Abstract
Two independent signals are necessary for neural crest (NC) induction in Xenopus, a Bmp signal, which must be partially attenuated by Bmp antagonists and a separate signal mediated either by a canonical Wnt or Fgf. The mesoderm underlying the NC-forming region has been proposed as a source of this second signal. Wnt8 and Fgf8a are expressed in this tissue around the time of NC induction and are therefore good candidate NC inducers. Loss-of-function studies indicate that these ligands are both necessary to specify the NC, however it is unclear whether these signaling molecules are operating in the same or in parallel pathways to generate the NC. Here we describe experiments addressing this outstanding question. We show that while Wnt8 expression can restore NC progenitors in Fgf8a-deficient embryos, Fgf8a is unable to rescue NC formation in Wnt8-depleted embryos. Moreover, the NC-inducing activity of Fgf8a in neuralized explants is strongly repressed by co-injection of Wnt8 or β-catenin morpholino, suggesting that the activity of these two signaling molecules is linked. Consistent with these observations Fgf8a is a potent inducer of Wnt8 in both whole embryos and animal explants, and Fgf8a knockdown results in a dramatic loss of Wnt8 expression in the mesoderm. We propose that Fgf8a induces NC indirectly through the activation of Wnt8 in the paraxial mesoderm, which in turn promotes NC formation in the overlying ectoderm primed by Bmp antagonists.
Keywords: Fgf8, Wnt8, Bmp, Neural Crest, Induction, Xenopus
Introduction
The neural crest (NC) is a population of cells unique to the vertebrate embryo. NC progenitors originate from the neural plate border, and as the neural tube closes undergo an epithelial-to-mesenchymal transition allowing them to migrate into the periphery and contribute to multiple lineages including the developing heart, the peripheral nervous system, and much of the craniofacial skeleton (LeDouarin et al., 2004). At the time of its induction the NC-forming region is flanked by the neural plate on one side and the non-neural ectoderm on the other side, and sits on top of the underlying paraxial mesoderm. Because of their position relative to the NC each one of these tissues has been proposed as a source of inducer of NC. The relative contribution of these tissues to NC induction appears to vary greatly from one species to another (reviewed in Knecht and Bronner-Fraser, 2002; Huang and Saint-Jeannet, 2004).
At least three major signaling pathways have been implicated in NC induction (reviewed in Jones and Trainor; 2005). Studies in frog and fish have shown that NC forms in region of the ectoderm where Bone Morphogenetic Protein (Bmp) signaling is partially attenuated by Bmp antagonists, such as Chordin, Noggin and Follistatin, derived from the axial mesoderm (Marchant et al., 1998; Nguyen et al., 1998; Tribulo et al., 2003). However it is also true that changes in Bmp signaling levels in the ectoderm are not sufficient for NC induction and that other signaling pathways are involved (LaBonne and Bronner-Fraser, 1998; Garcia-Castro et al., 2002). A large body of work indicates that signaling through the canonical Wnt pathway is critical to specify the NC in fish, frog and chick (Saint-Jeannet et al., 1997; LaBonne and Bronner-Fraser, 1998; Chang and Hemmati-Brivanlou, 1998; Bang et al., 1999; Deardorff et al., 2001; Garcia-Castro et al., 2002; Lewis et al., 2004; reviewed in (Wu et al., 2003; Heeg-Truesdell and LaBonne, 2007). The source of this Wnt signal has been proposed to reside in the paraxial mesoderm of frog and fish (Bang et al., 1999; Lewis et al., 2004) and in the ectoderm of birds (Garcia-Castro et al., 2002). In the mouse the situation is not as clearly defined. Genetic analyses suggest that Wnt signaling may have a role in NC lineages specification, rather than induction (Ikeya et al., 1997; Hari et al., 2002). However, because of functional redundancy an earlier role of Wnt in NC formation cannot be completely excluded.
Studies in Xenopus have shown that members of the fibroblast growth factor (Fgf) family are also involved in NC induction (Kengaku and Okamoto, 1993; Mayor et al., 1995; 1997; Villanueva et al., 2002; Monsoro-Burq et al., 2003). Expression of a dominant negative Fgf receptor blocks NC formation in the whole embryo (Mayor et al., 1997), and in animal explants recombined with paraxial mesoderm (Monsoro-Burq et al., 2003). Fgf8 is expressed in the paraxial mesoderm and is a likely candidate to mediate this activity (Monsoro-Burq et al., 2003). So far Xenopus is the only model organism in which Fgf signaling has been implicated in NC induction.
Therefore, in Xenopus NC induction depends on a Bmp signal, which must be partially attenuated by Bmp antagonists, and a separate signal mediated by either a canonical Wnt or Fgf. However it is unclear how Wnt and Fgf interact at the neural plate border to generate the NC. While there are suggestions that these pathways might be linked (LaBonne and Bronner-Fraser, 1998), there is also evidence that they may act independently (Monsoro-Burq et al., 2003; 2005). In this study, we present a comparative analysis of the NC-inducing activity of Wnt8 and Fgf8a, two candidates NC inducer in Xenopus. Loss- and gain-of-function studies indicate that these ligands share very similar properties. Individually, Fgf8a and Wnt8 are both necessary to specify the NC. Using a number of assays in the whole embryo and animal explants we also show that Fgf8a requires active canonical Wnt signaling to mediate its activity. Moreover Fgf8a is a potent inducer of Wnt8 and is required for Wnt8 expression in the paraxial mesoderm. These results indicate that Fgf8a induces NC indirectly through Wnt8 activation suggesting that these factors are functioning in the same pathway to specify the NC.
Materials and methods
Xenopus embryo injections, morpholinos and explants culture
Embryos were staged according to Nieuwkoop and Faber 1967). Wnt8 (25 pg; Wolda et al., 1993), Fgf8a (5 pg; Christen and Slack, 1997), and XFD (2 ng; Amaya et al., 1991) mRNAs were synthesized in vitro using the Message Machine kit (Ambion). Wnt8 (W8MO; AAAGTGGTGTTTTGCATGATGAAGG; 25–50 ng; Park and Saint-Jeannet, 2008), β-catenin (βCatMO; 25–50 ng; Heasman et al. 2000), and Fgf8a (F8MO; 50 ng; Fletcher et al., 2006) morpholino antisense oligonucleotides were purchased from Gene-Tools LLC (Philomath, OR). In whole embryo experiments synthetic mRNAs and antisense oligonucleotides were injected unilaterally into 2-cell stage embryos. For Wnt8 and β-catenin plasmid DNA was injected to avoid axis duplication (100 pg and 200 pg, respectively; Fig 2). Injected embryos were cultured in 0.1X normal amphibian medium (NAM). For animal explant experiments, both blastomeres at the 2-cell stage were injected in the animal pole region, with Wnt8 or Fgf8a mRNA alone or in combination with Chordin DNA (10 pg; Sasai et al., 1994), explants were then dissected at the late blastula stage and immediately cultured in vitro for several hours (5 hours or 10 hours) in 0.5X NAM. In some experiments 40 µM of U0126 (Calbiochem) was added to the culture medium to block the MAPK pathway (Kuroda et al., 2005). Animal explants were subsequently analyzed by Real Time RT-PCR for the expression of various marker genes (Hong and Saint-Jeannet, 2007).
