Abstract
Recent evidence has implicated the orbitofrontal cortex (OFC) in the pathophysiology of social deficits in autism. An MRI-based morphometric study of the OFC was conducted involving 11 children with autism (age range 8.1–12.7 years) and 18 healthy, age-matched controls (age range 8.9–12.8 years). Decreased grey matter volume in the right lateral OFC in the patient group was found, and correlations were observed between social deficits and white, but not grey, matter structures of the OFC. These findings support the role of OFC in autism and warrant further investigations of this structure using structural and functional methodologies.
Keywords: Autism, Grey Matter, White Matter, Orbitofrontal Cortex, MRI, Social Deficits
1. Introduction
Impairments in social interactions and behaviors are among the essential characteristic features of individuals with autism. A recent hypothesis proposed that abnormalities of the orbitofrontal-amygdala circuit may underlie several of the core features of this severe developmental disorder (Bachevalier and Loveland, 2006). The orbitofrontal cortex (OFC), and more specifically its right lateral subdivision, appears to play an important role in social cognition (O’Doherty et al., 2001; Vollm et al., 2005), with some evidence suggesting its role in the pathophysiology of autism (Bachevalier and Loveland, 2006; Dawson et al., 2002; Salmond et al., 2003). A morphometric MRI investigation reported decreased volume of the total (i.e., grey plus white matter) right lateral OFC in children and adolescents with autism, which contrasted with increased size in adults (Hardan et al., in press). Moreover, a recent fMRI study of healthy adults reported that processing theory of mind tasks, a function believed to be impaired in individuals with autism, was associated with increased activation of the right lateral OFC (Vollm et al., 2005). Finally, the potential relevance of the OFC in autism is highlighted by its role in the reward system (O’Doherty et al., 2001), with evidence suggesting that impairment in assigning and flexibly modifying social reward values may be related to some of the social deficits observed in pervasive developmental disorders (Dawson et al., 2002).
In light of the mounting evidence implicating the OFC in social functioning, this study was conducted in a sample comprised exclusively of children with autism to examine grey and white matter volumes of this structure, and to investigate the relationship between OFC structures and social deficits as measured by the Autism Diagnostic Interview-Revised (ADI-R) and Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1989; Lord et al., 1994). We hypothesized that right lateral OFC grey matter would be decreased in children with autism when compared with matched controls, and negative correlations would be observed between OFC grey and white matter structures and social deficits.
2. Methods
2.1. Participants
Subjects were 11 boys with autism and 18 healthy male controls between eight and 12 years of age. The autistic subjects represented all consecutive referrals to a research clinic who were eligible to participate in the study. The diagnosis of autism was established through the administration of the ADI-R and the ADOS, in addition to expert clinical evaluation. Subjects meeting diagnostic criteria for autism but without delayed or abnormal language development were considered to have Asperger’s Disorder and were not included in this study. Children with secondary autism related to a specific etiology such as tuberous sclerosis or Fragile X were excluded, as were potential subjects with evidence of genetic, metabolic, seizure, or infectious disorders. All subjects had a full-scale IQ (FSIQ) > 70.
Controls were children recruited from the community through advertisements in areas socioeconomically comparable to those of the families of origin of the autistic subjects. Socioeconomic status for all subjects was assessed using the Hollingshead method (Hollingshead, 1975). Control subjects were screened by face-to-face interviews, questionnaires, telephone interviews, and observation during psychometric tests, and individuals with a family history of any neuropsychiatric disorder, such as autism, learning disability, affective disorders, and schizophrenia, were not included. Potential subjects with a history of birth asphyxia, head injury, or a seizure disorder were also excluded.
All control subjects had a FSIQ > 70 and no learning disability as assessed by the Wide Range Achievement Test-R. Subjects were not matched for IQ since available evidence does not support the existence of IQ effects on brain size (Hazlett et al., 2005). Exclusions for control subjects and individuals with autism were based on history and physical examination as well as laboratory testing when indicated. The Wechsler Intelligence Scale for Children-III was administered to measure cognitive functioning including FSIQ for all participants. Methodology of the study was approved by the Institutional Review Board. Written informed consent was obtained from parents and assent was obtained from all children.
