig. 1A–C, the magnitude and rate of decay of the Ca2+ response to 10 nm LPA displayed significant intercellular heterogeneity, but in all cells consisted of a single peak, which then decayed at a variable rate. In contrast to the unstimulated condition, depolarization to −10 mV during the LPA response evoked a marked, transient [Ca2+]i increase in 75% of the oocytes tested (16 out of 20; peak 0.30 ± 0.05 Δf/f0, Fig. 1B and F) and a small [Ca2+]i decrease in the remaining 25% (4 out of 20; peak −0.09 ± 0.02 Δf/f0, Fig. 1C). A correlation existed between the magnitude of the response to LPA and the polarity and amplitude of the effect of depolarization (R=−0.63; P < 0.01, supplementary Fig. 1). Depolarization during small or average amplitude responses to 10 nm LPA generated an increased [Ca2+]i (Fig. 1B), but produced a fall in [Ca2+]i during larger agonist-evoked responses (Fig. 1C). Thus, the heterogeneity in the response to a voltage step can be explained by an increasing contribution of agonist-evoked Ca2+ influx as the LPA-mediated response becomes larger, since depolarization will reduce the driving force for Ca2+ entry. In the absence of external Ca2+, the average response to 10 nm LPA was similar in magnitude to the control situation (peak 0.81 ± 0.09 Δf/f0, n= 20, P= 0.37; Fig. 1D), as expected for a response that initially depends primarily on InsP3-dependent Ca2+ mobilization. Depolarization was still able to generate an increase of [Ca2+]i, and importantly this was observed in 100% of oocytes (20/20) (peak 0.17 ± 0.02 Δf/f0, n= 20; Fig. 1D and F). The average response to depolarization was slightly reduced compared to normal Ca2+-containing saline (P < 0.05; Fig. 1F), which may result from a reduced content of the Ca2+ stores after the initial LPA response. Nevertheless, in cells exposed to Ca2+-free medium, sufficient Ca2+ store content was available to observe multiple depolarization-dependent Ca2+ increases in the presence of LPA (Fig. 1D). The experiment in Fig. 1D also shows that the induction of these transient depolarization-evoked Ca2+ increases by LPA was readily reversed by removal of agonist. To assess whether the depolarization-dependent Ca2+ increase observed during stimulation by LPA was the result of a voltage modulation of the electrogenic Na+–Ca2+ exchanger, this protein was blocked by 5 mm extracellular Ni2+ (Schlief & Heinemann, 1995). Ni2+ did not block the depolarization-evoked [Ca2+]i increase (n= 6, Fig. 1F) induced by LPA receptor stimulation in a Ca2+-free medium, indicating that altered Ca2+ efflux via the Na+–Ca2+ exchanger is not required.
Figure 1. Effect of membrane voltage on LPA-evoked Ca2+ responses in Xenopus oocytes.
A–E, intracellular Ca2+ recordings from oocytes under two-electrode voltage clamp in normal (Ca2+-containing) Ringer solution (A–C) or Ca2+-free Ringer solution (D and E). Upper panels show the Fluo5, f/f0 ratio and lower panels the membrane voltage. A, typical recording showing the lack of effect of a 70 mV depolarizing voltage step (−80 to −10 mV, lower panel) on [Ca2+]i (top panel) in the absence of agonist and the typical response to 10 nm LPA held at −80 mV. B and C, typical recordings of the [Ca2+]i increase (B, 75% of cells) and [Ca2+]i decrease (C, 25% of cells) evoked by depolarization during exposure to LPA. D, recording showing the repetitive and reversible nature of the depolarization-evoked Ca2+ increase observed in 100% of oocytes in Ca2+-free medium. E, effect of membrane hyperpolarization (−50 to −120 mV) on the response to 10 nm LPA. F, bar graph showing the average depolarization-induced Ca2+ response (Δf/f0) in the absence (no agonist) or presence of 10 nm LPA in the presence and absence of external Ca2+ and in the presence of 5 mm Ni2+ in Ca2+-free Ringer solution. The depolarization-evoked response in Ca2+-free Ringer solution was significantly smaller than that observed in normal Ringer solution (asterisk, P < 0.05).
Together, these data indicate a major role for Ca2+ release from intracellular stores in the depolarization-evoked increase of [Ca2+]i during stimulation of LPA receptors. Furthermore, it suggests that the depolarization-evoked [Ca2+]i decrease observed in the presence of external Ca2+ in a proportion of oocytes was due to a reduction of the driving force for Ca2+ entry. The final change in [Ca2+]i in response to a depolarizing voltage step will vary in individual cells as a result of the balance between the depolarization-induced Ca2+ release and the reduction of the driving force for Ca2+ entry. For this reason, all subsequent experiments, which are aimed at investigating the properties and mechanism of the voltage-dependent Ca2+ release phenomenon, were performed in Ca2+-free external solutions.