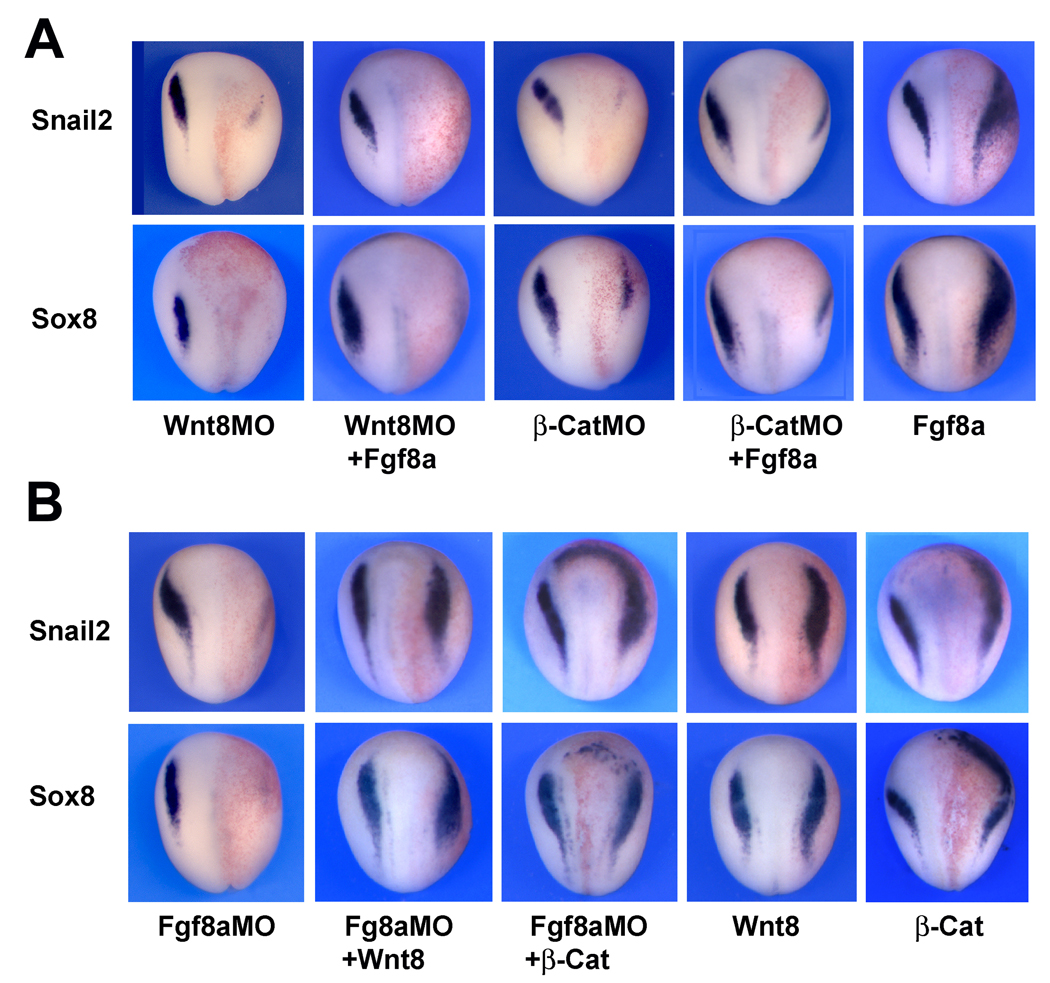
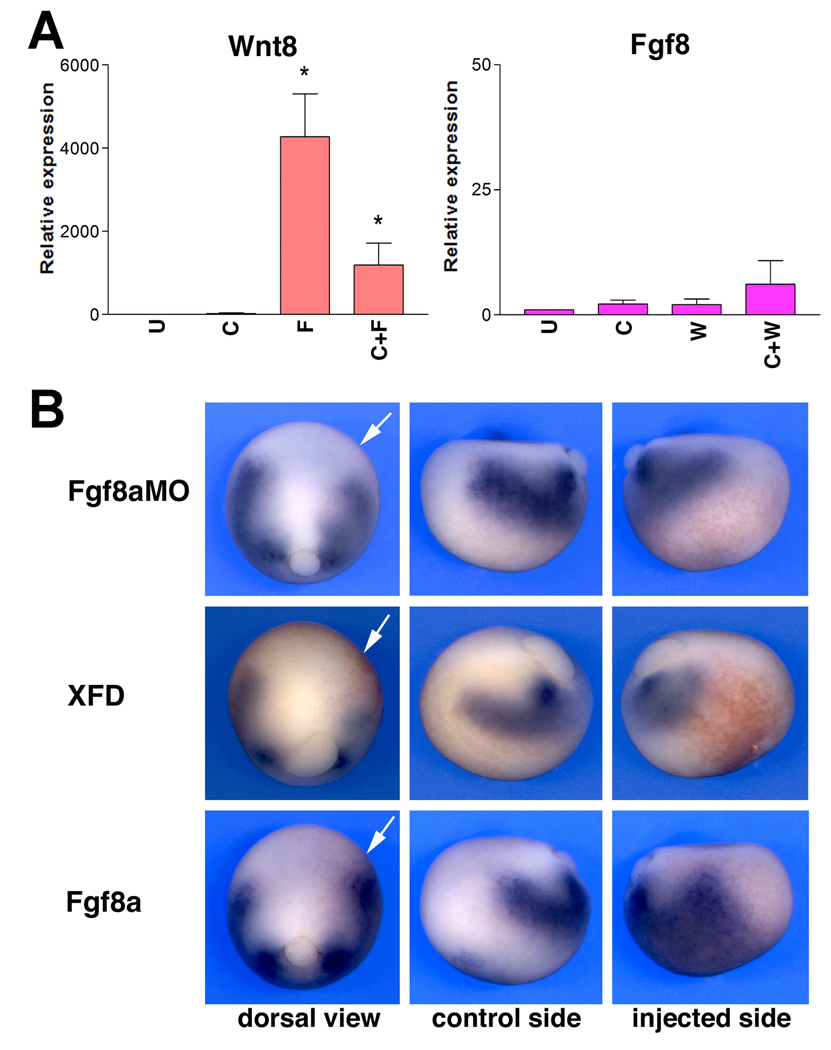
Figure 2. Fgf8a and Wnt8 differ in their ability to restore NC progenitors in Wnt8-and Fgf8a-deficient embryos.
(A) Fgf8a mRNA injection fails to rescue Snail2 and Sox8 expression at the neural plate border of embryos injected with Wnt8MO (25ng) or β-CatMO (25 ng). Single injection of Fgf8a mRNA (2.5 pg) expands Snail2 and Sox8 expression domains. (B) Conversely, Wnt8 (100 pg) or β-catenin (200 pg) plasmid DNA injection restores Snail2 and Sox8 expression in embryos injected with Fgf8aMO (50ng). Injection of Wnt8 or β-catenin in sibling embryos expanded Snail2 and Sox8 expression domains. In all panels, embryos are viewed from the dorsal side anterior to top. Injected side is to the right.
