Abstract
Despite the prevalence and importance of carbohydrate polymers, the molecular details of their biosynthesis remain elusive. Many enzymes responsible for the synthesis of carbohydrate polymers require a “primer” or “initiator” carbohydrate sequence. One example of such an enzyme is the mycobacterial galactofuranosyltransferase GlfT2 (Rv3808c), which generates an essential cell wall building block. We recently demonstrated that recombinant GlfT2 is capable of producing a polymer composed of alternating β-(1,5) and β-(1,6)-linked galactofuranose (Galf) residues. Intriguingly, the length of the polymers produced from a synthetic glycosyl acceptor is consistent with those found in the cell wall. To probe the mechanism by which polymer length is controlled, a collection of initiator substrates has been assembled. The central feature of the synthetic route is a ruthenium-catalyzed cross-metathesis as the penultimate transformation. Access to synthetic substrates has led us to postulate a new mechanism for length control in this template independent polymerization. Moreover, our investigations indicate that lipids possessing but a single galactofuranose residue can act as a substrate for GlfT2.
Keywords: galactan, carbohydrate polymer, glycosyl acceptor, galactofuranose, glycosyltransferase, cross metathesis, Mycobacterium tuberculosis
1. Introduction
Carbohydrate polymers mediate critical functions in all organisms, including energy storage, cellular structure and protection, cell differentiation and proliferation, and immune responses. Polysaccharides can vary in length from tens of residues to thousands, and polymer length appears to be important for function. For instance, polysaccharides that are involved in signaling tend to be shorter, whereas long polysaccharides are components of the extracellular matrix of eukaryotes and the protective capsules of bacteria.1, 2 Despite the ubiquity of polysaccharides, many of the details of their biosynthesis and function continue to elude researchers. For example, cellulose is composed of packed glucose polymers, which are primarily linked through β-(1,4)-linkages with a varying percentage of β-(1,6)-linkages. The biosynthesis of cellulose is catalyzed by cellulose synthase and thought to occur via a highly processive, template-independent mechanism. Interestingly, the factors that determine cellulose polymer length are unknown,3 highlighting the difficulty of understanding the control mechanisms that operate in template-independent polymerization reactions.
Chemical synthesis can provide probes to elucidate the mechanisms that underlie the biosynthesis of carbohydrate and glycan polymers. For example, Walker, Kahne and coworkers recently synthesized a collection of analogues of Lipid II, which is polymerized by glycosyltransferases to build bacterial peptidoglycan.4 The peptidoglycan glycosyltransferases use lipid-linked saccharides as both the donor and acceptor for polymerization, and the synthetic compounds provide the means to differentiate between the lipid requirement for the donor and acceptor sites. We have employed chemical synthesis to explore the acceptor requirements and the mechanisms that govern the assembly of a building block for the mycobacterial cell wall.
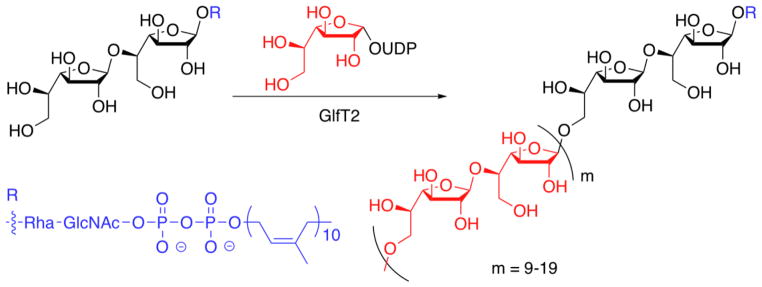
Because of the devastating consequences that can result from mycobacterial infection, our efforts to illuminate the mechanisms that govern the biosynthesis of glycan polymers have focused on the carbohydrate polymerase GlfT2 from Mycobacterium tuberculosis. GlfT2, encoded by the glfT2 (or RV3808c) gene in M. tuberculosis, is an essential galactofuranosyltransferase.5, 6 GlfT2 can catalyze the synthesis of a linear polymer of 20–40 Galf residues that are connected by alternating β-(1,5) and β-(1,6) glycosidic bonds (Fig. 1).7, 8 This polymer, termed the galactan, serves as a covalent bridge between the peptidoglycan and the protective mycolic acid-arabinan layer.9 We recently demonstrated that recombinant His6-GlfT2 is capable of producing galactan polymers of endogenous length from synthetic lipid-bearing acceptors.8 Fundamental to this study was the availability of synthetic acceptors. Herein, we describe a divergent synthetic route and demonstrate its utility for assembling a wide range of acceptors. These compounds can dissect the molecular features relevant for elongation and template-independent length control by GlfT2.