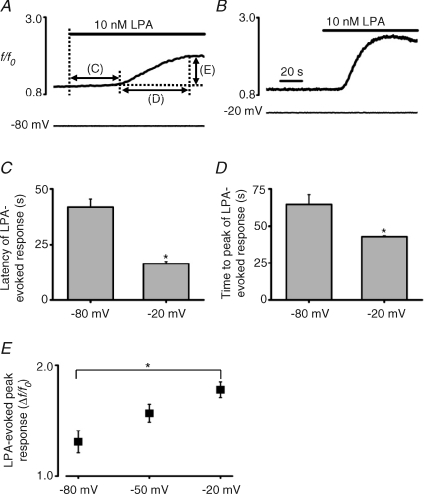
Previous studies showing voltage control of Ca2+ mobilization via native P2Y and Gαq-coupled muscarinic receptors have demonstrated that the effect is bipolar in nature (for review see Mahaut-Smith et al. 2008), such that membrane hyperpolarization causes a transient reduction in the agonist-evoked response. During stimulation of LPA receptors in Xenopus oocytes, the effect of hyperpolarization was less obvious, probably due to the transient nature of the agonist-evoked [Ca2+]i increase and the relatively slow speed of the voltage-dependent response compared to previous studies (see Fig. 1E, where a 70 mV hyperpolarizing pulse was applied during the LPA-mediated Ca2+ increase). However, in contrast to P2Y receptor-evoked responses in the megakaryocyte (Martinez-Pinna et al. 2005), the level of the steady holding potential had a marked effect on the initial Ca2+ response stimulated by LPA in oocytes. Overall, a shift of holding potential to a more depolarized level accelerated and amplified the agonist-evoked response. For example, the latency of the onset of a 10 nm LPA-evoked response was shorter at −20 mV compared to at −80 mV (14 ± 1 s vs. 39 ± 4 s, n= 9, P < 0.05; Fig. 2A–C). Furthermore, the time to peak of the response to 10 nm LPA was less at −20 mV compared to at −80 mV (40 ± 1 s vs. 62 ± 6 s, n= 9, P < 0.05; Fig. 2A, B and D). Finally, 10 nm LPA stimulated a larger peak Ca2+ increase at a holding voltage of −20 mV compared to at −80 mV (1.78 ± 0.1 Δf/f0vs. 1.31 ± 0.1 Δf/f0, n= 9, P < 0.05; Fig. 2A, B and E), with an intermediate amplitude increase being observed at −50 mV (1.56 ± 0.16 Δf/f0, n= 8, Fig. 2E). These results demonstrate that the voltage control of LPA-induced Ca2+ responses is substantially modulated in time course and amplitude over a physiologically relevant range of steady membrane potential, the response being enhanced and accelerated by a depolarizing shift in voltage.
Figure 2. Effect of the holding potential on LPA-evoked Ca2+ responses.
A and B, representative traces showing the influence of the holding potential on LPA-evoked Ca2+ release at −80 and −20 mV, respectively, in a Ca2+-free external solution. Time scale in A is the same as in B. The lines with arrows in A indicate the parameters quantified in panels C, D and E. The latency (C), the time to peak (D) and the amplitude (E) of LPA-evoked Ca2+ response were measured in 8–9 oocytes at the constant holding potential shown (−80, −50 and −20 mV). The values for the three parameters were significantly different (asterisk, P < 0.05) between holding potentials of −80 and −20 mV.