2.2. MRI Scans
Scans were obtained on a GE 1.5 Tesla Signa scanner. A T1-weighted SPGR sequence was first acquired with the following parameters: slice thickness=1.5mm, slice numbers=124; TE 5ms; TR 25ms; flip angle 40 degrees, NEX 1; FOV 24cm; matrix 256×192. Proton density and T2-weighted images were then obtained using a single double-echo protocol with the following parameters: slice thickness=5mm; TE 17ms for PD or 102ms for T2; TR 2500ms; NEX 1; FOV 24cm; matrix 256×192; total slices = 24. All images were obtained in the coronal plane. MRI data were identified by scan number to retain blindness and analyzed using Brain Research: Analysis of Images, Networks and Systems software (BRAINS) while applying previously published methodologies of total brain volume (TBV) measurements (Magnotta et al., 2002). The image processing was performed on a SGI workstation (Silicon Graphics Inc., Mountain View, CA) using the BRAINS2 (University of Iowa, Iowa City, IA, USA) software package. The image data was normalized to standard Talairach stereotactic three-dimensional space (Talairach and Tournoux, 1988) by identifying six brain-limiting points (anterior, posterior, superior, inferior, left, and right); the anterior-posterior commissure line specified the x-axis, a vertical line rising from the x-axis through the interhemispheric fissure specified the y-axis, and a transverse orthogonal line with respect to x and y coordinates specified the z-axis. Registration was performed by aligning the T2 and PD images with a resampled T1 image and then resampling the T2 and PD images themselves (Magnotta et al., 2002). After normalization to a standard three-dimensional space, the pixels representing grey matter, white matter, and cerebrospinal fluid were identified using a segmentation algorithm applied to the T1, T2, and PD image sequences as described elsewhere (White et al., 2003).
2.3. Tracing Guidelines
Detailed descriptions of the method utilized to measure the OFC and TBV have previously been published (Hardan et al., in press; Lacerda et al., 2003; Magnotta et al., 2002). The OFC was manually traced in the coronal plane (Fig 1). The posterior boundary was the tip of the genu of the corpus callosum; the anterior boundary was the most anterior coronal slice where brain tissue could be identified; the superior boundaries were the inferior border of the anterior cingulate (in subgenual regions) and the midpoint of the intercommissural line (in front of the genu of the corpus callosum); and the lateral and inferior boundaries were demarcated based on the lateral and inferior surfaces, respectively, of the frontal lobes. In each slice, lines were drawn connecting the superior boundary point to the intersections of the inferior and lateral surfaces of the frontal lobes. These lines were the lateral borders of the tracings. The inferior boundary was made by tracing along the inferior surface of the frontal lobe within the lateral boundaries. The OFC was also subdivided into medial and lateral parts by drawing a line from the superior boundaries to the olfactory sulcus. For reliability assessment, two raters (RRG and JJN) independently traced the OFC and its subdivisions in each hemisphere in 10 randomly selected scans and obtained an intraclass correlation coefficient (ICC) superior to 0.91. The ICCs for grey and white matter structures were all greater than 0.95. Tracings used for analysis were conducted by one rater (RRG) on only a subset of the available scans (11/14) in the autistic group due to poor image quality and movement artifact of the remaining MRI scans. Measurements of TBV were performed using the BRAINS2 masks as generated by a neural network and corrected by manual tracing (ICC > 0.90). TBV was defined as the whole supra- and infratentorial brain tissue superior to the foramen magnum while excluding cerebrospinal fluid.
Fig. 1.
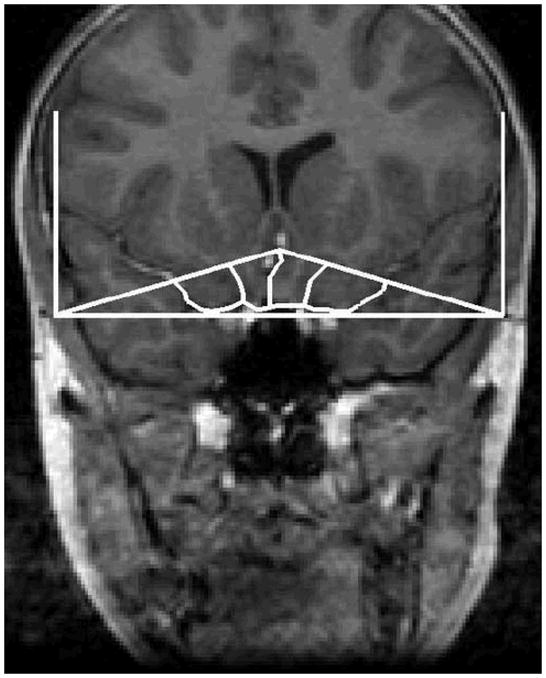
A tracing of the orbitofrontal cortex in the coronal plane.
