Abstract
Objective
Interoception is the perception of one's internal physiological, sensory, and emotional status. Extensive evidence supports a link between interoception and subjective experience. An altered ability to monitor or modulate interoception as it relates to subjective experience may provide a mechanistic explanation for the development of some forms of psychiatric illness.
Methods
We investigated which neural networks are activated when anticipating a change in affective (and thus interoceptive) state, which we term “affective set-shifting”, in women with posttraumatic stress disorder (PTSD) related to intimate partner violence, and in non-traumatized healthy volunteers.
Results
Although both groups activated the dorsolateral prefrontal cortex during affective set-shifting, the PTSD group showed significantly less activation in the right anterior insula than did the controls.
Conclusions
These findings may suggest that although individuals with PTSD are cognitively aware of the impending shift in interoceptive state, they fail to appropriately activate neural circuitry involved in modulating interoceptive responses.
Keywords: Interoception, emotional set-shifting, PTSD, insula, anticipation, DLPFC
INTRODUCTION
Interoception is the sense of the internal physiological and sensory status of the organism (1). Many conceptualizations of emotion (2–4) posit a connection between subjective experience and body state, suggesting that interoception is related fundamentally to emotion. An impaired ability to effectively monitor and modulate interoceptive status may result in emotional distress and contribute to the development of some forms of psychiatric illness (5, 6). The ability to regulate interoceptive state can be examined by analyzing brain responses to stimuli that predict changes in the physiological-emotional state.
We have proposed previously that one of the core deficits of anxiety disorders is the erroneous prediction of future interoceptive state, and an impaired capacity to adapt to this prediction (7). This deficit may be due to individuals with anxiety disorders viewing interoceptive sensation as dangerous or threatening (8).This suggests that individuals with anxiety disorders such as posttraumatic stress disorder (PTSD), which are characterized by avoidance and hyperarousal, may exhibit an impaired, slowed, or inadequate ability to change their interoceptive state in response to stimuli that signal an emotional set change. A network of structures, which includes, but is not limited to, the dorsal lateral prefrontal cortex (DLPFC) and insula is involved in emotion processing and may show altered activation in PTSD (9, 10). The current literature in PTSD often takes a regional focus which does not include the insula (9), however this area stands out in meta-analysis (11). There is significant overlap between this neural network and the structures involved in cognitive and interoceptive processing (1). Numerous studies reveal that the dorsal lateral prefrontal cortex (DLPFC) (12, 13) is critically involved in set-shifting, whereas the anterior insula (AI), specifically the right AI, is centrally involved in affecting changes in interoceptive state (5–7, 14). Specifically, the AI appears to represent the intensity of the predicted subjective state and not necessarily the valence of the related affect (15). The ability to preemptively modify interoceptive state during anticipation of a state change, such as a different emotional experience, may be instantiated in this network. The DLPFC may provide cognitive recognition and impetus whereas the AI may affect a change in physiology. A disconnect between the cognitive and the interoceptive set-shifting networks could cause individuals to just react to the cue, thus extending the negative interoceptive aspects of the stimulus into the anticipatory phase, rather than initiate adaptive preparatory adjustments to the stimulus. This disconnection could be due to excessive reaction to an intense affective cue, such that seen in PTSD subjects (10), that obscures adaptive anticipatory processes. We posit that the ability to preemptively modify interoceptive state is important for healthy adaptation to a changing environment, regardless of the valence direction of this change.
In a prior paper, we found that during anticipation of aversive stimuli, women with PTSD and non-traumatized controls showed increased activation of bilateral anterior insula and that activation within right anterior/middle insula was significantly greater in the PTSD relative to the control group (10). This suggested that women with PTSD were significantly more reactive to aversive anticipation than non-traumatized women in the key interoceptive region, i.e., right AI. To investigate the link between PTSD and the capacity to preemptively adapt interoceptive state to changes in the environment, we contrasted functional brain responses between a group of individuals with PTSD and a matched group of non-traumatized control (NTC) subjects during conditions when there was an anticipated change in an emotional experience brought on by anticipated viewing of aversive or positive images. We hypothesized that PTSD relative to NTC subjects would show: (1) no difference in DLPFC activation (indicating cognitive awareness of the affective set-shifting), and (2) reduced anterior insula activation (indicating a lack of adaptation of interoceptive state to the impending affective set-shifting).