Figure 1.
GlfT2 is a polymerizing glycosyltransferase responsible for the synthesis of the mycobacterial galactan polymer.
2. Results and discussion
GlfT2 was identified as a critical glycosyltransferase in mycobacterial galactan assembly.10–13 Recombinant GlfT2 is a soluble galactofuranosyl transferase that was first shown to add up to four new Galf residues to acceptors displaying galactofuranose-terminated oligosaccharides.14 Polymers of lengths comparable to those observed endogenously were absent. We sought to develop a synthetic route to rapidly generate acceptors that could be converted into polymers of Galf residues. We postulated that the ability to vary acceptor structure could illuminate the mechanisms involved in determining polymer length. Herein, we outline the logic of our synthetic route to disaccharide acceptors for GlfT2, describe its application to the synthesis of previously employed acceptors,8 as well as new acceptors, and compare the ability of GlfT2 to elongate these different compounds.
2.1. Rationale for acceptor substrate design
The galactan portion of the mycobacterial cell wall consists of alternating β-(1,5) and β-(1,6)-linked Galf residues. In principle, synthetic acceptors with either a terminal 1,5- or 1,6-linked sugar might be effective substrates for GlfT2. Studies with synthetic disaccharide acceptors suggested that acceptors with a 1,6-Galf linkage at the non-reducing end were better substrates than 1,5-terminated acceptors.14 Still, neither gave rise to full length polymers. Accordingly, we selected a lipid-linked 1,6-Galf disaccharide as a starting point for our mechanistic investigations.8 In particular, we were interested in probing the role of the lipid functionality present in the putative natural acceptor, which we suspected might be relevant to GlfT2 elongation.4, 15 As a result, altering the lipid became a major focus for our synthetic strategy.
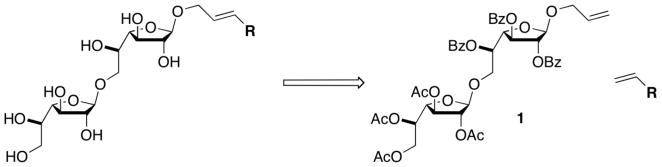
We were attracted by the possibility of a divergent synthesis in which different lipophilic substituents could be appended late in the synthetic scheme. To this end, we envisioned using an olefin cross-metathesis reaction between a sugar substrate equipped with an anomeric allyl group and an alkenyl-lipid in the penultimate step of the synthesis (Fig. 2).16–18 There are several advantages of this approach over conventional variation by glycosylation. First, it provides the means to rapidly diversify acceptors from the common intermediate disaccharide 1. Second, the outstanding compatibility of functional groups with metathesis reactions allows the installation of lipids with diverse functional groups. Third, in our experience, direct glycosylation reactions of hydroxyl-terminated lipids, especially with larger lipids, are more difficult to optimize. In contrast, the yields of cross metathesis reactions are less affected by the length of the alkenyl substituent. We envisioned that cross metathesis could provide compounds that could be used to explore how GlfT2 functions to control polysaccharide length and the molecular features19 of compounds that serve as GlfT2 acceptors.
Figure 2.
Retrosynthetic coupling strategy for glycolipid formation.