2.4. Data Analysis
Due to the small sample size, all of the analyses were conducted using non-parametric tests. The Mann-Whitney U test was used to compare the autistic and control groups on demographic measures and OFC tracings. Values were expressed as means, standard deviation (M ± SD) and, when indicated, median. Regression analyses were conducted to account for possible effects of confounding factors such as TBV. Additionally, despite the unclear effect of IQ on brain size in individuals with autism, analyses were conducted to account for this potential confounding variable (Hazlett et al., 2005). Spearman’s rho correlation coefficients were used to examine the relationship between OFC structures and FSIQ as well as ADI-R and ADOS scores from the social (ADI-R and ADOS), communication (ADI-R and ADOS), and stereotyped-repetitive behaviors (ADI-R) domains. A two-tailed statistical significance level was set at p < 0.05 for all analyses.
3. Results
No differences were observed between the demographic characteristics of patients and controls, except for FSIQ (table 1). Nine of the children with autism were being prescribed psychotropic medications and most of them were either taking a psychostimulant or a selective serotonin reuptake inhibitor, with only one subject receiving the atypical antipsychotic risperidone. Smaller grey matter volumes were observed in children with autism in the right lateral OFC (table 2). This finding remained unchanged after conducting a regression analysis to account for TBV (Beta = 0.42; t = 2.30; p = 0.03). No differences between the two groups were observed for white matter volumes. All total (i.e., grey plus white matter) OFC volumes were non-significantly smaller in the autistic group when compared to controls. Finally, no differences were observed between the two groups on any of the OFC measurements when controlling for FSIQ.
Table 1.
Subject characteristics
| Autistics (N = 11) | Controls (N = 18) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Median | Mean | SD | Range | Median | MWU | Z | p | |
| Age (years) | 10.6 | 1.3 | 8.1–12.7 | 10.7 | 10.4 | 1.2 | 8.9–12.8 | 10.7 | 98.50 | −0.02 | −0.98 |
| Full Scale IQ | 93.1 | 15.9 | 70–119 | 94.0 | 115.4 | 12.3 | 91–131 | 115 | 26.50 | −3.26 | 0.00 |
| SES | 4.45 | 0.52 | 4–5 | 4 | 4.39 | 0.61 | 3–5 | 4 | 95 | −0.21 | 0.88 |
| TBV (cc) | 1357 | 125 | 1131–1608 | 1339 | 1336 | 97 | 1207–1488 | 1318 | 73.00 | −0.74 | 0.48 |
| ADI-R Total Score | 55.5 | 6.5 | 43–62 | 58.0 | --- | --- | --- | --- | --- | --- | --- |
| ADOS Total Score | 15.6 | 2.6 | 11–19 | 16.0 | --- | --- | --- | --- | --- | --- | --- |
MWU: Mann-Whitney U test; SES: socio-economic status; TBV: total brain volume; ADI-R: Autism Diagnostic Interview-R; ADOS: Autism Diagnostic Observation Schedule.
Table 2.