METHODS
Participants
Fifteen women with Post Traumatic Stress Disorder (PTSD) (full or subthreshold; see below) and 15 healthy female subjects (NTC) who had never experienced a PTSD “Criterion A” event (i.e., significant trauma) completed a cued anticipation task during functional Magnetic Resonance Imaging (fMRI). The psychiatric diagnosis was established based on diagnostic criteria for PTSD according to a structured clinical interview for DSM IV, which was administered by a licensed psychologist and reviewed by a board certified psychiatrist (MBS). PTSD in these subjects was due to intimate partner violence. PTSD subjects were included if they had other comorbid affective or mood disorders, such as major depressive disorder, as long as PTSD was the primary diagnosis according to the psychiatric interview. Subjects were excluded if they met criteria for alcohol or substance abuse and/or dependence in the past year, a history of >2 years of alcohol abuse, use of psychotropic medication within the last 4 weeks (or fluoxetine within the last 6 weeks), irremovable ferromagnetic material, pregnancy, claustrophobia, bipolar disorder, or schizophrenia. All participants gave informed written consent to participate in this study, which was approved by the University of California San Diego Human Research Protection Program.
The groups were significantly different in level of education (mean (SD): NTC=15.57 (1.72); PTSD=13.13 (1.73); t=−3.87, p<0.001) but not age (NTC=37.13 (7.14); PTSD=34.33(7.83)). Twelve of the 15 subjects met DSM-IV criteria for PTSD and 3 had subthreshold PTSD (i.e., fulfilled Criterion A and the impairment/distress criterion, and had subthreshold Criteria B, C, and/or D symptoms). Excluding the 3 subjects with subthreshold PTSD did not change the results in any meaningful way and used the same sample as in the previous related publication (10). Therefore, we have reported results from the entire group of 15 subjects.
Stimulus and Apparatus
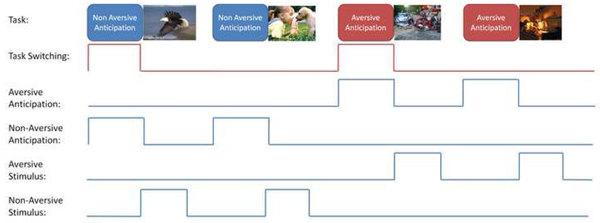
During fMRI, all subjects performed a previously published anxiety processing paradigm (16) that combined a continuous performance task (CPT) with the interspersed presentation of aversive affective stimuli (Figure 1). During the CPT, subjects were instructed to press a LEFT mouse button whenever they saw a blue circle and a RIGHT mouse button whenever they saw a blue square on the screen. The CPT was included to monitor and maintain subjects’ attention and to ensure similar cognitive engagement in both groups. Stimuli were presented at a visual angle of 4 degrees at a rate of 0.5Hz. Simultaneously, a 250 msec long 500Hz tone was presented at a rate of 2Hz. Subjects were instructed prior to the task that a switch to a green shape accompanied by a switch to a 250Hz tone indicated that a positive image was going to appear, and that a switch to a red shape accompanied by a switch to a 1000Hz tone indicated that a negative image was going to appear. The picture stimuli were 17 positive and 17 negative images from the International Affective Picture System (IAPS) (17). In 17 cases, the trial was preceded with a condition of matched valence (e.g., positive then positive) and in 16 cases the stimulus was in the opposite direction, i.e., affective set-shifting (e.g., positive then negative or vice versa). The initial stimulus was added to the latter group as it also required a change of interoceptive state (from the subjects’ pre-task condition). As positive to negative and negative to positive set-shifting required the same theoretical distance of regulation in the interoceptive state they were combined to maximize detection power. Negative images were selected to reflect the types of traumatic events experienced frequently by women (18). The total duration of the task was 580 seconds. Behavioral data related to performance of the CPT were collected and scored for accuracy and latency of response. No response from subjects was required when a picture stimulus was presented on the screen.