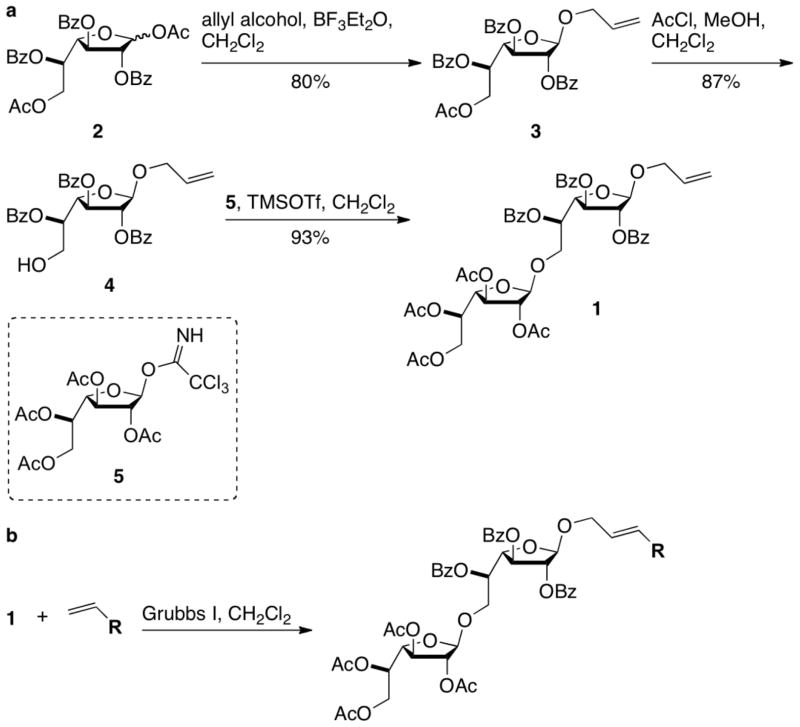
We chose allyl-bearing 1,6-linked Galf disaccharide 1 as the key substrate for the cross metathesis reaction. The assembly of this key building block began with the preparation of protected Galf derivative 2 (Scheme 1a), which was generated from D-galactose.20, 21 Fischer glycosylation with allyl alcohol provided 3, which could be converted into monosaccharide acceptor 4 by selective removal of the primary acetate protecting group (Scheme 1a). This reaction was accompanied by a small amount of benzoyl group migration to the primary alcohol. The extent of migration varied based on the number of equivalents of acetyl chloride added. Presumably, a minimum amount of acid is required for acetate hydrolysis, but increased amounts lead to acid-catalyzed migration. The major product (4) could be coupled to monosaccharide donor 522, 23 in the presence of trimethylsilyl trifluoromethanesulfonate (TMSOTf) to afford 1,6-linked Galf disaccharide 1.
Scheme 1.
(a) Route to β-1,6-linked Galf disaccharide 1. (b) Grubbs-catalyzed cross metathesis of 1 and a collection of lipids can provide protected glycolipids.
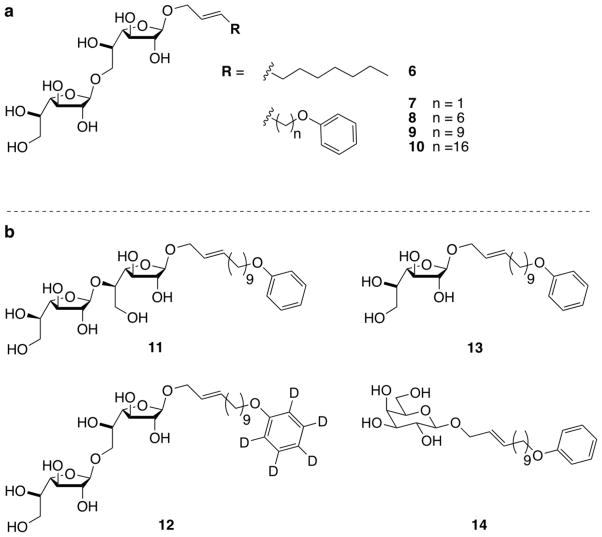
A series of phenoxy-terminated alkenyl lipids were prepared from the corresponding alkenyl bromides. We reasoned that the installation of phenoxy groups would add lipophilicity and provide a means to quantify acceptor substrate concentration in biological assays. Olefin cross-metathesis of lipids and disaccharide 1 (Scheme 1b), followed by removal of the ester protecting groups with sodium methoxide, provided a collection of acceptor analogues (Fig. 3a). The variation of lipophilicity of our acceptor analogues afforded key insights into the mechanism of GlfT2-catalyzed galactan polymerization.
Figure 3.
