Abstract
Colonic epithelial K+ secretion is a two-step transport process with initial K+ uptake over the basolateral membrane followed by K+ channel-dependent exit into the lumen. In this process the large-conductance, Ca2+-activated KCa1.1 (BK) channel has been identified as the only apparent secretory K+ channel in the apical membrane of the murine distal colon. The BK channel is responsible for both resting and Ca2+-activated colonic K+ secretion and is up-regulated by aldosterone. Agonists (e.g. adrenaline) that elevate cAMP are potent activators of distal colonic K+ secretion. However, the secretory K+ channel responsible for cAMP-induced K+ secretion remains to be defined. In this study we used the Ussing chamber to identify adrenaline-induced electrogenic K+ secretion. We found that the adrenaline-induced electrogenic ion secretion is a compound effect dominated by anion secretion and a smaller electrically opposing K+ secretion. Using tissue from (i) BK wildtype (BK+/+) and knockout (BK−/−) and (ii) cystic fibrosis transmembrane regulator (CFTR) wildtype (CFTR+/+) and knockout (CFTR−/−) mice we were able to isolate the adrenaline-induced K+ secretion. We found that adrenaline-induced K+ secretion: (1) is absent in colonic epithelia from BK−/− mice, (2) is greatly up-regulated in mice on a high K+ diet and (3) is present as sustained positive current in colonic epithelia from CFTR−/− mice. We identified two known C-terminal BK α-subunit splice variants in colonic enterocytes (STREX and ZERO). Importantly, the ZERO variant known to be activated by cAMP is differentially up-regulated in enterocytes from animals on a high K+ diet. In summary, these results strongly suggest that the adrenaline-induced distal colonic K+ secretion is mediated by the BK channel and probably involves aldosterone-induced ZERO splice variant up-regulation.
Introduction
Colonic K+ secretion contributes to the overall K+ homeostasis. Active epithelial K+ secretion requires basolateral uptake of K+ via the Na+/K+-ATPase and the Na+/K+/2Cl− (NKCC1) co-transporter (Sweiry & Binder, 1989). In a second step, cytosolic K+ leaves the cell via apical K+ channels. Recently, we have shown that resting and Ca2+-activated mouse distal colonic K+ secretion occurs via luminal KCa1.1 (BK) channels (gene name: KCNMA1) in mouse distal colon (Sausbier et al. 2006). In addition, we found that the aldosterone-induced colonic K+ secretion occurs via BK channels (Sorensen et al. 2008). Functional BK channels are heteromeric protein complexes formed by four pore-forming BK α-subunits and commonly have four associated β-subunits. Four different β-subunits are known (β1−4), which all differentially modulate the biophysical properties of this channel (Salkoff et al. 2006). In isolated murine colonic crypts we identified the exclusive expression of the β2-subunit (KNCMB2) suggesting that the secretory K+ channel is composed of the pore-forming α-subunit and the regulatory β2-subunit (Sorensen et al. 2008).
The BK α-subunit is known to be subject of molecular variations, which lead to differential regulation of the K+ channel. It shows extensive C-terminal pre-mRNA splicing (Xie & McCobb, 1998; Chen et al. 2005; Salkoff et al. 2006). A number of different BK α-subunit splice variants have been detected in different tissues (Xie & McCobb, 1998; Chen et al. 2005). One particular splice variation (the ZERO variant) repetitively occurs at the so-called C2 splice site and lacks the exon 18 (58 aa) (database information from ENSEMBL version 1/11-07 for the mouse KNCMA1 gene) (Xie & McCobb, 1998). The corresponding BK channel variant containing exon 18 is named stress axis regulated exon (STREX). Several regulatory and biophysical features make these two splice variants interesting targets of physiological research. As compared to ZERO, the STREX variant shows gain-of-function characteristics with current activation at more negative membrane voltages, and faster activation and slower deactivation kinetics (Xie & McCobb, 1998). Interestingly, STREX was found to be inhibited by increasing the second messenger cAMP, whereas the ZERO variant is activated by cAMP (Tian et al. 2001a,b; Friis et al. 2003; Chen et al. 2005).
Colonic K+ and Cl− secretion can in many ways be viewed as functionally and mechanistically parallel transepithelial transport processes. In the colonic crypts, the major sites of colonic exocrine secretion (Welsh et al. 1982; Greger et al. 1997), basolateral Cl− and K+ uptake occur via NKCC1 (Pena-Munzenmayer et al. 2005) and the luminal Cl− exit occurs predominantly via cystic fibrosis transmembrane conductance regulator (CFTR) (Greger et al. 1997). Increases of cAMP and Ca2+ are pivotal intracellular signalling events, which stimulate both the activation of Cl− and K+ secretion (Kunzelmann & Mall, 2002). It is currently not known which luminal K+ channel is responsible for cAMP-stimulated colonic K+ secretion. The current study was therefore designed to clarify the molecular nature of the cAMP-dependent apical K+ channel in mouse distal colon. For this purpose we use adrenaline as an agonist to promote cAMP-dependent K+ secretion. In mammalian colonic mucosa it is well established that adrenaline triggers an acute increase of K+ secretion via β-receptor activation and a subsequent increase of intracellular cAMP (Rechkemmer et al. 1996; Grotjohann et al. 1998; Horger et al. 1998). The results of this study show that the adrenaline-stimulated distal colonic K+ secretion is also conducted via apical BK channels.
Methods
Mice and diets
Mice deficient in the BK channel α-subunit (BK−/−) and their wildtype controls on a SV129×C57Bl6 hybrid background (F2 generation) (Sausbier et al. 2004) were bred in the Department of Pharmacology and Toxicology, Institute of Pharmacy, University of Tübingen, Germany. Mice lacking CFTR (CFTR−/−) and their wildtype controls on an NMRI background were bred in the Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Germany. To avoid intestinal obstruction CFTR mice were fed a fibre-free diet (C1013, Altromin, Lage, Germany) and received Oralav, (Braun, Melsungen, Germany) diluted 1:4 in tap water, a mild osmotic diarrheal solution containing polyethylene glycol 4000. Additional NMRI mice were obtained from Taconic (Ry, Denmark). Experiments were performed on age-matched animals or littermates (2–4 months old) of either sex. Mice were kept on two different diets both from Altromin: a standard diet (1310) with a K+ content of 10 g kg−1 or a high K+ diet (C1050) with a K+ content of 50 g kg−1 for a period of 4 days. Experiments were performed according to Danish legislation on the protection of animals.