Lineage tracing and in situ hybridization
In all experiments, embryos were co-injected with β-gal mRNA to identify the manipulated side. Embryos at the appropriate stage were fixed in MEMFA and successively processed for Red-Gal staining (Research Organics) and in situ hybridization. Antisense DIG-labeled probes (Genius kit, Roche) were synthesized using template cDNA encoding Sox8 (O’Donnell et al., 2006), Snail2 (Mayor et al., 1995), Sox2 (Mizuseki et al., 1998), Pax3 (Bang et al., 1997), Ap2 (Luo et al., 2003), Wnt8 (Smith and Harland, 1991), Fgf8 (Christen and Slack, 1997), Xbra (Smith et al., 1991) and Sox10 (Aoki et al., 2003). Whole-mount in situ hybridization was performed as previously described (Harland, 1991). For in situ hybridization on section, embryos at stage 12 and 12.5 were fixed in MEMFA for 1 hour, embedded in Paraplast+, and 12 µm serial sections hybridized with Sox8, Fgf8 or Wnt8 probe according to the procedure described by Henry et al. (1996). Sections were briefly counter stained with eosin.
TUNEL staining
TUNEL staining was carried as described (Hensey and Gautier, 1998). Morpholino-injected embryos fixed in MEMFA were rehydrated in PBT and washed in TdT buffer (Roche) for 30 min. End labeling was carried out overnight at room temperature in TdT buffer containing 0.5 µM DIG-dUTP and 150 U/ml TdT. Embryos were then washed for 2 hours at 65°C in PBS/1 mM EDTA. DIG was detected by anti-DIG Fab fragments conjugated to alkaline phosphatase (Roche; 1:2000) and the chromogenic reaction performed using NBT/BCIP (Roche).
Real-Time RT-PCR
For each sample total RNAs were extracted from 10 animal explants using an RNeasy micro RNA isolation kit (Qiagen) according to the manufacturer’s direction. During the extraction procedure the samples were treated with DNase I, to eliminate possible contamination by genomic DNA. The RNA amount was quantified by measuring the optical density using a spectrophotometer (Beckman). Real-time RT-PCR was performed as previously described using specific primer sets (Hong and Saint-Jeannet, 2007). In each case, EF1α was used as an internal reference (data not shown), and each bar on the histograms has been normalized to the level of EF1α expression. The histograms in each figure are presented as mean ± s.e.m of three independent experiments. A Student’s t-test was used to define statistically significant values in each group.
Results
Fgf8a and Wnt8 are both required for NC induction
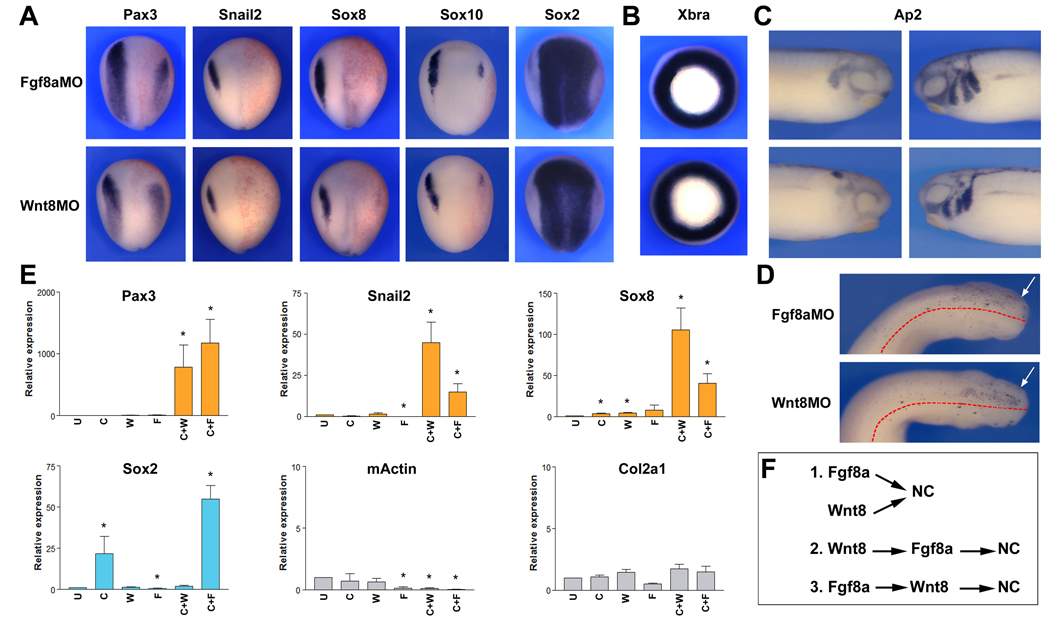
Fgf and Wnt signaling have been both implicated in NC induction in Xenopus. To better understand their relative contribution to this inductive process, we compared the activity of Fgf8a and Wnt8, two ligands expressed in the paraxial mesoderm around the time of NC induction (Christen and Slack, 1997; Monsoro-Burq et al., 2003; Smith and Harland, 1991; Bang et al., 1999). Morpholino-mediated knockdown of Fgf8a or Wnt8 resulted in a similar loss of NC progenitors at the neurula stage, as determined by the expression of four NC-specific genes, Pax3, Snail2, Sox8 and Sox10 (Fig 1A). Often this loss of the NC tissue was associated with an expansion of the neural plate (Sox2) on the injected side (Fig 1A). In these embryos lacking Fgf8a or Wnt8 function, mesoderm appeared to form normally as determined by the expression at the gastrula stage of the general mesoderm marker Xbra (Fig 1B). In both knockdowns the loss of early NC progenitors resulted into a severe reduction of migrating NC cells in the branchial arches at the tailbud stage (Fig 1C), due to increased cell death (Fig 1D). These results suggest that Fgf8a and Wnt8 are both required for NC formation.
Figure 1. Wnt8 and Fgf8a are necessary for NC formation.