Synthetic acceptor substrates for GlfT2. (a) A collection of β-1,6-linked Galf disaccharides used to reveal that GlfT2 forms galactan polymers and that lipid length is critical for activity. (b) Compounds 11–14 represent examples of an expanded collection of chemical tools for studying GlfT2.
Acceptor 9 provided the first evidence for the polymerase activity of GlfT2 (Table 1). MALDI-TOF MS analysis showed enzymatic reaction products that contained as many Galf residues as are found in the natural galactan (27 additional Galf residues).8 Because GlfT2 acts on a physiological substrate that can insert into membranes, it was possible that GlfT2 depends upon the presence of a hydrophobic environment. We therefore tested the ability of acceptor 9 to form micelles in dye solubilization assays and found that it could. This observation led us to question whether the ability of compound 9 to serve as a substrate depends upon its incorporation into micelles. To test this possibility, we used our synthetic route to vary the anomeric substituent and therefore alter the propensity of compounds to form micelles. We found that disaccharides 8 and 9 were polymerized by GlfT2 to similar extents (Table 1), but the former did not form micelles. Thus, the ability of compounds to act as substrates for polymerization by GlfT2 is not dictated by their ability to form micelles. Previous studies had demonstrated GlfT2 can elongate an octyl-linked trisaccharide.14 Therefore, we synthesized glycolipid 6 as a benchmark substrate for glycosyltransferase activity. When crude enzymatic reaction mixtures were analyzed by MALDI-TOF MS, disaccharide 6 afforded products elongated by, at most, four Galf residues (Table 1). Disaccharide 7 was synthesized to investigate how an increase in lipophilicity without an increase in lipid length would affect polymerization. Similar extents of elongation were observed with disaccharides 7 and 6 (Table 1), demonstrating that a threshold lipid length was required for galactan formation by GlfT2.
Table 1.
Summary of the ability of synthetic acceptors to act as substrates of GlfT2.
| Compound | Substrate | Polymerized? | Galf residues added |
|---|---|---|---|
| 6 | Yes | No | 4 |
| 7 | Yes | No | 12 |
| 8 | Yes | Yes N | 25 |
| 9 | Yes | Yes | 27 |
| 10 | Yes | Yes | 48 |
| 11 | Yes | Yes | 35 |
| 12 | Yes | Yes | 27 |
| 12 | Yes | Yes | 46 |
| 14 | No | No | 0 |
These results suggest that the lipid substituent, and not the saccharide functionality, is a key determinant of polymer length. We hypothesize that the acceptor substrate occupies not only the active site but also is tethered to a lipid-binding secondary site. If this prediction is accurate, the longer lipid present in compound 10 should bind with increased affinity to this lipid-binding site as compared to acceptor 9. Thus, if elongation occurs through bivalent substrate binding, the product distribution obtained from compound 10 versus compound 9 should be shifted to higher-molecular weight polymers. Our previous results indicate that compound 10 is elongated to give longer polymer products than those seen for acceptor 9 (Table 1). Thus, access to acceptors possessing anomeric substituents with a range of lengths allowed us to determine how GlfT2 recognizes and polymerizes acceptor substrates.
2.2. Expanding the armamentarium of chemical tools
Based on the insight afforded by examining the GlfT2-catalyzed elongation of 1,6-linked acceptors, we sought to expand the breadth of our acceptor collection to address additional questions regarding the mechanism of galactan polymerization. In addition to the ability of GlfT2 to control polymer length, the enzyme also was reported to be bifunctional, producing alternating β-(1,5) and β-(1,6) glycosidic bonds. 1,5-Linked synthetic acceptors with short hydrophobic anomeric substituents had been shown to serve as GlfT2 substrates, but only short oligomers were observed.14 Our results with 1,6-linked acceptors suggest that 1,5-linked acceptors equipped with a more lipophilic substitutent could give rise to longer polymers. Thus, acceptor 11 (Fig. 3b) was synthesized using a route analogous to that described above (Scheme 1). As previously mentioned, the conditions employed to remove the primary acetate group of 3 were not entirely selective; they afforded regioisomeric products that could be separated by column chromatography. In this way, an appropriate monosaccharide acceptor for the synthesis of 11 was obtained. Acceptor 11 is a substrate for GlfT2 (Fig. 4; Table 1), and longer polymeric products are observed. These data provide additional importance of lipid substitution and suggesting bifunctionality may be intrinsic to the recognition event.