Figure 1.
Task Switching Regressors: the immediate task demands are modeled by 4 primary regressors: aversive Anticipation, Non-Aversive Anticipation, Aversive Stimulus, and Non-Aversive Stimulus regressors. The set-shifting is modeled by a regressor that depends on the valence prior trial such that a trial is modeled when the valence of the stimulus is reverse as depicted in the top row.
Response accuracy and reaction times (RT) were obtained during: (1) performance of the CPT, (2) anticipation of a positive image, and (3) anticipation of a negative image. To examine the behavioral effect of anticipation, we examined differences in accuracy and reaction time (RT) between the non-shift and shift conditions in each of the groups.
Image Acquisition
During the task, an fMRI run sensitive to BOLD contrast was collected for each subject using a Signa EXCITE (General Electric Healthcare, Milwaukee) 3.0T scanner (T2* weighted echo planar imaging, repetition time=2000ms, echo time=32ms, field of view=250×250 mm3, 64×64 matrix, 30 2.6mm axial slices with a 1.4mm gap, 290 scans). fMRI acquisitions were time-locked to the onset of functional run. During the same experimental session, a high resolution T1-weighted image (interval time=450ms, repetition time=8ms, echo time=4ms, flip angle=12°, matrix=250×250, ~1 mm3 voxels) was obtained for anatomical reference thus brains could be warped to a common space based the more accurate anatomical scans.
Data were preprocessed and analyzed with the Analysis of Functional NeuroImages software package. Preprocessed time series data for each individual were analyzed using a multiple regression model. In this study we report only on one regressor of interest, i.e., affective set-shifting (see Figure 1). Non-set-shifing trials were not regressed as the combination of shift and non-shift would create co-linear matrices once the task regressors were included. The task behaviors were modeled using four regressors: 1) anticipation of a positive image, 2) anticipation of a negative image, 3) the positive image phase, and 4) the negative image phase. The results of the contrasts between positive and negative anticipation and stimulus are beyond the scope of the current study and are reported in a separate manuscript) (10). In addition, six nuisance regressors were entered into the linear regression model: three movement-related regressors used to account for residual motion (in the roll, pitch, and yaw direction), a white matter mask to control for physiological noise (19), and regressors for baseline and linear trends used to eliminate slow signal drifts. A Gaussian filter with full width- half maximum 6 mm was applied to the voxel-wise percent signal change data to account for individual variations in the anatomical landmarks. Data of each subject were normalized to Talairach coordinates as defined by pre-existent atlases.
Voxel-wise percent signal change for the affective set-shifting regressor for the whole brain was entered into three t-tests in order to examine activation during affective set-shifting between PTSD and NTC subjects in a two-sample t-test, as well as for each group alone using one-sample t-tests. A threshold adjustment method based on Monte-Carlo simulations was used to guard against identifying false positive areas of activation. Based on the whole brain analysis an a priori voxel-wise probability of p< 0.05 in a cluster of 1408 µL resulted in a posteriori cluster probability of p<0.05 (determined by AlphaSim). Finally, the average percent signal difference was extracted from regions of activation that were found to survive this whole brain threshold/cluster method. All analyses for the behavioral data were carried out with SPSS 12.0.
RESULTS
Behavioral Effects
The overall accuracy during the CPT was 90%. The average response latency during anticipation was 878ms. There were no significant differences in error rate or RT between groups during affective set-shifting or non-shifting conditions.