(A) Embryos injected with Fgf8a (Fgf8aMO; 50 ng) or Wnt8 (Wnt8MO; 40 ng) morpholino antisense oligonucleotides exhibit a strong reduction of Pax3, Snail2, Sox8, and Sox10 expression at the neurula stage, while the expression domain of the pan-neural marker Sox2 is expanded. Embryos are viewed from the dorsal side anterior to top. Injected side is on the right. (B) At the gastrula stage Fgf8aMO- and Wnt8MO-injected embryos show normal expression of the mesoderm marker, Xbra. Embryos are viewed from the vegetal pole. (C) At the tailbud stage the migration pattern of cranial NC cells is severely perturbed in both Fgf8aMO- and Wnt8MO-injected embryos, as revealed by expression of the cranial NC marker Ap2. Lateral views, dorsal to top. Anterior to the right (injected side, left panels) or to the left (control side, right panels). (D) TUNEL staining shows a similar increase in apoptotic cells in the cranial region of Fgf8aMO- and Wnt8MO-injected embryos at the tailbud stage (arrows). Embryos are viewed from the dorsal side, anterior to the right. The dotted lines indicate the position of the midline. (E) In animal explants Wnt8 (25 pg) or Fgf8a (5 pg) share the same ability to induce NC markers (Pax3, Snail2 and Sox8) when co-expressed with the Bmp antagonist Chordin (10 pg) (C+W and C+F, respectively). In these explants the induction of NC fate occurs in the absence of mesoderm formation (mActin and Col2a1). Fgf8a also synergizes with Chordin to induce neural tissue (Sox2). Values (n=3) are presented as mean ± s.e.m.; (*), p<0.05 versus uninjected animal explant (U). (F) The dual requirement of Fgf8a and Wnt8 suggests that these factors are either acting in parallel (1) or in the same pathway, one upstream of the other (2, 3) to generate the NC.
We also compared the ability of Wnt8 and Fgf8a to induce NC markers in blastula stage animal pole explants neuralized by Bmp attenuation (Chordin injection). The neuralization of these explants was assessed by the expression of the pan-neural gene Sox2. In this assay Fgf8a had the ability to enhance the neuralization mediated by Chordin (Fig 1E), as previously reported (Lamb and Harland, 1995). We observed that Fgf8a and Wnt8 had very similarly abilities to activate NC markers (Pax3, Snail2 and Sox8) in these explants (Fig 1E). Importantly, the induction of these NC-specific genes occurred independently of mesoderm formation. Marker genes for skeletal muscle (m-Actin) and notochord (Col2a1) were not significantly increased in these explants, suggesting that Wnt8 and Fgf8a are directly converting these cells from a neural (Sox2) to a NC (Pax3, Snail2 and Sox8) fate. Taken together these results indicate that individually, Fgf8a and Wnt8 are both necessary to generate NC progenitors in Xenopus. However, it is unclear whether this dual requirement reflects the fact that these two signaling molecules are operating in the same or in parallel pathways (Fig 1F).
NC induction by Fgf8a requires active canonical Wnt signaling
To determine whether Fgf8a and Wnt8 are functioning independently, we first compared the ability of Fgf8a and Wnt8 to restore NC progenitors in Wnt8- or Fgf8a-depleted embryos, respectively. While injection of Fgf8a mRNA expands Snail2 and Sox8 expression domains (Fig 2A), as previously reported (Monsoro-Burq et al., 2003; Hong and Saint-Jeannet, 2007), Fgf8a expression was unable to restore the expression of these NC markers in embryos injected with Wnt8 or β-catenin morpholino (Fig 2A). Conversely, injection of Wnt8 or β-catenin plasmid DNA was very efficient at restoring NC progenitors in Fgf8a-depleted embryos (Fig 2B). These results indicate that NC induction by Fgf8a requires active Wnt signaling in the embryo, whereas Wnt8 NC-inducing activity can occur independently of Fgf8a function.
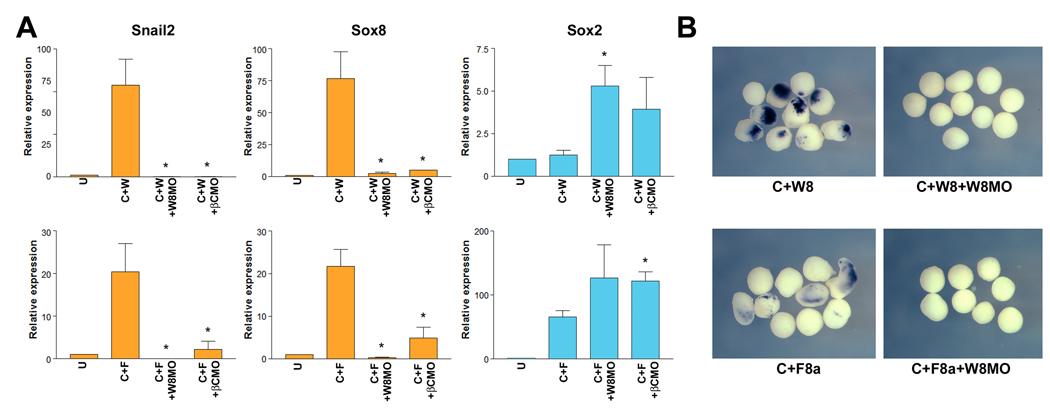
We also evaluated the relationship between Fgf8a and Wnt8 in animal explants. We found that the NC-inducing activity of Wnt8 and Fgf8a in neuralized explants was dramatically inhibited by co-injection of Wnt8 or β-catenin morpholinos, as visualized by Real-Time RT-PCR (Fig 3A). The loss of Snail2 expression in these explants co-injected with Wnt8 morpholino was also evaluated by whole-mount in situ hybridization (Fig 3B). Manipulating Wnt signaling in Fgf8a-injected explants did not significantly change the levels of expression of the neural plate marker Sox2 (Fig 3A). While in Wnt8-injected explants, inhibition of Wnt signaling restored Sox2 expression to levels similar to those observed in neuralized explants (Chordin injected; not shown). These results support the view that Fgf8a requires a functional canonical Wnt pathway to mediate its NC-inducing activity, suggesting that Fgf8a may act upstream of Wnt8 during NC induction.
Figure 3. NC induction by Fgf8a requires active canonical Wnt signaling in animal explants.
(A) In animal explants induction of NC markers (Snail2 and Sox8) by co-expression of Chordin (10 pg) and Wnt8 (25 pg) (C+W) or Chordin (10 pg) and Fgf8a (5 pg) (C+F) is dramatically reduced in the context of embryos injected with Wnt8MO (50 ng) or β-CatMO (50 ng). Interference with Wnt signaling pathway did not affect (C+F) or restored (C+W) the neuralization of these explants (Sox2). Values (n=3) are presented as mean ± s.e.m.; (*), p<0.05 versus C+W (upper graphs) or C+F (lower graphs) samples. (B) The expression of Snail2 detected by whole-mount in situ hybridization in Chordin and Wnt8 (C+W8) or Chordin and Fgf8a (C+F8a) treated animal explants is abolished by co-injection of Wnt8MO (W8MO; 50 ng).