Crypt preparations, RNA isolation and cDNA generation
Mice were killed by cervical dislocation. The preparation of isolated colonic crypts was described previously (Siemer & Gögelein, 1990). In brief, a 2 cm piece of mouse distal colon was inverted and rinsed with ice-cold Ca2+-free Ringer-type solution with the following composition (in mm): NaCl 127; KCl 5; sodium pyruvate 5; d-glucose 5; Hepes 10; EDTA 5; MgCl2 1; pH 7.4. Both ends were ligated to obtain a sac preparation and filled with Ca2+-free solution. The sacs were then incubated for 10 min at 37°C. Isolated crypts were obtained by shaking the sacs. Approximately 100 crypts were collected from each preparation. Crypts were centrifuged for 1 min at 1520 g and the pellet was immediately re-suspended in lysis buffer (RNeasy Mini Kit, Qiagen), vortexed and frozen in liquid nitrogen. Total RNA was extracted from isolated colonic crypts with the RNeasy Mini Kit (Qiagen). RNA was quantified with a Quant-iT RiboGreen RNA reagent (Stratagene), after treatment with RNase-free DNase (Qiagen). cDNAs were then synthesized from 300 ng of total RNA using SuperScript III reverse transcriptase (Invitrogen) and random decamer primers according to the manufacturer's instructions.
Analysis of BK α-subunit splice variants in isolated mouse colonic crypts
Reverse transcriptase-PCR was used to investigate if specific mRNA splice variants for the mouse BK α-subunit are present in mucosal epithelial cells. Primers were designed to bracket the known spliced exon 18 and neighbouring exons (15–20) using the published mouse sequences for the BK α-subunit (KCNMA1; ENSEMBL version 1/11-07). Note the controversy in numbering the exons of the KCNMA gene with previously studies investigating the same BK α-subunit C-terminal splice site. What was denoted as exon 21 e.g. in the publication of Chen et al. (2005) is now denoted exon 18. The sequence of the forward primer (5′→3′) was: CTTCGTGGGTCTGTCCTTCC and the reverse primer was (5′→3′): GATGACTTTCTCAATCTCCTTGGG. The cycling profile for each run was 95°C for 10 min and 25 cycles of 95°C for 15 s followed by 60°C for 1 min. The PCR products were isolated from a 2% agarose gel using QIAEX II gel extraction kit (Qiagen). The isolated products were sent to MWG Biotech (Germany) for sequencing. The same primer set used for the RT-PCR was used for the sequencing reaction at MWG Biotech.
Semi-quantitative PCR analysis of BK α-subunit splice variants
Semi-quantitative PCR was used to analyse the mRNA expression levels of the different BK α-subunits. TaqMan probes, labelled at the 5′ end with FAM and at the 3′ end with BHQ1, and qPCR primers (synthesized by MWG Biotech) were designed with the Beacon designer software. Specificity for either the STREX or ZERO variants was obtained selecting a STREX probe in exon 18 and the ZERO probe on the boundary and bridging over exon 17 and 19. The primers were validated with SYBR Green by generating a dissociation curve after the amplification reaction. qPCR with TaqMan probes was performed with ExTaq (Takara) on an Mx3000P Quantitative PCR System (Stratagene). All reactions were carried out in duplicate. Optimization of primer and probe concentrations was performed with 2-fold dilution series of cDNA from whole colonic crypts. Sequences and final concentrations of real-time primers and probes are shown in Supplemental Table S1. The reaction was hot started with 95°C for 10 min and followed by 45 cycles of 95°C for 15 s ending by 60°C for 1 min. The expression of the genes of interest was normalized to the reference genes hypoxanthine–guanine phosphoribosyl transferase (HPRT) and β-actin. As positive control we chose the gene for the aldosterone-regulated corticosteroid hormone-induced factor (CHIF, synonym FXYD4, i.e. one γ-subunit of the Na+/K+-ATPase), known to be expressed and regulated by aldosterone in distal colonic epithelium (Wald et al. 1996). The expression level of HPRT and ß-actin were similar in all groups. Expression of genes of interest was analysed in reference to the endogenous reference genes HPRT and β-actin.
Ussing chamber experiments
Mice of either sex were killed by cervical dislocation. The distal 2 cm of the entire (non-stripped) colonic sheet was mounted in an Ussing chamber. In one series of experiments, the effect of adrenaline was also investigated in stripped tissue, where the muscular layers were gently removed (Supplemental Fig. S1). The two halves of the chamber were continuously perfused by a bubble lift system. The solutions on the two sides were symmetrical and had the following composition (in mm): NaCl 120; NaHCO3 25; K2HPO4 1.6; KH2PO4 0.4; calcium gluconate 1.3; MgCl2 1; d-glucose 5, indomethacin (5 μm); pH 7.4. The reservoirs were bubbled with 5% CO2 and 95% O2 and kept at 37°C with water jackets. Initially, tetrodotoxin (TTX, 1 μm) was added to the serosal side to inhibit possible secretory activation by the enteric nervous system or other autonomous neurons. Subsequently, amiloride (100 μm) was added to the mucosal perfusate to abolish electrogenic Na+ absorption via the epithelial sodium channel (ENaC).
The experiments in Figs 2–7 were performed in open-circuit mode using chambers with an aperture of 0.283 cm2. The transepithelial voltage (Vte) is given in reference to the serosal side. Transepithelial resistance (Rte) was calculated from the voltage deflections (ΔVte) induced by short current pulses (25 μA, 0.6 s). These deflections were corrected by values obtained in an empty chamber. The equivalent short circuit current (Isc) was calculated by Ohm's law from Vte/Rte. The calculated Isc changes in Supplemental Table S2 were derived from peak values. After an equilibration period of 30 min adrenaline was added to the basolateral side. In the experiments with propranolol, the β-receptor blocker was added directly after mounting the tissue. The data in Fig. 9 were recorded as short circuited currents with tissue clamped to 0 mV.
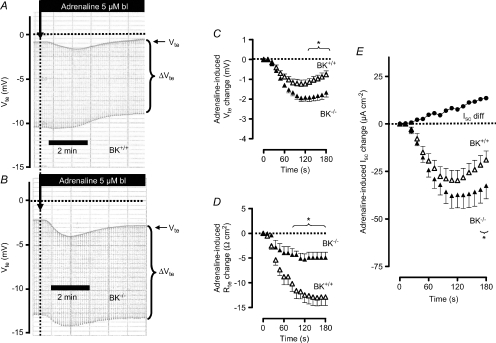
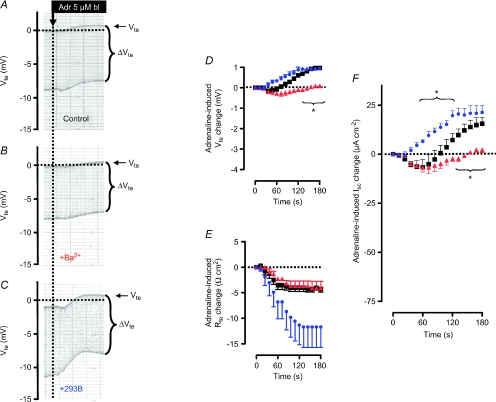
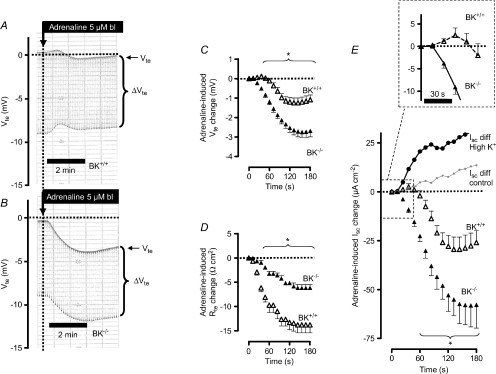
Figure 2. The effect of basolateral addition of adrenaline on ion secretion in distal colonic mucosa from BK+/+ and BK−/− mice (SV129×C57Bl6 hybrid background).