Figure 4.
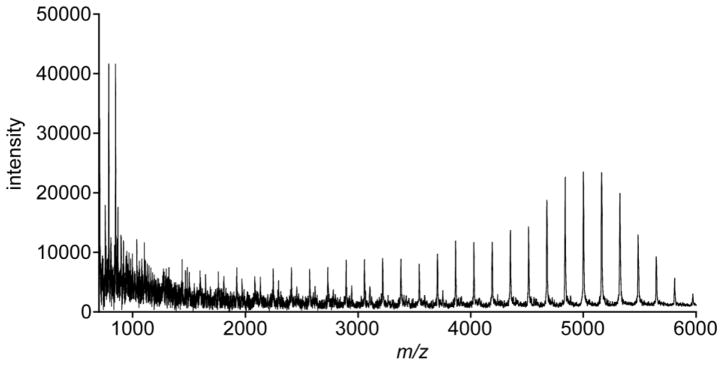
MALDI-TOF MS spectrum obtained from the reaction of His6-GlfT2, UDP-Galf, and 1,5-linked disaccharide 11.
The assays for evaluating GlfT2 activity rely on mass spectrometry analysis. Time-course assays and competition studies might benefit from an isotopically labeled substrate that would provide distinct peaks in a mass spectrometry trace compared to compound 9. To this end, we used our synthetic route to access compound 12 (Fig. 3b), which contains a d5-phenoxy-terminated lipid that can be distinguished from acceptor 9 in MALDI-TOF MS analysis of enzymatic reaction mixtures. We anticipate that this compound will be a valuable of GlfT2 catalysis.
We also used our synthetic strategy to generate compounds that could be used to investigate the role of the sugar substituent. In studies reported to date, di- or trisaccharide acceptors have been found to be substrates for GlfT2. As mentioned previously, others had shown GlfT2 to be capable of elongation of both di- and trisaccharides. Still, the production of full-length galactan formation by GlfT2 depends on the acceptor substrate attributes. In addition to exploiting our synthetic route to examine the substrate lipid requirements, we also wanted to probe the minimum requirements for the acceptor saccharide. To this end, we assembled monosaccharide Galf acceptor 13 (Fig. 3b) by exploiting the modularity of our synthetic route (Scheme 2). Fischer glycosylation of allyl alcohol and Galf pentaacetate (15) gave compound 16. Ruthenium-catalyzed cross-metathesis provided lipid-linked Galf 17, and sodium methoxide-catalyzed ester deprotection yielded 13.
Scheme 2.
Route for the synthesis of monosaccharide Galf acceptor 13.
We tested whether GlfT2 could elongate this simple acceptor. Analysis of crude enzymatic reaction mixtures using MALDI-TOF MS indicate that monosaccharide Galf acceptor 13 is a substrate (Fig. 5a). The products obtained possess up to 46 additional Galf residues, and this length is comparable to those generated from 1,6-linked disaccharide acceptors 8, 9, and 10 (Table 1). This result is particularly surprising because the putative endogenous substrate for GlfT2 is a tetrasaccharide terminated by a 1,5-linked Galf disaccharide. While these investigations do not assess how the number of Galf residues in the acceptor influence the efficiency of elongation, they do indicate that the disaccharide itself is not essential for GlfT2-catalyzed polymer formation.
Figure 5.