Structure volumes for grey matter (cc)
| Autistics (N = 11) | Controls (N = 18) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | Mann-Whitney U | Z | p | |
| Right Lateral OFC | 3.48 | 0.82 | 3.21 | 4.21 | 0.85 | 3.87 | 58 | −2.15 | 0.03 |
| Left Lateral OFC | 3.81 | 0.76 | 3.96 | 4.28 | 0.92 | 4.06 | 80 | −1.24 | 0.23 |
| Right Medial OFC | 4.60 | 1.22 | 4.48 | 5.19 | 1.31 | 4.79 | 86 | −0.99 | 0.34 |
| Left Medial OFC | 4.27 | 1.25 | 4.15 | 4.73 | 1.08 | 4.72 | 86 | −0.99 | 0.34 |
| Total OFC | 19.70 | 4.41 | 19.71 | 21.62 | 3.95 | 19.86 | 88 | −0.91 | 0.38 |
OFC = orbitofrontal cortex
The relationship between FSIQ and OFC was examined, and no correlations were observed for any OFC structure except for right lateral white matter in the control group (r = −0.50; p = 0.03). Interestingly, the examination of the relationship between OFC structures and clinical features of autism revealed the existence of negative correlations between several OFC white, but not grey, matter volumes and social deficits as measured by the ADI-R and ADOS (table 3). No correlations were observed between grey matter and white matter volumes of the OFC and the remaining domains of the ADI-R (Communication and Stereotyped-Repetitive Behaviors) and ADOS (Communication).
Table 3.
Correlations between white matter OFC volumes and social deficits (N = 11)
| ADI-R Social Domain | ADOS Social Domain | |||
|---|---|---|---|---|
| r | p | r | p | |
| Right Lateral OFC | −0.66 | 0.03 | −0.23 | 0.49 |
| Left Lateral OFC | −0.58 | 0.06 | −0.25 | 0.46 |
| Right Medial OFC | −0.43 | 0.19 | −0.28 | 0.40 |
| Left Medial OFC | −0.55 | 0.08 | −0.25 | 0.46 |
| Total OFC | −0.71 | 0.01 | −0.63 | 0.04 |
OFC = orbitofrontal cortex; ADI-R = autism diagnostic interview-revised; ADOS = autism diagnostic observation schedule
4. Discussion
Findings from this study revealed the existence of decreased size of the right lateral OFC in individuals with autism, which appears to be related to abnormalities in grey matter. This finding of morphometric alterations of the OFC is consistent with several (McAlonan et al., 2005; Salmond et al., 2003), but not all (Abell et al., 1999; Boddaert et al., 2004; Carper and Courchesne, 2005; Kwon et al., 2004; Waiter et al., 2004), imaging studies. Differences in the morphometric methodologies applied and sample sizes used could explain the inconsistencies in these findings, such as the absence of bilateral alterations in the present study in contrast to the observations of two recent voxel-based morphometry studies reporting structural abnormalities in several brain regions including right and left OFC (McAlonan et al., 2005; Salmond et al., 2003). Interestingly, results observed in the present investigation are also concordant with our recent finding, in a completely different sample, of smaller right lateral OFC in children and adolescents, but not in adults, with autism (Hardan et al., in press). Together, these observations suggest the possible existence of age-related structural abnormalities in this disorder and are thus consistent with several neuropathologic and neuroimaging investigations which similarly suggest neurodevelopmental changes in individuals with autism (Aylward et al., 2002; Courchesne et al., 2001; Hardan et al., 2001; Kemper and Bauman, 1998). This mounting evidence of these micro- and macroscopic developmental abnormalities highlights the importance of examining the clinical, neuropsychological, structural, and functional alterations in autism in specific age groups as well as in longitudinally-designed investigations.
In the present study, correlations were observed between several white matter regions of the OFC and social deficits as measured by the ADI-R and ADOS. However, the significance of these correlations is limited by the absence of actual abnormalities of the white matter volumes themselves, which could be due to the small sample size used in this study or to limitations inherent to the measurement methodology. Nevertheless, these relationships are consistent with neuropsychological (Dawson et al., 2002) investigations that have implicated the OFC in the pathophysiology of social deficits in individuals with autism. In addition, although only observed in white matter, these correlations are supported by a recent diffusion tensor imaging study that found reduced fractional anisotropy in white matter structures adjacent to the ventromedial prefrontal cortex in individuals with autism; alterations which could affect the neural pathways involved in social cognition (Barnea-Goraly et al., 2004). Further, our observation of alterations in only some subdivisions of the OFC is supported by fMRI studies (O’Doherty et al., 2001) and is consistent with findings of regional and asymmetric volumetric alterations of the OFC in several neuropsychiatric disorders with deficits in social cognition, such as schizophrenia (Hoptman et al., 2005), major depressive disorder (Lacerda et al., 2004), and obsessive-compulsive disorder (Kang et al., 2004), and could be related to the rich and diverse cytoarchitecture (Carmichael and Price, 1994) and connectivity (Carmichael and Price, 1995) of the OFC.