A and B, two original Ussing chamber recordings in BK+/+ (A) and in BK−/− tissue (B). Note the larger Vte effect in BK−/− tissue and the larger Rte effect (as seen in ΔVte effect) in BK+/+ tissue. C, summary of adrenaline-induced Vte effects in distal colonic mucosa from BK+/+ (n= 8) and BK−/− (n= 6) mice. D, summary of adrenaline-induced Rte effects in distal colonic mucosa from BK+/+ and BK−/− mice. E, summary of calculated adrenaline-induced Isc effects in distal colonic mucosa from BK+/+ and BK−/− mice. The positive-directed difference Isc curve was obtained by subtracting ΔIsc BK−/− from ΔIsc BK+/+. This positive current indicates electrogenic K+ secretion. * indicates statistical significance (P < 0.05) between BK+/+ and BK−/− curves.
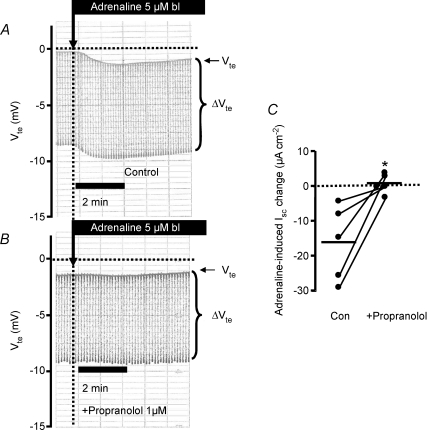
Figure 7. Basolateral (bl) adrenaline-induced ion transport effects in distal colonic mucosa of NMRI mice from untreated controls and propranolol-incubated (1 μm) tissues.
A and B, original Ussing chamber recordings in untreated controls (A) and in propranolol-incubated tissues (B). C, summary of calculated adrenaline-induced Isc effects in the absence and presence of propranolol. From each mouse two distal colonic, directly proximate mucosa pieces served as the paired control (* indicates statistical significance between groups). The connecting lines between the data points indicate data from the same mouse. Note that adrenaline-induced electrogenic ion transport effects were completely absent with propranolol. The horizontal bars indicate mean values.
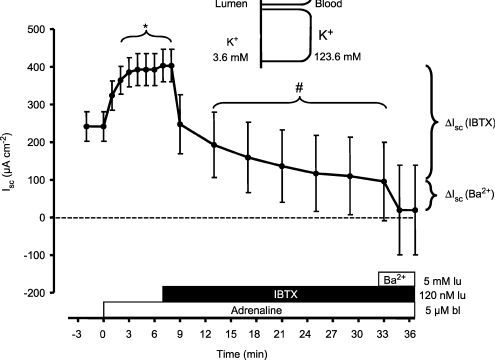
Figure 9. Basolateral adrenaline induced a sustained positive short circuit current (Isc) in mouse (NMRI) distal colonic mucosa.
The tissue was clamped to 0 mV and the basolateral side exposed to a high K+ concentration (123.6 mm). Luminal iberiotoxin (IBTX, 120 nm) inhibited the major fraction of the positive Isc. The residual positive Isc was blocked by 5 mm luminal BaCl2. * indicates statistical significance between pre-control Isc values and those after addition of adrenaline. # indicates statistical significance between the adrenaline-induced Isc values (7 min post-stimulation) and those after addition of iberiotoxin (n= 5).
Serum aldosterone determinations
Blood sampling for serum aldosterone measurements was performed at 11.00 h in a group of control animals and a group of animals kept on the high K+ diet for 4 days. The animals were anaesthetised with a 3% isoflurane gas mixture (in air), killed and completely drained of blood (approx. 1 ml). Blood was collected in Microtainers (Becton Dickinson) and the tubes centrifuged at 12,000 g for 2 min to separate serum from the blood cells. The total serum aldosterone was assayed using a commercial radioimmunoassay (RIA, Coat-A-Count) kit (Siemens Medical Solutions Diagnostics, Ballerup, Denmark).
Imaging of isolated mouse distal colonic crypts
The set-up comprised an inverted microscope (Axiovert 100TV, Zeiss, Jena, Germany) with the objective LD LCI Plan-Apochromat (oil, glycerine, water) 25×/0.8 NA (Zeiss), a monochromator (Polychrome IV, Till Photonics, Planegg, Germany) and a CCD camera (MicroMax, 5 MHz, Princeton Instruments, NJ, USA). Image acquisition and data analysis were performed with Metamorph/Metafluor (Universal Imaging, West Chester, PA, USA). Measurement of [Ca2+]i was performed with Fluo-4. Isolated crypts were transferred into a custom-made perfusion chamber and mechanically suspended between two holding pipettes. They were incubated in 20 μm Fluo-4 AM for 10 min at room temperature in control solution. Only those crypts that appeared intact in the transmission image with clear delineation of the luminal duct and the basolateral membranes before and after the experiment were selected. The Fluo-4 measured [Ca2+]i changes (excitation, 488 nm; emission, >510 nm) were expressed relative to stable baseline values (acquisition frequency, 0.5 Hz). To reduce potential photodamage, the excitation light intensity was controlled by neutral density filters in the excitation light path. In addition, the excitation light exposure time was minimized by 3-fold binning of the CCD camera output. Fluorescence was recorded from the entire crypt. The experiment was started 2–3 min after washout of Fluo-4. During the experiments the entire preparation was superfused at about 3–5 ml min−1, i.e. ∼10 bath exchanges per minute. The agonists adrenaline (5 μm) and ATP (100 μm) were prepared freshly, pH adjusted and added to the superfusion solution.
Solutions and chemicals
All chemicals were supplied by Sigma Aldrich or Merck.
Statistics
The data shown are mean ±s.e.m. n refers to the number of tissue preparations and one or two preparations were used from each animal. All data were tested for normal distribution (Kolmogorov–Smirnov test) and Student's t test was used to compare mean values between the experimental series. Two effects in the same tissue were tested paired (Fig. 7C) and effects obtained from different tissues were tested unpaired (Fig. 1C). One-way (Figs 8B and 9 and Supplemental Fig. S2) or two-way (Figs 2C–E, 3C–E, 4C–E, 5C–E and Supplemental Fig. S1) ANOVA was used to allow multiple comparisons. P values of <0.05 were accepted as statistically significant.