Effect of agonist concentration on the voltage-dependence of LPA receptor-evoked Ca2+ release
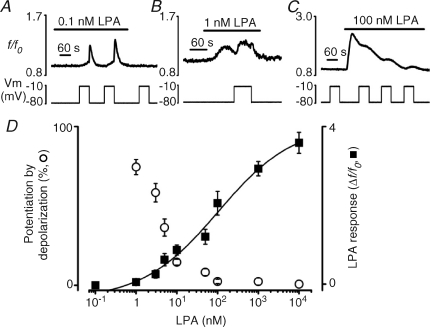
The experiments above have explored the effect of membrane potential changes during application of a single concentration of LPA of 10 nm, which is close to the reported EC50 value for Ca2+-dependent chloride current responses in Xenopus oocytes (Liliom et al. 1996) and close to the EC50 for Ca2+ signals in Jurkat T cells co-transfected with human LPA1 or LPA2 receptors and aequorin (An et al. 1998). The relationship between LPA concentration and LPA-dependent Ca2+ response in Xenopus oocytes was fitted with a sigmoid curve (Fig. 3D), with an estimated EC50 close to 100 nm. Depolarization-evoked Ca2+ increases were observed following exposure to a very wide range of LPA concentrations (Fig. 3B–D). Interestingly, the depolarization-dependent Ca2+ increase displayed an inverse relationship with LPA concentration, such that depolarization was more efficient at lower levels of receptor activation. The ratio of the [Ca2+]i increase elicited by depolarization to that evoked by agonist was 0.74 ± 0.05, 0.58 ± 0.06, 0.36 ± 0.05, 0.14 ± 0.03 and 0.08 ± 0.02 fold at 1 nm, 3 nm, 5 nm, 10 nm and 50 nm LPA, respectively (Fig. 3D). At higher concentrations of agonist, 100 nm, 1 μm and 10 μm LPA the efficiency of depolarization to potentiate LPA response was lower than 0.05-fold (Fig. 3D). A further striking observation was that, although all cells (8/8) failed to respond to 0.1 nm LPA, the lowest concentration of LPA tested, three of these (37.5%) displayed a marked depolarization-evoked [Ca2+]i increase (Fig. 3A). These results demonstrate that depolarization is most effective at modifying LPA-induced Ca2+ release at agonist concentrations in the nanomolar range, which, importantly, corresponds to the LPA levels found in plasma (Baker et al. 2001).
Figure 3. Potentiation of LPA receptor-evoked Ca2+ response by depolarization is greatest at low concentrations of LPA.
A–C, effect of a depolarizing pulse (−80 to −10 mV) on [Ca2+]i at a subthreshold (0.1 nm, A), near-threshold (1 nm, B) and suprathreshold (100 nm, C) concentration of LPA in Ca2+-free external solution. At 0.1 nm LPA (A), all oocytes failed to respond to the agonist alone, whereas 3 out of 8 cells displayed a subsequent depolarization-evoked [Ca2+]i increase. D, semilogarithmic concentration–response relationship for the depolarization-evoked [Ca2+]i increase (open circles, measured as a percentage of the corresponding LPA-evoked response) and LPA-evoked peak response (Δf/f0, filled squares), versus the LPA concentration. Potentiation by depolarization at 0.1 nm LPA is excluded due to lack of agonist response. LPA-evoked peak response data were best fitted with the logistic equation: 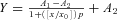 , where x0 is the middle value, p is the power and A1 and A2 are the initial and final Y values, respectively. Each point is the mean and s.e.m. of 4–13 cells.
, where x0 is the middle value, p is the power and A1 and A2 are the initial and final Y values, respectively. Each point is the mean and s.e.m. of 4–13 cells.
Requirement of functional InsP3 receptors and phospholipase-C activation for the voltage control of Ca2+ release
Activation of LPA receptors in Xenopus oocytes is coupled to the phosphatidylinositol second messenger system (Tigyi & Miledi, 1992). To investigate whether functional InsP3 receptors are required for the voltage-dependent Ca2+ release mechanism, experiments were undertaken with 2-aminoethyl diphenylborinate (2-APB), an inhibitor of InsP3 receptors (Chorna-Ornan et al. 2001). Depolarization to −10 mV during the LPA-induced [Ca2+]i transient (peak 0.60 ± 0.10 Δf/f0, n= 6; Fig. 4A) evoked an increase in [Ca2+]i in six oocytes (peak 0.13 ± 0.01 Δf/f0, n= 6; Fig. 4A), but this response was abolished in the presence of 100 μm 2-APB (Fig. 4A), indicating the requirement of functional InsP3 receptors for the voltage modulation of Ca2+ release.
Although 2-APB is widely used as an inhibitor of InsP3 receptors, it may interfere with a variety of ion channels and pumps (Parekh & Putney, 2005). Hence, we further assessed the requirement of InsP3 production in the voltage-dependent Ca2+ release during LPA receptor stimulation by incubation of oocytes with the aminosteroid U-73122 (1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione), an inhibitor of phospholipase-C activation (Noh et al. 1998). U-73122 (10 μm) decreased the response to depolarizing steps in the presence of LPA in a time-dependent manner (Fig. 4B). In these experiments, LPA was applied immediately prior to the depolarization; as expected, the response to LPA decreased over a similar time scale to the loss of response to depolarization (data not shown). In contrast, incubation with the inactive analogue U-73343 (1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino] hexyl]-2,5-pyrrolidinedione; 10 μm) had no significant effect on the [Ca2+]i increase elicited by either LPA (not shown) or by voltage steps in the presence of LPA (Fig. 4B). This indicates that activation of phospholipase-C, and thus InsP3 production, plays an essential role in the voltage-dependent Ca2+ release during LPA receptor stimulation.