There are several limitations of the current investigation including the small sample size, the reliance on arbitrary landmarks in obtaining OFC measurements, and the exclusion of girls and mentally retarded individuals with autism which may possibly limit the generalizability of the results. Additionally, adjustment of the p-value might be needed in light of the number of comparisons conducted. However, while p-value adjustments reduce the chance of making type I errors, they increase the chance of making type II errors, or require the increase of the sample size (Rothman, 1990; Perneger, 1998; Feise, 2002). Therefore, replication studies with larger samples are warranted to confirm these findings, and would complement our previous study of the OFC in a larger sample reporting similar results (Hardan et al., in press).
5. Conclusion
Findings from this investigation support the existence of morphometric abnormalities of the OFC, and in particular grey matter of the right lateral OFC, in children with autism. In addition, preliminary data presented here suggest a role for the OFC in the pathophysiology of social deficits in autism. However, in light of the above limitations, replication of these results is critical before any final statements can be made. Finally, future studies should also examine the age-related neurobiologic alterations of the OFC in a longitudinal design and their relationships with deficits in social cognition to further elucidate the role of this structure in the pathophysiology of autism.
Acknowledgments
This work was supported in part by NIMH grant MH 64027 (AYH) and NICHD grant HD 35469 (NJM). This study was also supported by an NICHD Collaborative Program of Excellence in Autism (CPEA).
Abbreviations
- ADI-R
Autism Diagnostic Interview-Revised
- ADOS
Autism Diagnostic Observation Schedule
- FSIQ
Full Scale IQ
- ICC
Intraclass Correlation Coefficient
- M ± SD
Mean Plus or Minus Standard Deviation
- MRI
Magnetic Resonance Imaging
- OFC
Orbitofrontal Cortex
- TBV
Total Brain Volume
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happe F, Frith C, Frith U. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RS, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dawson G, Munson J, Estes A, Osterling J, McPartland J, Toth K, Carver L, Abbott R. Neurocognitive function and joint attention ability in young children with autism spectrum disorder versus developmental delay. Child Dev. 2002;73:345–358. doi: 10.1111/1467-8624.00411. [DOI] [PubMed] [Google Scholar]
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Lacerda ALT, Yorbik O, Kilpatrick M, Keshavan MS, Minshew NJ. An MRI Study of the Orbitofrontal Cortex in Autism. J Child Neurol. doi: 10.1177/08830738060210100701. (in press) [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. J Child Neurol. 2001;16:421–424. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- Hoptman MJ, Volavka J, Weiss EM, Czobor P, Szeszko PR, Gerig G, Chakos M, Blocher J, Citrome LL, Lindenmayer JP, Sheitman B, Lieberman JA, Bilder RM. Quantitative MRI measures of orbitofrontal cortex in patients with chronic schizophrenia or schizoaffective disorder. Psychiatry Res. 2005;140:133–145. doi: 10.1016/j.pscychresns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Kim JJ, Choi JS, Kim YI, Kim CW, Youn T, Han MH, Chang KH, Kwon JS. Volumetric investigation of the frontal-subcortical circuitry in patients with obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2004;16:342–349. doi: 10.1176/jnp.16.3.342. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kwon H, Ow AW, Pedatella KE, Lotspeich LJ, Reiss AL. Voxel-based morphometry elucidates structural neuroanatomy of high-functioning autism and Asperger syndrome. Dev Med Child Neurol. 2004;46:760–764. doi: 10.1017/s0012162204001306. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Hardan AY, Yorbik O, Keshavan MS. Measurement of the orbitofrontal cortex: a validation study of a new method. Neuroimage. 2003;9:665–673. doi: 10.1016/s1053-8119(03)00137-x. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry. 2004;55:353–358. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Yip L, Murphy DG, Chua SE. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments? BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Salmond CH, de Haan M, Friston KJ, Gadian DG, Vargha-Khadem F. Investigating individual differences in brain abnormalities in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:405–413. doi: 10.1098/rstb.2002.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54:418–426. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]