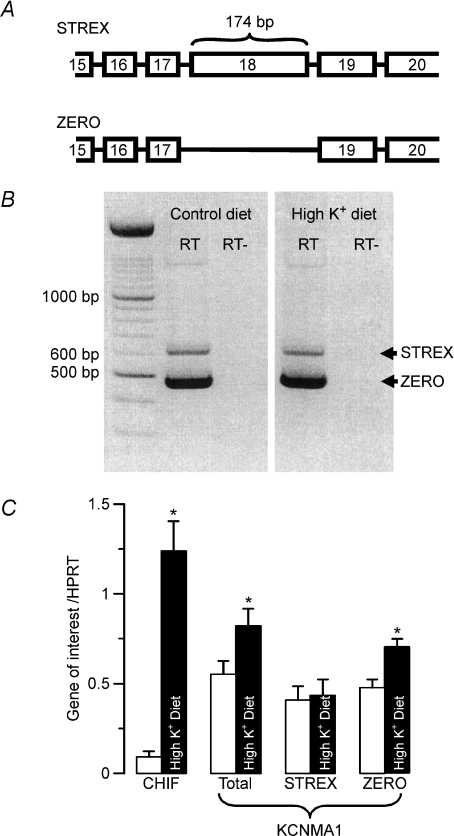
Figure 1. Identification and regulation of the STREX and ZERO splice variants of the BK α-subunit in enterocytes of distal colon from NMRI mice on control and high K+ diets.
A, schematic view of the C-terminus exon information of both splice variants. B, the STREX (longer) and ZERO (shorter) PCR products of BK channel mRNA. The identical pattern was seen after high K+ diet (n= 6). RT and RT– indicate the presence or absence of reverse transcriptase during the generation procedure of cDNA. C, semi-quantification of BK channel splice variant transcripts STREX and ZERO in isolated colonic crypts from control mice and mice fed a high K+ diet (n= 7 in each group). The amount of mRNA of the genes of interest is shown relative to the reference gene HPRT. Control data: open bars; high K+ diet data: filled bars. Corticosteroid hormone-induced factor (CHIF) served as a positive control for the effect of aldosterone. * indicates statistical significance (P < 0.05) between groups.
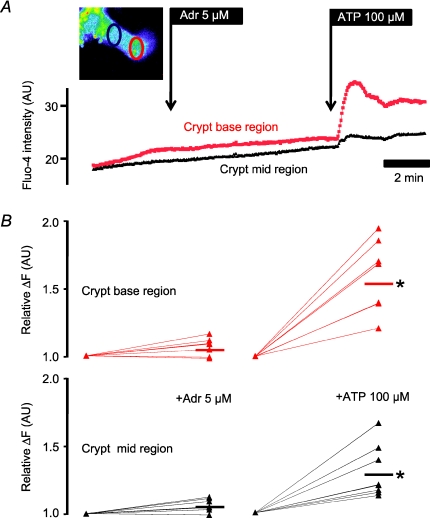
Figure 8. Intracellular Ca2+ measurements in isolated NMRI mouse colonic crypts.
A, Fluo-4 fluorescence (original recording) measured in crypt base region (red) and mid-crypt region (black). Adrenaline (5 μm) did not increase [Ca2+]i. In contrast, 100 μm ATP increased [Ca2+]i especially in the crypt base region. Inset: a single Fluo-4 loaded crypt displayed in pseudo-colour. The crypt base measured region of interest is marked in red and the crypt mid-region in black. B, summary of the relative increase of Fluo-4 fluorescence as a function of agonist addition (n= 8). The short horizontal bars indicate mean values and the * a significant [Ca2+]i increase.
Results
Identification of STREX and ZERO BK splice variants in distal colonic enterocytes
Figure 1A shows a schematic view of the C-terminus exon information of the mouse KCNMA gene. RT-PCR was performed with mRNA extracted from isolated distal colonic crypts. KCNMA primers spanning from exon 14 to exon 20 resulted in two different PCR products. These two PCR products had a size difference of approximately 150–200 base pairs (Fig. 1B) (6 mice in each group). The sequencing of both PCR products revealed that the disparity between these products was a 174 base pair segment identical to exon 18. Exon 18 is the splice target defining the splice variants STREX (with Exon 18) and ZERO (without Exon 18). Thus, both the STREX and ZERO splice variants are detected in mouse colonic enterocytes.
Regulation of BK splice variants in mouse colonic crypts
We have recently shown that a high K+ diet increases the amount of mRNA coding for the BK channel α-subunits in mouse distal colonic enterocytes (Sorensen et al. 2008). First we investigated if the above-described splicing pattern is altered in mice kept on a high K+ diet. We found similar PCR products in tissues from control mice as compared to those on a high K+ diet (Fig. 1B, right). Subsequently, we investigated which of the different BK α-subunit splice variants were affected by a high K+ diet. We designed primers for semi-quantitative RT-PCR of the STREX and ZERO variants and the total BK α-subunit mRNA. The corticosteroid hormone-induced factor was used as positive control for the aldosterone responsiveness of the tissue (Wald et al. 1996), and β-actin and HPRT were used as reference genes. Corticosteroid hormone-induced factor mRNA levels were greatly up-regulated (11-fold) in animals on a high K+ diet. K+-loaded animals show a significant increase of the total (a factor of 1.8) and ZERO variant (a factor of 1.6) mRNA. The STREX variant was, however, not regulated, regardless of whether β-actin or HPRT mRNA was used as reference transcript. Thus, a high K+ diet leads to the differential up-regulation of the ZERO splice variant of the BK channel α-subunit.
Adrenaline induces a BK channel-dependent electrogenic K+ secretion
In non-epithelial tissue, the ZERO variant was found to be activated via an increase of intracellular cAMP (Tian et al. 2001a,b; Friis et al. 2003; Chen et al. 2005). Importantly, adrenaline induces a sustained electrogenic K+ secretion in distal colon from different species, which involves an increase of intracellular cAMP in enterocytes (Rechkemmer et al. 1996). The following experiments were performed to investigate if the known adrenaline-activated mammalian distal colonic K+ secretion is conducted via the BK channel. In the Ussing chamber the adrenaline-induced K+ secretion is seen as a lumen-positive deflection of the transepithelial voltage (Vte). In terms of the transepithelial currents, adrenaline induced persistent K+ secretion in guinea pig (Rechkemmer et al. 1996), rabbit (Halm & Frizzell, 1986) and rat distal colon (Grotjohann et al. 1998). These results appear to contrast with our observations in mice. As shown in Fig. 2A, addition of basolateral adrenaline (5 μm) resulted in a transient Vte change towards lumen negative values from −1.14 ± 0.13 to −2.36 ± 0.29 mV (n= 8) within the first 2 min. After approximately 5 min Vte had returned to pre-control values. By this time, the transepithelial resistance had decreased significantly (see Fig. 2D and Supplemental Table S2) to a new stable value. This finding is by no means surprising because cAMP-elevating agonists commonly activate both electrogenic K+ and Cl− secretion simultaneously. Thus, the shift of Vte to lumen-negative values demonstrates that adrenaline predominantly induces transcellular anion secretion in mouse colonic epithelia. This finding was consistent regardless of whether the muscle layer was mechanically removed from the preparation or left intact (see Supplemental Fig. S1).