Lack of voltage-dependent Ca2+ release after direct elevation of InsP3 or heterotrimeric G-protein activation
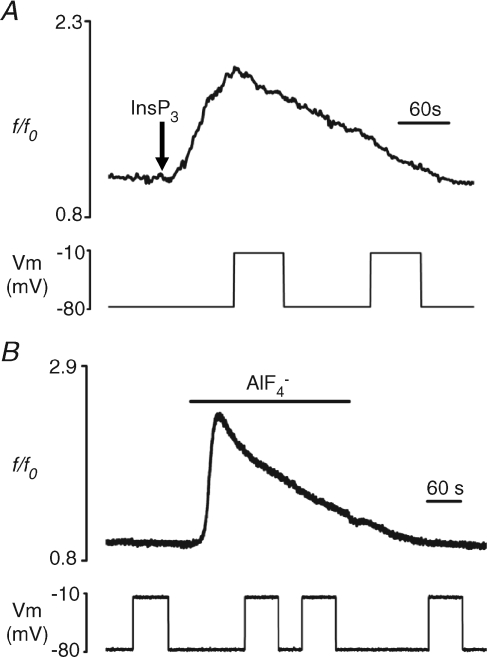
The voltage dependence of Ca2+ release during activation of a GPCR can be explained by a direct sensitivity to membrane potential of the receptor itself or of any downstream molecules activated in the second messenger cascade (Ganitkevich & Isenberg, 1993; Mahaut-Smith et al. 1999; Ben Chaim et al. 2003; Martinez-Pinna et al. 2005; Ben Chaim et al. 2006). To assess whether activation of InsP3 receptors alone can induce a voltage dependence of Ca2+ release in our cell model, for example by configurational coupling between InsP3 receptors on the intracellular stores and ion channels on the plasma membrane (Kiselyov et al. 1998), we tested the effects of directly elevating the cytosolic InsP3 level by pressure injection (see Methods). The pressure and duration applied to the intracellular micropipette were adjusted so that a single bolus of 1 mm InsP3 generated a Ca2+ response (peak 0.80 ± 0.16 Δf/f0, n= 5; Fig. 5A) of similar magnitude to that evoked by 10 nm LPA, a concentration at which depolarization-evoked Ca2+ increases are of a sizeable amplitude (see above). During this InsP3-evoked response, depolarization was unable to stimulate a further Ca2+ increase (Fig. 5A), excluding the possibility that the voltage dependence during LPA GPCR activation results from an event induced by direct activation of InsP3 receptors or a subsequent step in the signalling cascade.
Figure 5. Lack of depolarization-evoked Ca2+ response following direct elevation of InsP3 or the heterotrimeric G-protein activation with aluminium tetrafluoride.
A, lack of effect of depolarizing pulses (lower panel) on [Ca2+]i (top panel) during the response to ∼500 fmol of intracellularly injected InsP3 (see Methods). Arrow indicates the point of InsP3 injection. B, cells were exposed to the heterotrimeric G-protein activator AlF4− using a mixture of 10 mm NaF and 200 μm AlCl3. Exposure to AlF4− reversibly induced a [Ca2+]i increase (top panel) but depolarizing pulses (lower panel) had no effect on [Ca2+]i. All experiments were performed in Ca2+-free external solution.
Extracellular application of aluminium tetrafluoride is employed as a tool to activate heterotrimeric G-proteins in the absence of agonist (Sternweis & Gilman, 1982; Martinez-Pinna et al. 2005). Superfusion of oocytes with AlF4− resulted in an increase in [Ca2+]i (peak 0.99 ± 0.08 Δf/f0, n= 8; Fig. 5B), as a consequence of Gαq-subunit activation and hence phospholipase-C stimulation and InsP3 production (Moon et al. 1997). However, as observed following direct injection of InsP3, depolarizing pulses were unable to stimulate a Ca2+ increase during AlF4−-induced responses (Fig. 5B). This indicates that the primary site of action of membrane voltage in the control of LPA GPCR-dependent Ca2+ mobilization is upstream of the heterotrimeric G-proteins.