BOLD Activation during Affective Set-Shifting Condition
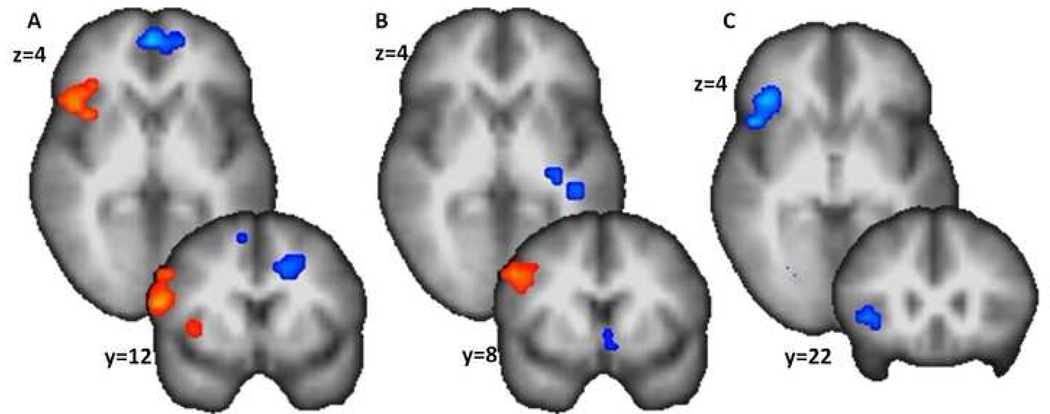
One-sample t-tests in PTSD and NTC groups indicated that both groups showed significant BOLD increases within right inferior frontal gyrus (IFG) and dorsal lateral prefrontal cortex (DLPFC) during the affective set-shifting. However, the NTC group showed additional BOLD increase within right anterior insula (AI). Between-group comparison using a two-sample t-test showed that right AI, thalamus, right cerebellum, and right anterior cingulate showed significantly less activation in the PTSD group than the NTC group (see Table 1 and Figure 2).
Table 1.
Group and Task effect for interoceptive set-shifting.
| ROI | BA | Vol (μl) |
X | Y | Z | Tvalue |
|---|---|---|---|---|---|---|
| NTC > PTSD | ||||||
| Set-shifting activation | ||||||
| Thalamus | 2240 | 2 | −31 | 15 | 4.10‡ | |
| Right Cerebellum | 2176 | 27 | −69 | −13 | 4.36‡ | |
| Right Anterior Insula | 13 | 1728 | 39 | 23 | 0 | 3.51† |
| Right Anterior Cingulate | 32 | 1472 | 18 | 34 | 13 | 3.57† |
| PTSD Only | ||||||
| Set-shifting activation | ||||||
| Right Inferior Frontal Gyrus | 9 | 2176 | 47 | 4 | 32 | 4.51‡ |
| Set-shifting deactivation | ||||||
| Cingulate Gyrus | 31 | 6656 | −2 | −54 | 29 | −3.47† |
| Left Middle Temporal Gyrus | 39 | 5824 | −41 | −62 | 31 | −3.92† |
| Left Postcentral Gyrus | 3 | 4160 | −28 | −21 | 46 | −3.78† |
| Left Caudate | 3456 | −22 | −28 | 16 | −3.05† | |
| Anterior Cingulate | 32 | 2368 | 5 | 20 | −7 | −3.41† |
| Paracentral Lobule | 5 | 2240 | −4 | −32 | 53 | −3.27† |
| NTC Only | ||||||
| Set-shifting activation | ||||||
| Right Inferior Frontal Gyrus and Anterior | 45/13 | 7488 | 47 | 23 | 16 | 6.37‡ |
| Insula | ||||||
| Set-shifting deactivation | ||||||
| Left Medial Frontal Gyrus | 6 | 16832 | −17 | −16 | 47 | −3.56† |
| Right Precentral Gyrus | 4 | 6208 | 18 | −18 | 52 | −3.45† |
| Medial Frontal Gyrus | 10 | 2944 | 0 | 52 | 1 | −4.42‡ |
| Right Paracentral Lobule | 5 | 2560 | 18 | −40 | 50 | −3.39† |
Note.
p<0.01 and
p<0.001.
Figure 2.
Anticipatory switching in NTC (A), IPV-PTSD (B), and IPV-PTSD v. NTC (C). Both groups show DLPFC activations (in red) as visible in the coronal slices (i.e., y=..). However, only the NTC group shows anterior insula activation (as seen in the axial or × slice). This region showed a significantly greater activation for the NTC group than for the IPV-PTSD group (in blue) as depicted in the axial and coronal slices (C).