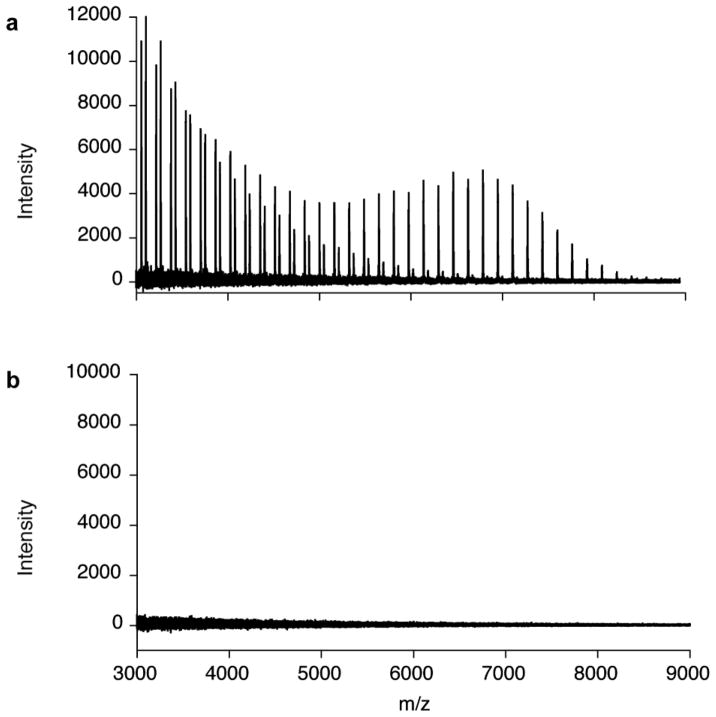
MALDI-TOF MS spectrum obtained from the reaction of His6-GlfT2, UDP-Galf, and monosaccharide acceptor substrates. (a) Monosaccharide Galf acceptor (13) is sufficient for galactan polymerization by GlfT2. Reaction products range from those resulting from the addition of 2 to 46 Galf residues. (b) Monosaccharide Galp acceptor (14) is not a substrate for GlfT2 under identical conditions.
The apparent promiscuity of GlfT2 for simple lipid-linked saccharides led us to probe the requirements for GlfT2 elongation further. The preceding enzyme in the pathway, GlfT1, is homologous to GlfT2, and the former acts on a substrate terminated with a pyranose residue. Thus, GlfT2 might be capable of elongating a substrate with a pyranose sugar. To test that GlfT2 requires at least one Galf residue for activity, the galactopyranose (Galp) analogue of acceptor 13 was synthesized in a manner analogous to that described for compound 13. As expected, Galp acceptor 14 (Fig. 3b) is not a substrate for GlfT2, and no elongation was observed (Fig. 5b; Table 1). Thus, a lipid bearing a terminal galactofuranose substituent is the minimal requirement for GlfT2-catalyzed polysaccharide synthesis. As expected, the galactofuranose residue serves as a key attribute for GlfT2 acceptors.
That GlfT2 can recognize a single Galf residue suggests there is variation or flexibility in the endogenous acceptor substrate required by GlfT2. To probe this possibility requires access to the natural substrate and analogs. Efforts to generate the requisite acceptors are ongoing. Because of its ease of synthesis, acceptor 13 is attractive as a substrate for studies that do not require differentiation between 1,5-linked and 1,6-linked acceptor substrates, including inhibitor screens. Inhibitors of mycobacterial galactan biosynthesis may be valuable leads for tuberculosis therapies because the gene encoding GlfT2 is essential for mycobacterial growth.
3. Conclusion
Our interest in the mechanics of polysaccharide biosynthesis led us to study the mycobacterial galactofuranosyltransferase GlfT2. Interrogation of the mechanism and activity of GlfT2 required a chemical biology approach to elucidate factors that influence galactan length. A divergent and modular synthetic strategy allowed for incorporation of lipids to explore the role of the lipid and the saccharide of GlfT2 acceptors. Interestingly, a lipid with a single galactofuranose residue was capable of serving as an acceptor, suggesting a relaxed specificity for GlfT2. We anticipate that the synthetic acceptors generated by our cross-metathesis strategy will continue to provide insight into the mechanism of GlfT2 and other enzymes that act on glycolipid acceptors.