Developmental expression of Fgf8 and Wnt8
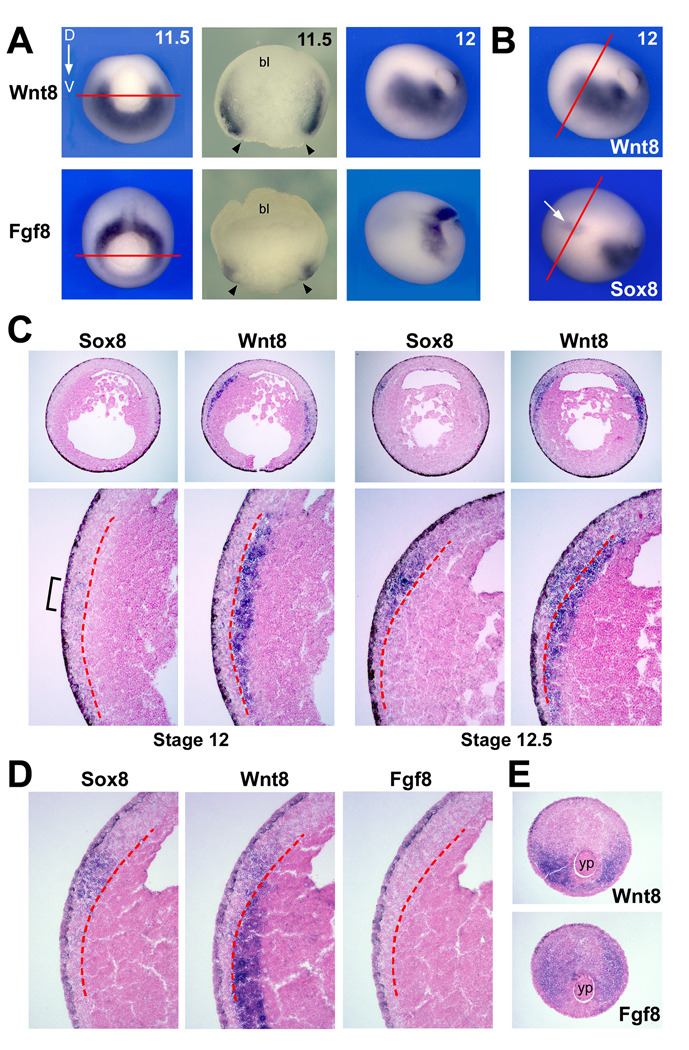
While Wnt8 and Fgf8a are good candidate NC inducers, a detailed analysis of their expression pattern as it relates to NC induction has not been reported. At the mid-gastrula stage (stage 11.5) Fgf8 and Wnt8 are expressed around the blastopore in a complementary pattern in the dorso-lateral and ventro-lateral mesoderm, respectively (Fig 4A). Their expression overlaps in the lateral region of the mesoderm (Fig 4A). At stage 12, while Fgf8 remains confined to the posterior mesoderm, Wnt8 expression domain extends anteriorly as the mesoderm involutes (Fig 4A, B). It is around stage 12 that early NC markers, such as Sox8 (O’Donnell et al., 2006), are first activated in the prospective NC tissue (Fig 4B).
Figure 4. Developmental expression of Wnt8 and Fgf8.
(A) Comparison of Wnt8 and Fgf8 expression at the gastrula stage. At the mid-gastrula stage (11.5) Wnt8 and Fgf8 have a complementary expression pattern in the ventro-lateral and dorso-lateral mesoderm, respectively. The embryos are oriented dorsal (D) to the top. Hemi-sections (red lines in the left panels) of these embryos along the animal-vegetal axis reveal that both genes are co-expressed in the lateral mesoderm. The arrowheads indicate the position of the lateral lip of the blastopore (bl; blastocoel). At stage 12, Wnt8 expression domain expands anteriorly into the involuting mesoderm, future paraxial mesoderm, while Fgf8 remains confined to the posterior mesoderm. (B) Comparative expression of Wnt8 and Sox8 at stage 12. Sox8 expression in the ectoderm (arrow) is adjacent to Wnt8 expression in the mesoderm. The red lines indicate the level of the serial sections shown in the next panels. (C) Expression of Sox8 and Wnt8 on adjacent sections of stage 12 embryo highlights the mesoderm expression of Wnt8 underlying the first Sox8-positive cells in the NC-forming region (bracket). At stage 12.5, Sox8 expression is stronger in the NC domain, and Wnt8 becomes more broadly expressed in both the ectoderm and the mesoderm layers. The red dotted lines in the lower panels demarcate the separation between the ectoderm and the mesoderm layers. Lower panels are higher magnifications of the upper panels. (D) Comparative expression of Sox8, Wnt8 and Fgf8 on adjacent sections of a stage 12/12.5 embryo confirms that Fgf8 is not co-expressed with Wnt8 in the mesoderm underlying the NC forming region. The red dotted lines indicate the separation between the ectoderm and the mesoderm layers. (E) In the posterior region of the same embryo Fgf8 is detected in the dorso-lateral mesoderm, around the yolk plug (yp), while Wnt8 is confined to the ventro-lateral region. Dorsal to top.
Adjacent transverse sections of stage 12 and stage 12.5 embryos were hybridized with Sox8 or Wnt8 probes to further evaluate their spatial relationship (Fig 4C). At stage 12, Wnt8 is detected in the mesoderm immediately contiguous to the NC-forming region where the first Sox8-positive cells are detected (Fig 4C). At stage 12.5 Sox8 is greatly increased in the ectoderm adjacent to Wnt8 expression in the mesoderm. At this stage Wnt8 is no longer confined to the mesoderm and is also detected in the ectoderm layer as previously reported (Bang et al., 1999). Hybridization of adjacent serial sections with Sox8, Wnt8 and Fgf8 probes confirms that Fgf8 is never co-expressed with Wnt8 in the mesoderm underlying the NC forming region (Fig 4D). Fgf8 expression is restricted to the posterior mesoderm at this stage (Fig 4E). With the understanding that we are looking at the mRNA expression of two secreted factors, and in the absence of appropriate antibodies to further evaluate the localization of the corresponding proteins, these data suggest that as compared to Fgf8a the spatio-temporal expression of Wnt8 is more consistent with a role in NC induction.
Fgf8a is a potent inducer of Wnt8 and is required for Wnt8 expression in the paraxial mesoderm
Our results so far indicate that Fgf8a requires an intact canonical Wnt pathway to activate NC-specific genes in whole embryos and in animal explants, suggesting that Fgf8a may act upstream of Wnt8 during NC induction. Moreover, the expression pattern of these two factors is also consistent with this view. These observations directly imply that Fgf8a must have the ability to activate Wnt8 expression. We tested this possibility in the context of animal explants, and found that expression of Fgf8a alone or in combination with Chordin was a very potent inducer of Wnt8 (Fig 5A), while Wnt8 expression had virtually no effects on Fgf8 expression levels, supporting the idea of a unidirectional relationship between these two ligands. Further we showed that the induction of Wnt8 by Fgf8a in neuralized explants was mediated through the MAPK pathway, as Wnt8 expression is severely reduced in the presence of the MAPK inhibitor, U0126 (Fig S1A).
Figure 5. Fgf8a is a strong inducer of Wnt8 in animal explants and in whole embryos.
(A) In animal explants derived from embryos injected with Fgf8a (F) or a combination of Fgf8a and Chordin (C+F) show a strong up-regulation of Wnt8 after 4 hours in culture. For comparison, Wnt8 (W) or Wnt8 and Chordin (C+W) co-injection had little effects on the expression levels of Fgf8. U; uninjected animal explant. Values (n=3) are presented as mean ± s.e.m.; (*), p<0.05 versus uninjected animal explant (U). (B) In whole embryos loss of Fgf function by injection of Fgf8aMO (50 ng) or a dominant negative Fgf receptor (XFD; 2 ng) results in a reduction of Wnt8 expression in the involuting mesoderm at stage 12. Conversely, Fgf8a (5 pg) mis-expression strongly up-regulates Wnt8. For all injections, dorsal and lateral views (control and injected sides) of the same embryo are shown. Dorsal views, anterior to top, injected side to the right (arrows). Lateral views, dorsal to top, anterior to the left (control side) or to the right (injected side).