Brain Behavior Relationship
For the NTC group the IFG activation for the affective set-shifting condition had a significant negative correlation with response latency differences between affective set-shifting versus non-shifting trials (Spearman’s rho=−.609, p<.05), whereas no significant relationship between IFG activation and RT was seen in the PTSD group (Spearman’s rho=−.063, NS).
DISCUSSION
In this study, we provide initial evidence that individuals with PTSD related to IPV relative to NTC show a reduced right anterior insula and a similar right DLPFC response during affective set-shifting, i.e., when a stimulus predicts affective valence shift. These results are consistent with the idea that although individuals with PTSD are cognitively aware of the impending shift in physiological/emotional state, they fail to appropriately activate neural circuitry involved in generating preparatory interoceptive changes.
One advantage of the task used in this study is that it cues the subject to an upcoming shift in the affective valence of a stimulus, and therefore a potential change in the individual’s interoceptive, emotional and cognitive states. Studies examining cognitive set-shifting (e.g., Wisconsin Card Sorting Test) consistently show DLPFC activation (13, 20). Consistent with this evidence, both groups in the current study showed increased DLPFC activation when they were cued for a shift in the upcoming affective image. This finding may suggest that previous trauma does not disrupt the function of neural circuitry involved in the cognitive representation of set-shifting.
Increased activation in AI during the anticipation of strong emotions has been observed in several studies (21–23). Neuroanatomical and functional neuroimaging evidence shows that the right AI plays a major role in detecting the mismatch between cognitive and interoceptive states (1, 24, 25). Decreased activation in this region in the PTSD group may indicate the inability to detect such a mismatch. This inability is probably not due to decreased interoceptive awareness (26, 27), but rather to an inability to preemptively modify interoceptive state. Recent evidence shows that AI activation can be effectively modulated by placebo (28) or biofeedback (29) in healthy control subjects. Further studies need to examine whether this process can be learned in anxiety disorders.
Other regions that showed differences between the two groups include the thalamus, right anterior cingulate, and right cerebellum. The thalamus is a key physiological center and relays interoceptive information to the AI as well as the anterior cingulate via Lamina I homeostatic pathway (1). Decreased activation within several nodes of the pathway that subserves feelings and motivations associated with changes in the body’s physiological condition and with the autonomic responses and behaviors that occur in order to restore an optimal balance (30) suggests that PTSD may interfere with interoceptive adaptation induced by changes in the affective value of the upcoming stimuli. These activation differences appear to be related to the reduction in interoceptive adaptation seen in the PTSD group.
In the NTC group, right IFG/DLPFC activation was related to a shorter response latency during affective set-shifting trials. This relationship was not observed in the PTSD group, suggesting that behavioral responses may be less tightly linked to IFG/DLPFC activation in PTSD. Thus in the NTC group greater IFG/DLPFC may suggest more effective preparation for emotional set-shifting and the interoceptive repercussions. However no such relationship was observed in the PTSD group which when taken in conjunction with significantly less right AI activation may indicate that IFG activation did not limit the disruptive effects of the task in this group. The activation of the cognitive (i.e., IFG/DLPFC) but not interoceptive (i.e., AI) set-shifting regions in the PTSD group suggests that while cognitively attentive to the anticipated affective shifts, PTSD may not be associated with an inability to appropriately activate brain regions necessary for generating physiological responses during such shifts.
The current findings require replication both in other groups of individuals with PTSD related to IPV as well as in groups of individuals with PTSD related to other types of trauma. In addition, work in male and mixed gender groups, as well as other anxiety and/or different psychiatric conditions could help in development of this model. In future work, it will be important to better understand the relationship between brain activation and physiological arousal, as well as metabolic changes (i.e., adrenergic increases) during affective set-shifting in individuals with PTSD, in order to describe a comprehensive model of interoceptive processing in this common and debilitating disorder. While the focus of this paper was to examine the capacity to preemptively modify interoceptive state, it is also important to understand how stimulus valence impacts affective set-shifting (i.e., shifts from negative to positive versus positive to negative). In the current study, we did not have sufficient trials to appropriately explore this hypothesis. Additionally, future studies employing neurofeedback and/or biofeedback as part of “interoceptive training” treatment program for PTSD might help test this model.