Discussion
In this study we have demonstrated for the first time a sensitivity of LPA receptors to physiological changes in membrane potential. This extends previous work demonstrating an ability of membrane potential to directly modulate Ca2+ signalling via a number of GPCRs coupled to Gαq-proteins and the phosphatidylinositol second messenger system. InsP3-induced intracellular Ca2+ release mediates the action of many hormones, neurotransmitters and other messengers (Berridge, 1993; Clapham, 1995), including LPA, which plays a principal role in fundamental biological processes such as development, differentiation, angiogenesis, pain inflammation and carcinogenesis (Moolenaar et al. 2004; Ishii et al. 2004; Noguchi et al. 2009). Therefore, the fact that LPA signalling can be modulated by membrane potential may have important functional consequences. Of particular interest is the fact that LPA receptor expression is regulated throughout brain development (Fukushima et al. 2001) and that LPA induces cortical growth and folding (Kingsbury et al. 2003). Thus, the modulation of LPA signalling by voltage could be particularly relevant during neuronal growth and differentiation in the developing nervous system, where changes in the level of membrane potential are constantly occurring in the form of synaptic and action potentials. Furthermore, LPA is an inducer of cell proliferation, migration and survival and its plasma levels are increased in malignant secretions, indicating a role for LPA in the initiation and progression of cancer (Mills & Moolenaar, 2003). This, together with the increased expression of voltage-sensitive ion channels associated with a malignant potential (Fiske et al. 2006), makes the voltage dependence of LPA signals a factor to be considered in cancer pathophysiology. Voltage control of LPA receptors could also influence development, since this agonist is known to control cortical actin assembly and cytoarchitecture in Xenopus embryos (Lloyd et al. 2005).
Two LPA receptor subtypes are expressed in Xenopus oocytes, XLPA1-1 and XLPA1-2, which are both highly homologous (∼89% identity) to mammalian LPA1 receptors (Kimura et al. 2001). Indeed, the LPA1 receptor shows a high degree of sequence conservation among many animals, including fish, amphibia (e.g. Xenopus), chicken and mammals (Chun et al. 2002). It is therefore possible that LPA receptors from different phyla show the voltage dependence. We have performed preliminary experiments in a human cell type (HEK-293 cells) which endogenously expresses LPA receptors to address this issue. In simultaneous whole-cell patch clamp and fura-2 fluorescence recordings, depolarization in the absence of agonist caused either no change, or a small reduction in Ca2+. However, in the presence of 1 μm LPA, depolarizations were able to repeatedly stimulate an increase in [Ca2+]i (supplementary Fig. 2 and unpublished results). This result indicates that the voltage dependence of Ca2+ signals during activation of LPA receptors is not restricted to lower vertebrates but is a widespread phenomenon.
Previous studies in a variety of cell types support the concept that voltage modulation is a common property of a number of GPCRs (Mahaut-Smith et al. 2008). Our results also suggest that the voltage sensor in the control of LPA GPCR-dependent Ca2+ release lies at the level of the receptor protein itself, since activation of G-proteins by aluminium tetrafluoride, in the absence of agonist, fails to induce voltage modulation of Ca2+ release. Moreover, InsP3 receptors or events downstream of InsP3-dependent Ca2+ release are not the voltage-sensing site, because direct intracellular injection of InsP3 causes a [Ca2+]i increase that cannot be modulated by voltage. Nevertheless, depolarization-induced Ca2+ release requires InsP3 production and InsP3-dependent Ca2+ release, as block of InsP3 receptors with 2-APB (Chorna-Ornan et al. 2001) or inhibition of phospholipase-C activity abolished the response to LPA and the depolarization-induced Ca2+ release. These results indicate the essential role of the phosphatidylinositol second messenger system and that the modulation is upstream of InsP3 production. A similar conclusion was derived in previous work on P2Y1 and muscarinic receptors in megakaryocytes and smooth muscle, respectively, which is consistent with the hypothesis that depolarization stimulates InsP3 production during activation of these GPCRs (Ganitkevich & Isenberg, 1993; Mahaut-Smith et al. 1999; Martinez-Pinna et al. 2005). Direct sensitivity of the GPCR to the membrane potential has been suggested by a series of observations on P2Y1 receptors and also by observations of voltage-dependent charge movements, analogous to gating currents of voltage-gated channels, in two prototypical GPCRs, the m2 and m1 muscarinic receptors, expressed in Xenopus oocytes (Ben Chaim et al. 2006). In the proposed mechanism, the intracellular loop that couples m2 and m1 receptors to their G-protein has a crucial function in voltage sensing, thereby influencing the affinity of the receptor for the agonist. On the other hand, any putative role of the electrogenic Na+–Ca2+ exchanger in the voltage modulation of [Ca2+]i during LPA application was ruled out in our experiments by superfusing the cell with a Ca2+-free Ringer solution in the presence of Ni2+, a blocker of the electrogenic Na+–Ca2+ exchanger. To date, all GPCRs shown to exhibit voltage dependence fall into class A, which constitutes ∼90% of all members of this superfamily of receptors (Pierce et al. 2002), including purinergic P2Y1, thromboxane A2, serotoninergic 5-HT2A, glutamatergic mGlu3 and mGlu1, muscarinic m2, m1 and m3, dopaminergic D2 (Ganitkevich & Isenberg, 1993; Ben Chaim et al. 2003; Martinez-Pinna et al. 2005; Ben Chaim et al. 2006; Ohana et al. 2006; Sahlholm et al. 2008; Liu et al. 2009) and Xenopus LPA (this study). Noticeably, all these receptors have multiple clusters of positively charged residues, mainly arginine and lysine, which resemble the voltage sensor in voltage-dependent ion channels (Bezanilla, 2000). Although these residues are located in the second and third intracellular loops of the GPCR structure, rather than within transmembrane regions that contain the main voltage sensors in other proteins, it is still possible they are involved in voltage sensing (Ben Chaim et al. 2006; Mahaut-Smith et al. 2008).