In previous studies, we used BK channel α-subunit-deficient mice (BK−/−) to identify the crucial role of this channel in distal colonic K+ secretion. Here we followed the same strategy to test if the BK channel is responsible for the cAMP-activated K+ secretion in colonic epithelia. Figure 2A and B shows two original Ussing chamber traces of adrenaline-induced ion transport effects in tissue from BK+/+ and BK−/− mice, respectively. Two interdependent observations can be extracted: (1) The transient adrenaline-induced deflection of Vte towards lumen-negative voltages was significantly larger in BK−/− tissue (Fig. 2C, Supplemental Table S2). 2. The reduction of Rte induced by adrenaline was significantly smaller in tissue lacking BK channels (Fig. 2D, Supplemental Table S2). Thus, the adrenaline-induced short circuit current was significantly larger in BK−/− mice (Fig. 2E, Supplemental Table S2). These results suggest that adrenaline in the presence of the luminal BK channels activates K+ secretion in parallel with a CFTR-mediated anion secretion. These processes electrically oppose each other. In the absence of K+ secretion, the larger change in Vte and Isc probably reflects electrically isolated CFTR-mediated anion secretion. The experiments were performed to electrically identify the adrenaline-stimulated distal colonic K+ secretion. By subtracting the two currents induced by adrenaline in BK+/+ and BK−/− mice, the adrenaline-induced K+ secretion is unmasked. These results indicate that the BK channel conducts the adrenaline-activated K+ secretion.
The difference of the adrenaline-induced short circuit currents between BK+/+ and BK−/− mouse tissue served to isolate the activated K+ secretion. These findings can be substantiated using the K+ channel blocker Ba2+. Figure 3A and B show representative recordings and Fig. 3D–F quantify the effects of BaCl2 on adrenaline-induced changes in Vte, Rte and Isc. In Fig. 3A, B and F one can see that the lumen-positive component of the adrenaline-induced Isc effect is significantly reduced by BaCl2. These data are thus in agreement with those presented in Fig. 2 using BK−/− mice.
Figure 3. The effects of luminal BaCl2 (5 mm) and basolateral (bl) 293B (5 μm) on adrenaline (Adr)-induced changes in Vte, Rte and Isc in mouse distal colonic mucosa from NMRI mice on control diet conditions.
A–C show representative recordings and D–F quantify the effects. The effects of BaCl2 and 293B are shown in red and blue, respectively. * indicates statistical significance (P < 0.05) between treated tissues and control curves.
Basolateral K+ channel activation is an important regulator of epithelial ion secretion. Anion secretion is promoted and, reciprocally, cation secretion is diminished by basolateral K+ channel activation. In distal colonic epithelia, it is well described that inhibition of basolateral K+ channels augments luminal K+ exit (Halm & Frizzell, 1986; Flores et al. 2007). Therefore, we used 293B which specifically blocks the basolateral KCNQ1/KCNE3 K+ channels to augment the apical K+ and in parallel reduced luminal anion exit. Activation of this ion channel is necessary to allow the stimulation of cAMP-dependent anion secretion. In the presence of 293B the adrenaline-induced Isc response is monophasic and continuous in the lumen-positive direction (Fig. 3C and F). This finding is consistent with a now predominant K+ secretory effect. It indicates that 293B has unmasked an adrenaline-induced K+ secretion.
Adrenaline-induced K+ secretion is increased in mice on a high K+ diet
Another way of stimulating the total BK channel activity is to increase its expression in the apical membrane via aldosterone. We have previously shown that mice on a high K+ diet for 4 days have a substantial increase in plasma aldosterone (Sorensen et al. 2008). In the following experiments we used this protocol to investigate the adrenaline-induced ion transport in distal colon from BK+/+ and BK−/− mice (Sorensen et al. 2008). The functional differences between BK+/+ and BK−/− tissue (Fig. 2A–E) were much more pronounced in the colon from animals on a high K+ diet (Fig. 4A–E, Supplemental Table S2). The differential effects become most apparent in the time course of the adrenaline-induced Isc effect, where the adrenaline-induced Isc change initially becomes positive in the BK+/+ tissue (see inset in Fig. 4E). First after a lag time of 52.3 ± 5.5 s the induced Isc change shifted polarity and became lumen-negative (see inset in Fig. 4E). This shows that the K+ current in animals on a high K+ diet is amplified to an extent where the compound effect is forced in the positive direction. In contrast, BK−/− tissue responded with a prompt monophasic change of Isc in the lumen-negative direction, a consequence of a lack of K+ secretion. The maximal adrenaline-induced Isc effect in the BK−/− mice was not significantly different in animals fed control diet as compared to a high K+ diet. The subtraction of the currents induced by adrenaline in BK+/+ and BK−/− mice unmasks a now much enlarged adrenaline-induced K+ secretion (Fig. 4E). Thus, these results support the idea that the adrenaline-induced K+ secretion is mediated via the BK channel and that this K+ secretion is increased in mice on a high K+ diet.
Figure 4. The effect of a high K+ diet on adrenaline-stimulated ion secretion in mouse distal colonic mucosa from BK+/+ and BK−/− mice (SV129×C57Bl6 hybrid background).
A and B, two original Ussing chamber recordings in BK+/+ (A) and in BK−/− tissue (B). Note the difference of a larger Vte effect in the BK−/− tissue. Note also that adrenaline induces a biphasic change of Vte with an initial change into the lumen-positive direction in the BK+/+ tissue. C, summary of adrenaline-induced Vte effects (n= 8). D, summary of adrenaline-induced Rte effects. E, summary of calculated adrenaline-induced Isc effects. The positive-directed difference Isc curve was obtained by subtracting ΔIsc BK−/− from ΔIsc BK+/+. This positive current indicates electrogenic K+ secretion. For comparison with the data in Fig. 2E the grey line shows this differential current from control mice. Note the up-regulation of apparent BK conducted electrogenic K+ secretion in mice treated on a high K+ diet. Note also the inset to enlarge the initial period of the adrenaline-induced Isc effect. * indicates statistical significance (P < 0.05) between BK+/+ and BK−/− curves.