In the whole embryo, targeted injection of Fgf8aMO or expression of a dominant negative Fgf receptor (XFD; Amaya et al., 1991) resulted in a reduction of Wnt8 expression in the paraxial mesoderm of late gastrula stage embryos (76%; n=73 and 91%; n=40, respectively; Fig 5B). Conversely, over-expression of Fgf8a dramatically expanded Wnt8 expression domain in most injected embryos (98%; n=83; Fig 5B). These results indicate that Fgf8a is required for Wnt8 expression in the paraxial mesoderm consistent with the proposal that Fgf8a functions upstream of Wnt8 during NC induction.
Fgf8a promotes NC fate at the anterior neural fold by up-regulating Wnt8
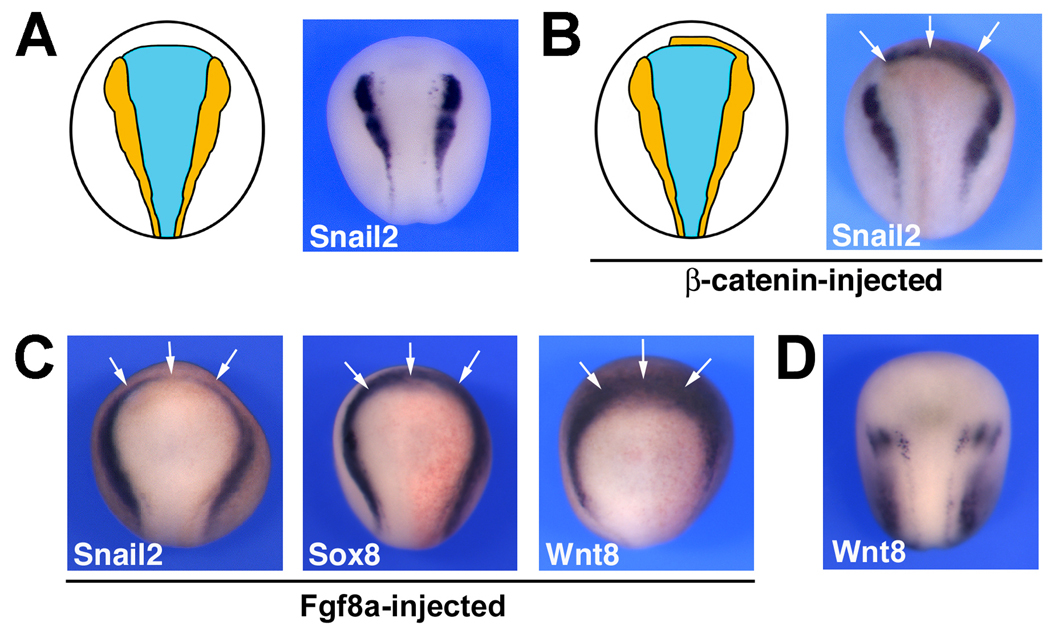
The absence of NC tissue at the anterior edge of the neural plate (Fig 6A) is believed to depend on the activity of an endogenous Wnt inhibitor, Dkk1, whose function is to prevent Wnt-mediated expansion of the NC tissue in this region of the ectoderm (Carmona-Fontaine et al., 2007). Consistent with this view, inhibition of Dkk1 function expands the NC domain anteriorly (Carmona-Fontaine et al., 2007), and excess Wnt signaling in this region of the embryo results in ectopic NC formation at the anterior neural fold (Wu et al., 2004; Voigt and Papalopulu, 2006; Carmona-Fontaine et al., 2007; Fig 6B). Surprisingly, several labs have also reported that Fgf mis-expression can also induce the expression of NC markers in this NC-free domain (Villanueva et al., 2002; Monsoro-Burq et al., 2003; 2005; Fig 6C) suggesting that a mechanism independent of Dkk1 may preclude NC formation in this region. Our findings placing Fgf8a upstream of Wnt8 may help resolve this apparent discrepancy. We observed that Fgf8a-mediated induction of Snail2 and Sox8 at the anterior neural fold was associated with a dramatic up-regulation of Wnt8 anteriorly (Fig 6C), as compared to control embryos (Fig 6D), suggesting that the activity of Wnt inhibitor Dkk1 can fully account for the exclusion of NC from the anterior neural fold.
Figure 6. Fgf8a induces NC at the anterior neural fold indirectly.
(A) The anterior neural plate is devoid of NC tissue as a result of the activity of a Wnt inhibitor, Dkk1 (Carmona-Fontaine et al., 2007). (B) β-catenin mis-expression (200 pg) can overcome this inhibition to induce NC markers (Snail2) at the anterior neural fold (arrows). (C) Fgf8a (5 pg) misexpression can also induce NC markers (Snail2 and Sox8) at the anterior neural fold (arrows), an activity that is mediated by up-regulation of Wnt8 in this region of the embryo (arrows). (D) Normal pattern of expression of Wnt8 in a control embryo at the same stage. In all panels the embryos are viewed from the dorsal side, anterior to top.
Discussion
In this study we have addressed the outstanding question of the relative contribution of Fgf and Wnt signaling pathways to the induction of the NC in Xenopus by comparing the activity of Wnt8 and Fgf8a, two putative NC inducers expressed in the paraxial mesoderm. Using a number of assays in the whole embryo and animal explants we demonstrate that Fgf8a induces NC indirectly through the activation of Wnt8 in the paraxial mesoderm, suggesting that signaling through Wnt8 can fully account for the NC-inducing activity of the paraxial mesoderm in Xenopus. How to reconcile these observations with other studies that have implicated an Fgf, rather than a Wnt signal, as the paraxial mesoderm-derived signal required for NC induction (Mayor et al., 1997; Monsoro-Burq et al., 2003)?
The existence of a paraxial mesoderm-derived Wnt signal in NC induction which was first proposed almost 10 years ago (Bang et al., 1999) has been recently challenged (Monsoro-Burq et al., 2003). In this study, the authors proposed that by interfering with Wnt signaling extracellularly, using Wnt antagonists such as dominant negative Wnt8 (LaBonne and Bronner-Fraser, 1998; Bang et al., 1999), or Nfz8, a truncated and diffusible form of the Wnt receptor, Frizzled 8, (Monsoro-Burq et al., 2003), NC formation was impaired not by blocking the activity of a Wnt signal derived from the paraxial mesoderm, but rather indirectly by altering the character of the mesoderm and therefore changing its signaling properties. In support of this view, these authors reported that interfering with the response of the ectoderm to Wnt signaling by means of intracellular Wnt antagonists, such as GSK3 and dominant negative TCF3, did not prevent the induction of NC markers by the paraxial mesoderm (Monsoro-Burq et al., 2003). However, in these studies we cannot exclude the possibility that these intracellular inhibitors were not fully active at blocking Wnt signaling (Huang and Saint-Jeannet, 2004). Moreover, these findings are conflicting with other studies that have clearly demonstrated that interfering with reception of Wnt signal in the ectoderm using dominant negative forms of Frizzled 3 (Fz3), Frizzled 7 (Fz7) and their co-receptor Lrp6 or morpholino-mediated knockdown of Fz3, Fz7, Lrp6, Kermen and β-catenin was sufficient to block NC formation in the whole embryo (Tamai et al, 2000; Deardorff et al., 2001; Wu et al., 2004; Abu-Elmagd et al. 2006; Hassler et al., 2007).