The present study not only provides evidence that changes in membrane potential can regulate Ca2+ signalling via LPA-receptors, but also indicates conditions under which a change in membrane voltage may exert its most significant effect on signalling via this class of GPCR. Firstly, experiments in Ca2+-free medium showed that the greatest potentiating effect of voltage is observed at the lowest effective agonist concentrations. This characteristic is shared by purinergic P2Y1 receptors (Gurung et al. 2008) and can be explained by an action of voltage on the ligand-bound, inactive or partially activated form of the receptor (Gurung et al. 2008). Previous work in oocytes has shown that a certain threshold amount of InsP3 is required to generate a detectable response and that this level of InsP3 can be achieved by facilitation between two temporally separated subthreshold InsP3 events (Parker & Miledi, 1989). In the present study, a subthreshold LPA concentration and a depolarizing voltage step may act in a similar cooperative manner since both stimulate release of InsP3. Secondly, at a constant concentration of LPA in the presence of external Ca2+, the depolarization-evoked Ca2+ response decreased in magnitude as the agonist-evoked Ca2+ response increased (see supplementary Fig. 1 showing this clear inverse correlation). Since the response to depolarization became a net decrease in intracellular Ca2+ for oocytes displaying the largest responses to agonist, the most likely explanation for this trend is a shift in balance from predominantly Ca2+ release for low LPA-evoked responses towards a greater contribution from Ca2+ influx as the agonist response increases (Putney, 1986). Thus, for the larger responses, the dominant effect of depolarization becomes a reduction in inward driving force for Ca2+ influx through store-operated and other pathways.
Given the widespread presence of LPA-receptors in adult and developing tissues (Ishii et al. 2004; Noguchi et al. 2009), this effect of membrane potential represents a potentially important mechanism whereby LPA responses can be modified by electrogenic influences. Depolarizing steps are also able to induce Ca2+ responses in the presence of a subthreshold agonist concentration (Fig. 3A) and, therefore, voltage control of GPCRs can be considered as a mechanism of coincidence detection between chemical signals and electrical stimuli. Consequently, in cellular structures such as dendrites, where low levels of neurotransmitters acting on GPCRs are released and changes in membrane potential are constantly occurring in the form of post-synaptic potentials and backpropagated action potentials, the voltage dependence of GPCR-mediated Ca2+ responses could play a major role in signalling.
In contrast to the clear bipolar nature of the voltage dependence of Ca2+ release during stimulation of muscarinic and P2Y1 receptors in mammalian cells (Ganitkevich & Isenberg, 1993; Mahaut-Smith et al. 1999; Martinez-Pinna et al. 2004), only depolarizing voltage steps could be shown to have a marked effect during LPA stimulation in the present study (Fig. 1D and E). The lack of a clear effect of hyperpolarizing voltage steps is most likely due to the transient nature of the Ca2+ response to LPA and the slow speed of the voltage-dependent response in oocytes compared to previous studies. However, we cannot rule out that differences in the structures of GPCRs account for their relative sensitivity to hyperpolarizing voltage steps. A further difference between the effect of voltage on P2Y1 receptors in megakaryocytes compared to LPA receptors in Xenopus oocytes was the influence of the holding potential on the initial agonist-evoked [Ca2+]i response. The peak response to LPA was accelerated and enhanced in a graded manner as the holding potential was shifted from −80 mV to −20 mV whereas P2Y1 receptor responses to 1 μm ADP were reduced in amplitude by a depolarizing shift in holding potential (Gurung et al. 2008). At present it is unclear precisely why LPA receptor responses are affected by holding voltage and P2Y1 receptors are not. However, the most likely explanation is that the Ca2+ content of the megakaryocyte stores is more susceptible to short periods of reduced Ca2+ influx that result from depolarization, together with the slower nature of the Ca2+ increases in the oocytes. The latter could allow a tonic effect of voltage on the initial agonist-evoked Ca2+ response to be unmasked. Despite a definitive answer to this issue, the ability of a constant holding voltage to modulate responses through a Gαq-coupled GPCRs significantly extends the extent to which membrane potential can influence signalling through this important family of surface proteins.