Adrenaline induces electrogenic K+ secretion in CFTR−/− mice
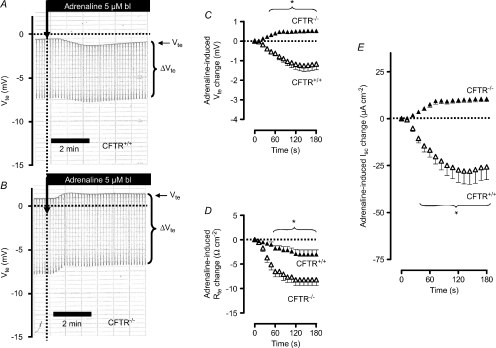
In the above experiments, we used BK−/− mice to identify the adrenaline-stimulated distal colonic K+ secretion. An alternative approach to electrically isolate the adrenaline-induced K+ secretion would be to remove the CFTR-conducted anion secretion and therefore we used a CFTR−/− mouse. In Fig. 5A the adrenaline-induced effect in a CFTR+/+ mouse is shown and was found to be nearly identical to that described in Fig. 2A (Supplemental Table S2). We could verify that CFTR−/− colonic tissue does not respond to elevation of cAMP (e.g. with forskolin, data not shown) with an increase of anion secretion, seen as an increase of the lumen-negative Vte (Weiner et al. 2008). In the absence of inducible anion secretion, adrenaline now triggered an ion secretion in the lumen-positive direction (Fig. 5B, C and E). Similar observations with other cAMP-increasing manoeuvres were made by other investigators using CFTR−/− mice (Weiner et al. 2008). Of note, the positive current in the CFTR−/− mice is similar to the subtracted Isc curve (BK−/− minus BK+/+) in Figs 2E and 6. This supports the idea that adrenaline simultaneously activates two opposing electrogenic ion transport processes, i.e. anion secretion and K+ secretion. In summary, the results of these experiments further support the hypothesis that the adrenaline-induced lumen-positive current is caused by K+ secretion.
Figure 5. Basolateral (bl) adrenaline-induced ion transport effects in distal colonic mucosa from CFTR+/+ and CFTR−/− mice (NMRI background).
A and B, original Ussing chamber recordings in CFTR+/+ (A) and in CFTR−/− tissue (B). Note that adrenaline induces Vte deflections in CFTR−/− tissue in the positive direction. C, summary of adrenaline-induced Vte effects in distal colonic mucosa from CFTR+/+ (n= 10) and CFTR−/− (n= 11) mice. D, summary of adrenaline-induced Rte effects in distal colonic mucosa from CFTR+/+ and CFTR−/− mice. E, summary of calculated adrenaline-induced Isc effects in distal colonic mucosa from CFTR+/+ and CFTR−/− mice. * indicates statistical significance (P < 0.05) between CFTR+/+ and CFTR−/− curves.
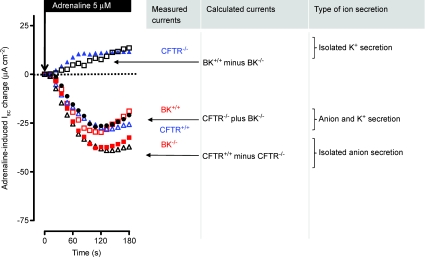
Figure 6. Adrenaline-induced Isc effect in BK+/+ (open red squares), BK−/− (filled red squares), CFTR+/+ (open blue triangles) and CFTR−/− tissues (filled blue triangles) (re-plotted fromFigs 2 and 5).
Adrenaline activates an isolated K+ secretion in CFTR−/− mice and an isolated anion secretion in BK−/− mice. The open black square curve shows the differential currents when subtracting ΔIsc BK−/− from BK+/+. This differential current is positive, reflects adrenaline-stimulated K+ secretion and is similar to that induced in CFTR−/− mice. The open black triangle curve shows the differential currents when subtracting ΔIsc CFTR−/− from CFTR+/+. This differential current is large and negative, reflects adrenaline-stimulated anion secretion and is similar to that induced in BK−/− mice. The filled black circles show the magnitude of current achieved by adding ΔIsc CFTR−/− to ΔIsc BK−/−. This current is the same as the control CFTR+/+ and BK+/+ currents.
Figure 6 compiles the original adrenaline-induced Isc effects of BK+/+, BK−/−, CFTR+/+ and CFTR−/− into one graph. In addition this figure also displays the ‘calculated’ adrenaline effects (subtracted Isc currents). We argue that the summation of the isolated K+ secretion (seen in CFTR−/−) and the isolated anion secretion (seen in BK−/−) should be similar in magnitude to those data where both conductances are stimulated at the same time in wildtype colonic tissues. Exactly this can be seen in the filled black circle curve. This figure supports an idea which assumes a clear separability of the two opposing ion conductances.
Propranolol inhibits the adrenaline-induced ion secretion
The stimulatory effect of adrenaline on ion secretion was completely inhibited in tissues pre-incubated for 30 min with 1 μm of the non-selective β-receptor blocker propranolol (Fig. 7). This confirms previous results from other mammalian species (Binder & Sandle, 1994) and strongly supports the involvement of an increase of cytosolic cAMP in this response. We also investigated if adrenaline could increase intracellular Ca2+ as reported in guinea pig distal colonic (Del Castillo et al. 1999). Figure 8 demonstrates that this was clearly not the case: adrenaline (5 μm) was without effect on [Ca2+]i. By contrast, the purinergic receptor agonist ATP (100 μm) robustly increased [Ca2+]i preferentially in crypt base cells as previously documented for isolated rat colonic crypts (Leipziger et al. 1997).
Effect of basolateral adrenaline on the apical membrane K+ conductance
The above-described functional data imply that adrenaline is able to activate apical BK channels. To substantiate this we chose the technique of basolateral membrane voltage depolarization in the Ussing chamber. By increasing the basolateral K+ concentration from 3.6 to 123.6 mm the basolateral membrane is electrically eliminated. Under short-circuited conditions only the chemical K+ gradient from the basolateral to the mucosal side will drive a K+ current across the apical membrane (Fuchs et al. 1977; Schultheiss & Diener, 1997; Schultheiss et al. 2003). Figure 9 shows the results of adrenaline-induced K+ secretion under these conditions. After substituting Na+ with K+ in the basolateral fluid compartment, novel steady-state conditions with a stable positive Isc (242 ± 39 μA cm−2, n= 5) were reached after ∼30 min. Addition of basolateral adrenaline promptly increased the positive Isc and after 2–3 min a novel, stable level of 403 ± 43 μA cm−2 (n= 5) was measured. Subsequent addition of 120 nm luminal iberiotoxin (IBTX) inhibited the major fraction of this positive Isc. Finally, addition of 5 mm BaCl2 to the luminal side inhibited the residual current. These data support the idea that adrenaline triggered the opening of apical BK channels in distal colonic mucosa. They also confirm previous results showing that luminal IBTX is able to inhibit the major fraction of the resting and stimulated distal colonic K+ secretion. These data substantiate the central role of the apical BK channel in adrenaline-induced distal colonic K+ secretion.
Discussion
Distal colonic K+ secretion is an essential component of K+ homeostasis. It is present under resting conditions, is enhanced by aldosterone and is activated by agonists that increase either intracellular Ca2+ or cAMP (Foster et al. 1983, 1985; Rechkemmer & Halm, 1989; Sausbier et al. 2006). Until recently, the precise nature of the distal colonic secretory K+ channel was unknown, but was suggested to be the large conductance Ca2+-activated BK channel (Butterfield et al. 1997). Our preceding publications used BK−/− mice to unequivocally demonstrate that the BK channel is responsible for resting, Ca2+- and aldosterone-induced distal colonic K+ secretion (Sausbier et al. 2006; Sorensen et al. 2008).