The same study proposed that an Fgf rather than a Wnt signal was in fact responsible for the NC-inducing activity of the paraxial mesoderm (Monsoro-Burq et al., 2003). This finding was based on the observation that a piece of dorso-lateral marginal zone (DLMZ), which normally induce NC markers in the ectoderm (Bonstein et al., 1998), was unable to induce NC when recombined with animal explants made refractory to Fgf signaling by expression with a dominant negative Fgf receptor (XFD). However, these experiments do not take into account the fact that intact Fgf signaling is required for neuralization of the ectoderm by Bmp antagonists (Launay et al., 1996; Delaune et al., 2004; Kuroda et al., 2005). Therefore, and because neural and NC induction are tightly linked, an alternative interpretation would be that NC induction was not blocked as a result of the inability of a DLMZ-derived Fgf ligand to signal in the ectoderm, but rather indirectly because the neuralization of these explants was impaired by expression of XFD (Launay et al., 1996). Consistent with this possibility, and as previously described (Kuroda et al., 2005), we observed that the MAPK inhibitor, U0126, blocks neuralization by Chordin (Fig S1B, C). Moerover, animal explants co-injected with Chordin and Fgf8a or Chordin and Wnt8 and cultured in the presence of U0126, show reduced expression of the NC marker Snail2 (Fig S1B, C). These results confirm previous observations on the active role played by Fgf/MAPK signaling in neuralization of the ectoderm by Bmp antagonists (Launay et al., 1996; Delaune et al., 2004; Kuroda et al., 2005). Further these observations suggest that the loss of NC in Fgf8a- and Wnt8-injected explants treated with the MAPK inhibitor (Fig S1B, C) or in explants injected with XFD and recombined with DLMZ (Monsoro-Burq et al., 2003) is likely to be secondary to the inability of Bmp antagonists to neuralize the ectoderm in the absence of an active MAPK pathway.
Other evidence suggesting that Wnt and Fgf signaling may function independently during NC induction, came from the observation that these factors differed in their ability to regulate the expression of two neural plate border specifier genes, Pax3 and Msx1 (Monsoro-Burq et al., 2005). However other studies have shown that Pax3 expression at the neural plate border is not only dependent on a Wnt signal (Monsoro-Burq et al., 2005), but is also tightly regulated by Fgf8a signaling (Sato et al., 2005; Hong and Saint-Jeannet, 2007). Similarly, Msx1 expression in the ectoderm is controlled by either Fgf8 (Monsoro-Burq et al., 2005) or Wnt8 signaling (Bang et al., 1999; Tribulo et al., 2003; Hong and Saint-Jeannet, 2007). The differences in the activity of Wnt8 and Fgf8a reported by different labs could be explained by subtle differences in the type of reagents or assays used to evaluate the expression of these genes.
It has been previously shown that co-expression of Chordin and eFGF induces Snail2 in animal explants, and that this activity is inhibited by expression of a dominant negative Wnt8, raising the possibility that the induction of Snail2 by Fgf signaling might be indirect (LaBonne and Bronner-Fraser, 1998). However, because eFgf is also a potent mesoderm inducer (Isaacs et al., 1992), in these experiments we cannot exclude the possibility that Snail2 activation is secondary to the production of a mesoderm-derived Wnt signal (LaBonne and Bronner-Fraser, 1998). In contrast Fgf8a does not induce mesoderm in animal explants (Fig 1E; Fletcher et al., 2006), suggesting that Fgf8a NC-inducing activity is directly linked to its ability to regulate Wnt8 expression.
Spatially Wnt8 is contiguous to Sox8 expression domain around the time of NC induction, these factors being confined to the paraxial mesoderm and the ectoderm, respectively. On the other hand Fgf8 remains restricted to the posterior mesoderm and never comes in close proximity to the NC-forming region (Fig 4). While we cannot exclude the possibility that Fgf8a protein may diffuse to induce NC, at the mRNA level the expression pattern of these molecules is more consistent with a role of Wnt8 in NC induction. Specific antibodies will be needed to further document the expression of Wnt8 and Fgf8a and the extent to which these molecules diffuse within and across germ layers.
In Xenopus, Fz3 and Fz7 are expressed in the ectoderm including the NC-forming region and are therefore excellent candidates to mediate Wnt8 activity. Fz3-and Fz7-depleted embryos completely lack NC progenitors (Deardorff et al., 2001; Abu-Elmagd et al., 2006), and Snail2 induction by Wnt8 in neuralized animal explants is blocked in the absence of Fz7 function (Abu-Elmagd et al. 2006). Nuclear accumulation of β-catenin has been reported in the region of the ectoderm fated to form the NC (Schohl and Fagotto, 2002), consistent with a direct role of Wnt signaling in NC induction. Additionally, β-catenin has been shown to induce NC markers in animal explants in a cell autonomous manner (LaBonne and Bronner-Fraser, 1998), and the Snail2 promoter in both Xenopus laevis and tropicalis has functional Tcf/Lef binding sites (Vallin et al., 2001) providing further evidence that canonical Wnt signaling induces NC directly.
In summary, this study addresses the outstanding question of the relative contribution of Fgf and Wnt signaling to neural crest induction in Xenopus. Our results provide evidence that while Fgf8a and Wnt8 are both required to induce the NC, the NC-inducing activity of Fgf8a is indirect through the activation of Wnt8 expression in the paraxial mesoderm. We therefore propose that Fgf8a and Wnt8 are part of the same signaling cascade to specify the NC in Xenopus.
Supplementary Material
Acknowledgements
We are grateful to Dr. Peter Klein for comments on the manuscript and to Beth Aksim for technical assistance. C-S H was supported by a research grant (2007) from Daegu University. This work was supported by a grant from the National Institutes of Health to J-P S-J (RO1-DE014212).