A further important consideration when comparing the present results with previous studies of GPCR voltage dependence is cell geometry. Oocytes have a diameter of ∼1 mm and hence the ratio of plasma membrane surface to cytoplasmic volume is reduced compared to other cell types. This could explain the low relative speed of the [Ca2+]i changes recorded in oocytes. Another morphological feature of the oocyte is the numerous microvilli within the plasma membrane, which significantly increase the cell membrane surface, as indicated by the high apparent specific membrane capacitance (5 μF cm−2) of these cells (Zhang & Hamill, 2000). These infoldings could increase the ratio of receptors to sub-plasma-membrane InsP3 receptors and thereby contribute to the robust nature of the depolarization on [Ca2+]i during LPA exposure in oocytes. Interestingly, in megakaryocytes, where the demarcation membrane system extends the surface plasma membrane throughout the extranuclear volume of the cell, the specific membrane capacitance is also high (average 8 μF cm−2) and we have previously speculated that this may account for the extremely robust nature of the voltage dependence of Gαq-coupled receptor Ca2+ signals by increasing the ratio of receptors to available InsP3-dependent Ca2+ release sites (Mahaut-Smith et al. 1999, 2003, 2008).
In conclusion, we have shown that stimulation of metabotropic LPA receptors in Xenopus oocytes confers a voltage sensitivity to the InsP3-dependent Ca2+ release from the intracellular stores. This influence is present over a wide range of LPA concentrations with the greatest effect of depolarizing voltage steps at near-threshold levels of agonist. The level of the membrane potential is also able to modulate the initial LPA-evoked Ca2+ release in a graded manner. Our results suggest that the principal voltage sensor lies within the receptor itself, rather than a downstream pathway. Given that LPA has widespread biological actions, voltage modulation of LPA signalling arises as an important mechanism for modulation of Ca2+ signals both in excitable and non-excitable cells.
Acknowledgments
The authors thank to Dr Isabel Ivorra for helpful comments on the manuscript and Mr Simon Moya for technical support. This work was supported by grants GV04-A57 and GV06/323 from the Generalitat Valenciana and BFU2006-04781 and CSD2008-00005 from the MICINN (Spain). I.S.G. and M.M.-S. were supported by the Medical Research Council, UK (G0301031).
Glossary
Abbreviations
- 2-APB
2-aminoethyl diphenylborinate
- GPCR
G-protein-coupled receptor
- LPA
lysophosphatidic acid
Author contributions
All authors contributed to conception, design, analysis and interpretation of data, and drafting the article and revising it critically for important intellectual content. All authors approved the version to be published. All experiments were done in the Laboratory of Physiology at Universidad de Alicante, Spain.
Supplemental material
Supplemental figure 1
Supplementary figure 2
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- An S, Bleu T, Zheng Y, Goetzl EJ. Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization. Mol Pharmacol. 1998;54:881–888. doi: 10.1124/mol.54.5.881. [DOI] [PubMed] [Google Scholar]
- Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem. 2001;292:287–295. doi: 10.1006/abio.2001.5063. [DOI] [PubMed] [Google Scholar]
- Ben Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109. doi: 10.1038/nature05259. [DOI] [PubMed] [Google Scholar]
- Ben Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003;278:22482–22491. doi: 10.1074/jbc.M301146200. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- Billups D, Billups B, Challiss RA, Nahorski SR. Modulation of Gq-protein-coupled inositol trisphosphate and Ca2+ signalling by the membrane potential. J Neurosci. 2006;26:9983–9995. doi: 10.1523/JNEUROSCI.2773-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorna-Ornan I, Joel-Almagor T, Ben Ami HC, Frechter S, Gillo B, Selinger Z, Gill DL, Minke B. A common mechanism underlies vertebrate calcium signalling and Drosophila phototransduction. J Neurosci. 2001;21:2622–2629. doi: 10.1523/JNEUROSCI.21-08-02622.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signalling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernhout BJ, Dijcks FA, Moolenaar WH, Ruigt GS. Lysophosphatidic acid induces inward currents in Xenopus laevis oocytes; evidence for an extracellular site of action. Eur J Pharmacol. 1992;213:313–315. doi: 10.1016/0014-2999(92)90698-4. [DOI] [PubMed] [Google Scholar]
- Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 2006;25:493–500. doi: 10.1007/s10555-006-9017-z. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Ishii I, Contos JJ, Weiner JA, Chun J. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001;41:507–534. doi: 10.1146/annurev.pharmtox.41.1.507. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung IS, Martinez-Pinna J, Mahaut-Smith MP. Novel consequences of voltage-dependence to G-protein-coupled P2Y1 receptors. Br J Pharmacol. 2008;154:882–889. doi: 10.1038/bjp.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signalling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- Ivorra I, Morales A. Membrane currents in immature oocytes of the Rana perezi frog. Pflugers Arch. 1997;434:413–421. doi: 10.1007/s004240050415. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Schmitt A, Fukushima N, Ishii I, Kimura H, Nebreda AR, Chun J. Two novel Xenopus homologs of mammalian LPA1/EDG-2 function as lysophosphatidic acid receptors in Xenopus oocytes and mammalian cells. J Biol Chem. 2001;276:15208–15215. doi: 10.1074/jbc.M011588200. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6:1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Kusano K, Miledi R, Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977;270:739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Liliom K, Murakami-Murofushi K, Kobayashi S, Murofushi H, Tigyi G. Xenopus oocytes express multiple receptors for LPA-like lipid mediators. Am J Physiol Cell Physiol. 1996;270:C772–C777. doi: 10.1152/ajpcell.1996.270.3.C772. [DOI] [PubMed] [Google Scholar]
- Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, Wang YX. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci U S A. 2009;106:11418–11423. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd B, Tao Q, Lang S, Wylie C. Lysophosphatidic acid signalling controls cortical actin assembly and cytoarchitecture in Xenopus embryos. Development. 2005;132:805–816. doi: 10.1242/dev.01618. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Hussain JF, Mason MJ. Depolarization-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte. J Physiol. 1999;515:385–390. doi: 10.1111/j.1469-7793.1999.385ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008;29:421–429. doi: 10.1016/j.tips.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Thomas D, Higham AB, Usher-Smith JA, Hussain JF, Martinez-Pinna J, Skepper JN, Mason MJ. Properties of the demarcation membrane system in living rat megakaryocytes. Biophys J. 2003;84:2646–2654. doi: 10.1016/S0006-3495(03)75070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pinna J, Gurung IS, Vial C, Leon C, Gachet C, Evans RJ, Mahaut-Smith MP. Direct voltage control of signalling via P2Y1 and other Gαq-coupled receptors. J Biol Chem. 2005;280:1490–1498. doi: 10.1074/jbc.M407783200. [DOI] [PubMed] [Google Scholar]
- Martinez-Pinna J, Tolhurst G, Gurung IS, Vandenberg JI, Mahaut-Smith MP. Sensitivity limits for voltage control of P2Y receptor-evoked Ca2+ mobilization in the rat megakaryocyte. J Physiol. 2004;555:61–70. doi: 10.1113/jphysiol.2003.056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A, Tan YP. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. J Physiol. 1989;419:665–687. doi: 10.1113/jphysiol.1989.sp017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R, Parker I. Latencies of membrane currents evoked in Xenopus oocytes by receptor activation, inositol trisphosphate and calcium. J Physiol. 1989;415:189–210. doi: 10.1113/jphysiol.1989.sp017718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Parker I, Sumikawa K. In: Fidia Research Foundation Neuroscience Award Lecture Series. Smith J, editor. Vol. 3. New York: Raven Press; 1989. pp. 57–90. [Google Scholar]
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signalling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- Moon C, Fraser SP, Djamgoz MB. G-protein activation, intracellular Ca2+ mobilization and phosphorylation studies of membrane currents induced by AlF4− in Xenopus oocytes. Cell Signal. 1997;9:497–504. doi: 10.1016/s0898-6568(96)00092-7. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol. 2009;9:15–23. doi: 10.1016/j.coph.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Noh SJ, Kim MJ, Shim S, Han JK. Different signalling pathway between sphingosine-1-phosphate and lysophosphatidic acid in Xenopus oocytes: functional coupling of the sphingosine-1-phosphate receptor to PLC-xβ in Xenopus oocytes. J Cell Physiol. 1998;176:412–423. doi: 10.1002/(SICI)1097-4652(199808)176:2<412::AID-JCP20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ohana L, Barchad O, Parnas I, Parnas H. The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. J Biol Chem. 2006;281:24204–24215. doi: 10.1074/jbc.M513447200. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Parker I, Miledi R. Nonlinearity and facilitation in phosphoinositide signalling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989;9:4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Sahlholm K, Nilsson J, Marcellino D, Fuxe K, Arhem P. Voltage-dependence of the human dopamine D2 receptor. Synapse. 2008;62:476–480. doi: 10.1002/syn.20509. [DOI] [PubMed] [Google Scholar]
- Schlief T, Heinemann SH. H2O2-induced chloride currents are indicative of an endogenous Na+-Ca2+ exchange mechanism in Xenopus oocytes. J Physiol. 1995;486:123–130. doi: 10.1113/jphysiol.1995.sp020796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis PC, Gilman AG. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982;79:4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992;267:21360–21367. [PubMed] [Google Scholar]
- Zhang Y, Hamill OP. On the discrepancy between whole-cell and membrane patch mechanosensitivity in Xenopus oocytes. J Physiol. 2000;523:101–115. doi: 10.1111/j.1469-7793.2000.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.