Colonic K+ secretion is markedly elevated by increments of cAMP in enterocytes (Foster et al. 1983; Rechkemmer et al. 1996) and adrenaline is an important agonist for cAMP-mediated electrogenic K+ secretion in mammalian distal colon (Rechkemmer et al. 1996; Grotjohann et al. 1998; Horger et al. 1998). However, the secretory ion channels involved in the adrenaline-induced K+ secretion remained to be defined. In this context, it is noteworthy that the BK channel is a known target for cyclic nucleotide-dependent protein kinases. In different cellular systems the increase of cAMP and cGMP leads to the activation of BK currents (Schubert & Nelson, 2001; Chen et al. 2005). Thus, we hypothesized that colonic adrenaline-induced K+ secretion could be conducted via the BK channel. Using the BK−/− mouse, we now provide evidence that adrenaline-induced K+ secretion is absent in distal colonic mucosa from these mice. The electrical visibility of adrenaline-induced K+ secretion is hidden within the total induced current, which comprises a larger and opposing anion secretion and K+ secretion. By eliminating the K+ secretory component in the BK−/− tissue or vice versa the anion secretory component in CFTR−/− tissue, we isolated the electrogenic K+ secretion in mouse distal colon. In the original data measured in BK−/− and CFTR−/− mice (Figs 2 and 5), the adrenaline-stimulated drop of Rte is significantly reduced as compared to wildtype controls. These findings are probably best explained by the lack of an adrenaline-induced opening of the relevant apical BK-dependent K+ or CFTR-mediated Cl− conductance. The data in Fig. 3 showed that luminal BaCl2 was qualitatively able to mimic the data obtained in BK−/−; however, we did not observe a significant difference in the adrenaline-induced drop of Rte in the presence and absence of luminal BaCl2. Currently, we have no explanation for this discrepancy but one possibility is that 5 mm Ba2+ might still leave some apical BK channel open.
In BK+/+ animals fed a high K+ diet, the adrenaline-induced K+ secretion showed a 2-fold increase and the initial Isc effect was lumen-positive. This K+ secretory effect is also seen when the K+ secretion pathway is stimulated separately by luminal nucleotides (ATP/UTP) and, as mentioned, is strictly dependent on the BK channel (Sausbier et al. 2006). As expected for CFTR−/− tissue, adrenaline-induced anion secretion was absent. Instead, a slow and sustained positive Isc effect was observed, a phenomenon consistent with the activation of K+ secretion. We also attempted to up-regulate K+ secretion in CFTR−/− mice. To avoid serious gastrointestinal symptoms, CFTR mice received an oral solution that provokes a mild degree of osmotic diarrhoea (Oralav) (Clarke et al. 1996). Oralav contains a significant amount of Na+ (125 mm), which prevented the plasma aldosterone levels to increase when the animals are fed a high K+ diet (see Supplementary Fig. S2). In these mice, we did not observe an increase in the amiloride-sensitive Na+ absorption after feeding a high K+ diet (see Supplementary Fig. S2). The very low amiloride-inhibitable Isc is indicative of low plasma aldosterone. Thus, the oral osmotic solution, probably through its significant Na+ content, suppresses the high K+ diet-induced increase of aldosterone. We did carry out experiments with CFTR−/− mice supplied with normal tap water.
Further experiments were conducted to evaluate if adrenaline is able to increase the apical K+ conductance in mouse distal colon. For this purpose we used an established method that eliminates the critical role of the basolateral membrane voltage (Fuchs et al. 1977; Schultheiss & Diener, 1997; Schultheiss et al. 2003). By substituting basolateral Na+ with K+ and clamping the mucosa to 0 mV, the serosal-to-mucosal K+ gradient provides the driving force for transepithelial K+ secretion. Under these conditions the influence of transepithelial Na+ and Cl− ion fluxes are minimal (ENaC inhibited with luminal amiloride; a very small Cl− gradient). Therefore, the positive Isc shown in Fig. 9 most certainly reflects a sustained K+ secretion. It is clearly seen that the largest fraction of this K+ secretion is blocked by luminal IBTX, probably indicating that the major fraction of transepithelial K+ secretion occurs via a transcellular route and uses the BK channel in the apical membrane. The remaining positive current is inhibited with luminal Ba2+ and this further supports the idea that the measured positive Isc under these conditions reflects transepithelial K+ secretion. Taken together, our data strongly indicate that the adrenaline-induced distal colonic K+ secretion is conducted via apical BK channels.
Adrenergic β-receptor stimulation of colonic mucosa is well described to elicit the activation of ion secretion in mammalian distal colon (Rechkemmer et al. 1996; Grotjohann et al. 1998; Horger et al. 1998). Interestingly, the stress-induced diarrheal response is believed to involve mucosal β-receptors (Lennane et al. 1975). β-Receptors in general are well established to cause an increase of cAMP and we have used adrenaline in the assumption that its action is primarily mediated via activation of mucosal β-receptors. Our data with propranolol as non-selective β-receptor antagonist supports and confirms that adrenaline triggers its effect via β-receptor activation, which in all likelihood involves an increase of cAMP in the enterocytes. This is supported by the lack of adrenaline-induced [Ca2+]i increments in isolated colonic crypts.
Frequently, the two C-terminal STREX and ZERO splice variants of the BK channel are found in BK-expressing tissues (Tian et al. 2001a,b; Friis et al. 2003; Chen et al. 2005). Here we document that both splice variants are present in mouse distal colonic enterocytes. This finding could be highly relevant regarding agonist-stimulated colonic K+ secretion. The STREX variant is inhibited and the ZERO variant activated by cAMP (Chen et al. 2005). Thus, if a cAMP agonist (e.g. adrenaline) stimulates K+ secretion, the effect is probably mediated by the ZERO splice variant. Intriguingly, we observed that the ZERO variant was significantly up-regulated in animals fed a high K+ diet. This selective up-regulation tempts us to speculate that aldosterone could be an up-stream regulator of the nuclear spliceosome. Aldosterone has not previously been shown to have any effects on the spliceosome, though this has been reported for other steroid hormones (MacDonald et al. 2004). These molecular data support the functional data and indicate that the adrenaline-induced distal colonic K+ secretion is conducted via luminal BK channels and that an increase of cAMP probably involves the activation of the ZERO splice variant.
In conjunction with our preceding work, the present study provides a comprehensive understanding of colonic K+ secretion via one channel. Independent of the mechanism of activation, colonic K+ secretion is always mediated by the apical BK channel and this channel appears to be the only functionally relevant K+ channel for distal colonic K+ secretion.
Acknowledgments
We thank Anne B. Strandsby and Edith B. Møller for expert technical assistance and Professor M. Diener for valuable advice about acquiring the data for Fig. 9. The project received financial support from the Desirée and Niels Ydes Foundation and the Danish Medical Research Foundation, DFG SE460/13-3 and SFB621/C9. No conflict of interest exists.