References
- Abu-Elmagd M, Garcia-Morales C, Wheeler G. Frizzled 7 mediates canonical Wnt in neural crest induction. Dev. Biol. 2006;298:285–298. doi: 10.1016/j.ydbio.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Saint-Germain N, Gyda M, Magner-Fink E, Lee Y-H, Credidio C, Saint-Jeannet J-P. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev. Biol. 2003;259:19–33. doi: 10.1016/s0012-1606(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Bang AG, Papalopulu N, Kintner C, Goulding MD. Expression of Pax3 is initiated in the early neural plate by posteriorizing signals produced by the organizer and by posterior non-axial mesoderm. Development. 1997;124:2075–2085. doi: 10.1242/dev.124.10.2075. [DOI] [PubMed] [Google Scholar]
- Bang AG, Papalopulu N, Goulding MD, Kintner C. Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from the posterior non-axial mesoderm. Dev. Biol. 1999;212:366–380. doi: 10.1006/dbio.1999.9319. [DOI] [PubMed] [Google Scholar]
- Bonstein L, Elias S, Frank D. Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Dev. Biol. 1998;193:156–168. doi: 10.1006/dbio.1997.8795. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Acuna G, Ellwanger KC, Mayor R. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev. Biol. 2007;309:208–221. doi: 10.1016/j.ydbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou A. Neural crest induction by Xwnt7B in Xenopus. Dev. Biol. 1998;194:129–134. doi: 10.1006/dbio.1997.8820. [DOI] [PubMed] [Google Scholar]
- Christen B, Slack JMW. FGF-8 is associated with anteroposterior patterning and limb regeneration in Xenopus. Dev. Biol. 1997;192:455–466. doi: 10.1006/dbio.1997.8732. [DOI] [PubMed] [Google Scholar]
- Deardorff MA, Tan C, Saint-Jeannet J-P, Klein PS. A role for frizzled-3 in neural crest development. Development. 2001;128:3655–3663. doi: 10.1242/dev.128.19.3655. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signaling in addition to BMP inhibition. Development. 2004;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirement of β-catenin in neural crest development. J. Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hassler C, Cruciat CM, Huang YL, Kuriyama S, Mayor R, Niehrs C. Kremen is required for neural crest induction in Xenopus and promotes LRP6-mediated Wnt signaling. Development. 2007;134:4255–4263. doi: 10.1242/dev.005942. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. β-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev. Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell E, LaBonne C. Multiples roles for Wnt signaling in the development of the vertebrate neural crest. Adv. Dev. Biol. 2007;17:204–221. [Google Scholar]
- Henry GL, Brivanlou IH, Kessler DS, Hemmati-Brivanlou A, Melton DA. TGF-beta signals and a pattern in Xenopus laevis endodermal development. Development. 1996;122:1007–1015. doi: 10.1242/dev.122.3.1007. [DOI] [PubMed] [Google Scholar]
- Hensey C, Gautier J. Programmed cell death during Xenopus development: a spatio-temporal analysis. Dev. Biol. 1998;203:36–48. doi: 10.1006/dbio.1998.9028. [DOI] [PubMed] [Google Scholar]
- Hong C-S, Saint-Jeannet J-P. The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Mol. Biol. Cell. 2007;18:2192–2202. doi: 10.1091/mbc.E06-11-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Saint-Jeannet J-P. Induction of the neural crest and the opportunities of life on the edge. Dev. Biol. 2004;275:1–11. doi: 10.1016/j.ydbio.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SMK, Johnson JE, McMahon AP, Takada S. Wnt signaling is required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Tannahill D, Slack JMW. Expression of a novel FGF in the Xenopus embryos. A new candidate inducing factor for mesoderm and anteroposterior specification. Development. 1992;114:711–720. doi: 10.1242/dev.114.3.711. [DOI] [PubMed] [Google Scholar]
- Jones NC, Trainor PA. Role of morphogens in neural crest cell determination. J. Neurobiol. 2005;64:388–404. doi: 10.1002/neu.20162. [DOI] [PubMed] [Google Scholar]
- Knecht AK, Bronner-Fraser M. Induction of the neural crest: a multigene process. Nat. Rev. Genet. 2002;3:453–461. doi: 10.1038/nrg819. [DOI] [PubMed] [Google Scholar]
- Kengaku M, Okamoto H. Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development. 1993;119:1067–1078. doi: 10.1242/dev.119.4.1067. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Fuentealba L, Ikeda A, Reversade B, DeRobertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes & Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development. 1995;121:3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- Launay C, Fromentaux V, Shi D-L, Boucaut J-C. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996;122:869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, Raible DW. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- Luo T, Lee Y-H, Saint-Jeannet J-P, Sargent TD. Induction of neural crest in Xenopus by transcription factor AP2α. Proc. Natl. Acad. Sci. (USA) 2003;100:532–537. doi: 10.1073/pnas.0237226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev. Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- Mayor R, Morgan R, Sargent MG. Induction of the prospective neural crest of Xenopus. Development. 1995;121:767–777. doi: 10.1242/dev.121.3.767. [DOI] [PubMed] [Google Scholar]
- Mayor R, Guerrero N, Martinez C. Role of FGF and noggin in neural crest induction. Dev. Biol. 1997;189:1–12. doi: 10.1006/dbio.1997.8634. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–3124. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publishing Co.; 1967. [Google Scholar]
- O’Donnell M, Hong CS, Huang X, Delnicki RJ, Saint-Jeannet JP. Functional analysis of Sox8 during neural crest development in Xenopus. Development. 2006;133:3817–3826. doi: 10.1242/dev.02558. [DOI] [PubMed] [Google Scholar]
- Park B-Y, Saint-Jeannet J-P. Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Dev. Biol. 2008 doi: 10.1016/j.ydbio.2008.09.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jeannet J-P, He X, Varmus HE, Dawid IB. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl. Acad. Sci. (USA) 1997;94:13713–13718. doi: 10.1073/pnas.94.25.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Sasai N, Sasai Y. Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development. 2005;132:2355–2363. doi: 10.1242/dev.01823. [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 mRNA acts early in Xenopus embryos to promote formation of vegetal dorsalizing center. Cell. 1991;67:735–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony RF, Liu C, Katsuyama Y, Hess F, Saint-Jeannet J-P, He X. LDL receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Ayba MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J. Biol. Chem. 2001;276:30350–30358. doi: 10.1074/jbc.M103167200. [DOI] [PubMed] [Google Scholar]
- Villanueva S, Glavic A, Ruiz P, Mayor R. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 2002;241:289–301. doi: 10.1006/dbio.2001.0485. [DOI] [PubMed] [Google Scholar]
- Voigt J, Papalopulu N. A dominant-negative form of the E3 ubiquitinligase Cullin-1 disrupts the correct allocation of cell fate in the neural crest lineage. Development. 2006;133:559–568. doi: 10.1242/dev.02201. [DOI] [PubMed] [Google Scholar]
- Wolda SL, Moody CJ, Moon RT. Overlapping expression of Xwnt-3a and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev. Biol. 1993;155:46–57. doi: 10.1006/dbio.1993.1005. [DOI] [PubMed] [Google Scholar]
- Wu J, Saint-Jeannet J-P, Klein PS. Wnt-frizzled signaling in neural crest formation. Trends Neurosci. 2003;26:40–45. doi: 10.1016/s0166-2236(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang J, Klein PS. Neural crest induction by the canonical Wnt pathway can be dissociated from anterior-posterior neural patterning in Xenopus. Dev. Biol. 2004;279:220–232. doi: 10.1016/j.ydbio.2004.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.