In summary, affective set-shifting was regressed out of an affective set-shifting task and the brain correlates where contrasted between PTSD and NTC individuals. The PTSD group was notable by a marked lack of activation in the right AI/IFG. This activation pattern provides initial evidence to back a previous conceptualization of interoceptive anxiety (5, 7). While these results provide compelling evidence of the neural underpinnings of interoceptive anxiety, they require both replication and effective monitoring of physiological changes.
Acknowledgments
This work was supported by the Veterans Administration via Merit Grants (to MPP and MBS), by grants from NIMH (MH65413 and MH64122 to MBS), and NARSAD Young Investigator Award (ANS, IAS, & SCM).
Acronyms
- AI
anterior insula
- BOLD
Blood Oxygenation Level Dependent
- CPT
continuous performance task
- DLPFC
dorsal lateral prefrontal cortex
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- fMRI
function magnetic resonance imaging
- IAPS
International Affective Picture System
- IFG
inferior frontal gyrus
- IPV
intimate partner violence
- mm
millimeters
- ms
milliseconds
- NTC
non-traumatized control
- PTSD
posttraumatic stress disorder
- RT
reaction times
REFERENCES
- 1.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. NatRevNeurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 2.Damasio AR. Towards a neurobiology of the emotions. International Journal of Psychology. 1996;31:4002. [Google Scholar]
- 3.James W. The Principles of Psychology. Vol. 1. Dover Publications; 1950. [Google Scholar]
- 4.Lange CG, James W. The Emotions. Williams & Wilkins Company; 1922. [Google Scholar]
- 5.Craig AD. Interoception: the sense of the physiological condition of the body. CurrOpinNeurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 6.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 7.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. BehavResTher. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 9.Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- 10.Simmons AN, Paulus MP, Thorpe SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64:681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. The American journal of psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafritz KM, Kartheiser P, Belger A. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. NeuroImage. 2005;25:600–606. doi: 10.1016/j.neuroimage.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 14.Craig ADB. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Gray MA, Harrison NA, Wiens S, Critchley HD. Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS ONE. 2007;2:e546. doi: 10.1371/journal.pone.0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 17.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): instruction manual and affective ratings. Gainsville University of Florida, The Center for Research in Psychophysiologys. 1999 [Google Scholar]
- 18.Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behaviour research and therapy. 2000;38:619–628. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 19.Strigo I, Simmons A, Craig AD, Paulus MP. Society for Neuroscience. Atlanta, GA: 2006. Breathing and BOLD fMRI: watch out. [Google Scholar]
- 20.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. NeuroImage. 2006;30:1038–1049. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- 22.Porro CA. Functional imaging and pain: behavior, perception, and modulation. Neuroscientist. 2003;9:354–369. doi: 10.1177/1073858403253660. [DOI] [PubMed] [Google Scholar]
- 23.Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- 24.Critchley HD, Taggart P, Sutton PM, Holdright DR, Batchvarov V, Hnatkova K, Malik M, Dolan RJ. Mental stress and sudden cardiac death: asymmetric midbrain activity as a linking mechanism. Brain. 2005;128:75–85. doi: 10.1093/brain/awh324. [DOI] [PubMed] [Google Scholar]
- 25.Gray MA, Critchley HD. Interoceptive Basis to Craving. Neuron. 2007;54:183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 27.Bechara A, Naqvi N. Listening to your heart: interoceptive awareness as a gateway to feeling. Nature neuroscience. 2004;7:189–195. doi: 10.1038/nn0204-102. [DOI] [PubMed] [Google Scholar]
- 28.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science (New York, NY. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 29.Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N. Regulation of anterior insular cortex activity using real-time fMRI. NeuroImage. 2007;35:1238–1246. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]