Supplemental material
Suppl. Figure 1
Suppl. Fig. 2
Suppl. tab. 1
Suppl. tab. 2
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 2133–2171. [Google Scholar]
- Butterfield I, Warhurst G, Jones MN, Sandle GI. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. J Physiol. 1997;501:537–547. doi: 10.1111/j.1469-7793.1997.537bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Tian L, Macdonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) α-subunits generated from a single site of splicing. J Biol Chem. 2005;280:33599–33609. doi: 10.1074/jbc.M505383200. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46:612–618. [PubMed] [Google Scholar]
- Del Castillo Jr, Arevalo JC, Burguillos L, Sulbaran-Carrasco MC. β-Adrenergic agonists stimulate Na+-K+-Cl− cotransport by inducing intracellular Ca2+ liberation in crypt cells. Am J Physiol Gastrointest Liver Physiol. 1999;277:G563–G571. doi: 10.1152/ajpgi.1999.277.3.G563. [DOI] [PubMed] [Google Scholar]
- Flores CA, Melvin JE, Figueroa CD, Sepulveda FV. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel Kcnn4. J Physiol. 2007;583:705–717. doi: 10.1113/jphysiol.2007.134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ES, Jones WJ, Hayslett JP, Binder HJ. Role of aldosterone and dietary potassium in potassium adaptation in the distal colon of the rat. Gastroenterology. 1985;88:41–46. doi: 10.1016/s0016-5085(85)80130-x. [DOI] [PubMed] [Google Scholar]
- Foster ES, Sandle GI, Hayslett JP, Binder HJ. Cyclic adenosine monophosphate stimulates active potassium secretion in the rat colon. Gastroenterology. 1983;84:324–330. [PubMed] [Google Scholar]
- Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O. Molecular and functional identification of cyclic AMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells. Circ Res. 2003;93:213–220. doi: 10.1161/01.RES.0000085041.70276.3D. [DOI] [PubMed] [Google Scholar]
- Fuchs W, Larsen EH, Lindemann B. Current–voltage curve of sodium channels and concentration dependence of sodium permeability in frog skin. J Physiol. 1977;267:137–166. doi: 10.1113/jphysiol.1977.sp011805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R, Bleich M, Leipziger J, Ecke D, Mall M, Kunzelmann K. Regulation of ion transport in colonic crypts. News Physiol Sci. 1997;12:62–66. [Google Scholar]
- Grotjohann I, Gitter AH, Kockerling A, Bertog M, Schulzke JD, Fromm M. Localization of cAMP- and aldosterone-induced K+ secretion in rat distal colon by conductance scanning. J Physiol. 1998;507:561–570. doi: 10.1111/j.1469-7793.1998.561bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm DR, Frizzell RA. Active K+ transport across rabbit distal colon; a relation to Na+ absorption and Cl− secretion. Am J Physiol Cell Physiol. 1986;251:C252–C267. doi: 10.1152/ajpcell.1986.251.2.C252. [DOI] [PubMed] [Google Scholar]
- Horger S, Schultheiss G, Diener M. Segment-specific effects of epinephrine on ion transport in the colon of the rat. Am J Physiol Gastrointest Liver Physiol. 1998;275:G1367–G1376. doi: 10.1152/ajpgi.1998.275.6.G1367. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- Leipziger J, Kerstan D, Nitschke R, Greger R. ATP increases [Ca2+]i and ion secretion via a basolateral P2Y receptor in rat distal colonic mucosa. Pflügers Arch. 1997;434:77–83. doi: 10.1007/pl00008079. [DOI] [PubMed] [Google Scholar]
- Lennane RJ, Peart WS, Shaw J. Adrenergic influences on the electrical potential across the colonic mucosa of the rabbit. J Physiol. 1975;250:367–372. doi: 10.1113/jphysiol.1975.sp011059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PN, Dowd DR, Zhang C, Gu C. Emerging insights into the coactivator role of NCoA62/SKIP in vitamin D-mediated transcription. J Steroid Biochem Mol Biol. 2004;89–90:179–186. doi: 10.1016/j.jsbmb.2004.03.097. [DOI] [PubMed] [Google Scholar]
- Pena-Munzenmayer G, Catalan M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci. 2005;118:4243–4252. doi: 10.1242/jcs.02525. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G, Frizzell RA, Halm DR. Active potassium transport across guinea-pig distal colon: action of secretagogues. J Physiol. 1996;493:485–502. doi: 10.1113/jphysiol.1996.sp021398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechkemmer G, Halm DR. Aldosterone stimulates K+ secretion across mammalian colon independent of Na+ absorption. Proc Natl Acad Sci U S A. 1989;86:397–401. doi: 10.1073/pnas.86.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol. 2006;17:1275–1282. doi: 10.1681/ASN.2005101111. [DOI] [PubMed] [Google Scholar]
- Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- Schultheiss G, Diener M. Regulation of apical and basolateral K+ conductances in rat colon. Br J Pharmacol. 1997;122:87–94. doi: 10.1038/sj.bjp.0701353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss G, Ribeiro R, Schafer KH, Diener M. Activation of apical K+ conductances by muscarinic receptor stimulation in rat distal colon: fast and slow components. J Membr Biol. 2003;195:183–196. doi: 10.1007/s00232-003-0618-y. [DOI] [PubMed] [Google Scholar]
- Siemer C, Gögelein H. Nonselective cation-channels in the basolateral membrane of crypt cells from rat distal colon. Pflügers Arch. 1990;415:R46. doi: 10.1007/BF00374465. [DOI] [PubMed] [Google Scholar]
- Sorensen MV, Matos JE, Sausbier M, Sausbier U, Ruth P, Praetorius HA, Leipziger J. Aldosterone increases KCa1.1 (BK)-channel-mediated colonic K+ secretion. J Physiol. 2008;586:4251–4264. doi: 10.1113/jphysiol.2008.156968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweiry JH, Binder HJ. Characterization of aldosterone-induced potassium secretion in rat distal colon. J Clin Invest. 1989;83:844–851. doi: 10.1172/JCI113967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001a;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- Tian L, Hammond MS, Florance H, Antoni FA, Shipston MJ. Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. J Physiol. 2001b;537:57–68. doi: 10.1111/j.1469-7793.2001.0057k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald H, Goldstein O, Asher C, Yagil Y, Garty H. Aldosterone induction and epithelial distribution of CHIF. Am J Physiol Renal Physiol. 1996;271:F322–F329. doi: 10.1152/ajprenal.1996.271.2.F322. [DOI] [PubMed] [Google Scholar]
- Weiner SA, Caputo C, Bruscia E, Ferreira EC, Price JE, Krause DS, Egan ME. Rectal potential difference and the functional expression of CFTR in the gastrointestinal epithelia in cystic fibrosis mouse models. Pediatr Res. 2008;63:73–78. doi: 10.1203/PDR.0b013e31815b4bc6. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Smith PL, Fromm M, Frizzell RA. Crypts are the site of intestinal fluid and electrolyte secretion. Science. 1982;218:1219–1221. doi: 10.1126/science.6293054. [DOI] [PubMed] [Google Scholar]
- Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.