4. Materials and Methods
4.1. General Synthetic Methods
All chemicals used were reagent grade from Sigma-Aldrich. Reactions were carried out under argon in oven-dried glassware. Methanol (MeOH) was distilled from magnesium and dichloromethane (CH2Cl2) was distilled from calcium hydride. Analytical TLC was carried out on E. Merck TLC plates precoated with silica gel 60 F254 (250-μm layer thickness). Analyte visualization was accomplished by using a UV lamp and charring with a solution of p-anisaldehyde (3.5 mL), acetic acid (15 mL), H2SO4 (50 mL), and ethanol (350 mL). Flash chromatography was performed on Scientific Adsorbents silica gel (32–63 m; 60-Å pore size) by using reagent-grade hexanes, ACS-grade ethyl acetate (EtOAc), MeOH or CH2Cl2. 1H NMR spectra were recorded on Bruker AC-300 spectrometers. 1H chemical shifts are reported relative to tetramethylsilane (0.00; CDCl3) or CD3OD (3.30; CD3OD). Peak multiplicity is reported as singlet (s), doublet (d), doublet of doublets (dd), triplet (t), doublet of triplets (dt), etc. Coupling constants (J) are listed in Hertz. High-resolution electrospray ionization mass spectrometry (HRESI MS) was performed on a Micromass LCT instrument. Synthetic procedures and characterization data for compounds 1–10 have been reported previously.8
4.2. 12-Phenoxy-dodec-2-enyl-β-D-galactofuranosyl-(1,5)-β-D-galactofuranoside (11)
Allyl 2,3,6-tri-O-benzoyl-5-hydroxy-β-D-galactofuranoside was obtained as a minor migration side product from the deacylation of allyl 6-O-acetyl-2,3,5-tri-O-benzoyl-β-D-galactofuranoside described previously (in 8% yield).8
1H NMR (CDCl3): δ 8.09–8.03 (m, 6H, Ar); 7.59–7.39 (m, 9H, Ar); 5.99–5.86 (m, 1H); 5.66 (dd, J = 4.8, 0.8, 1H); 5.55 (d, J = 1.3, 1H); 5.32 (s, 1H, H-1), 5.36–5.28 (m, 1H); 5.22–5.17 (m, 1H); 4.65–4.58 (dd, J = 12.8, 8, 1H); 4.52–4.46 (m, 2H); 4.41 (dd, J = 4.8, 2.1, 1H); 4.29–4.22 (m, 1H); 4.12–4.04 (m, 1H); 2.71 (d, J = 8.2, 1H, 5-OH).
The acceptor monosaccharide (71 mg, 0.13 mmol) and ethyl 2,3,5,6-tetra-O-acetyl-1-thio-β-D-galactofuranoside24 (57 mg, 0.15 mmol) were dissolved in CH2Cl2 (4.7 mL) with activated 4Å molecular sieve beads in an ice bath. N-Iodosuccinimide (41 mg, 0.18 mmol) and silver triflate (8 mg, 0.03 mmol) were added to the stirring solution. The reaction was stirred for 1.5 h in an ice bath. The mixture was filtered to remove the molecular sieves, and the organic layer was washed successively with 10% Na2S2O3 (5 mL), saturated Na2HCO3 (5 mL), water (5 mL), and brine (5 mL). The organic layer was dried over MgSO4 and concentrated under reduced pressure. Purification by flash chromatography [40% (vol/vol) EtOAc in hexanes] provided 62 mg (55%) of allyl 2,3,6-tri-O-benzoyl-β-D-galactofuranosyl-(1,5)-2,3,5,6-tetra-O-acetyl-β-D-galactofuranoside as a white crystalline solid.
1H NMR (CDCl3): δ 8.15–8.03 (m, 6H, Ar); 7.62–7.41 (m, 9H, Ar); 6.01–5.88 (m, 1H); 5.73 (d, J = 5.1, 1H); 5.55 (d, J = 1.1, 1H); 5.51 (s, 1H, H-1′); 5.38–5.31 (m, 2H); 5.31 (s, 1H, H-1); 5.23–5.19 (m, 2H); 5.00 (dd, J = 5.8, 2.4, 1H); 4.72–3.80 (m, 9H); 2.04, 2.01, 1.99, 1.78 (4s, 12H, 4xCH3CO).
A solution of Grubbs 1st generation catalyst (4.1 mg, 0.005 mmol) in CH2Cl2 (0.2 mL) was added to a stirring solution of allyl β-(1,5)-disaccharide (62 mg, 0.072 mmol) and 11-phenoxy-1-undecene8 (80 mg, 0.32 mmol) in CH2Cl2 (0.52 mL). The mixture was heated at reflux for 17 h and concentrated under reduced pressure. The crude product was purified by flash chromatography [0–40% (vol/vol) gradient EtOAc:hexanes] to yield 60 mg (77%) of 12-phenoxy-dodec-2-enyl 2,3,6-tri-O-benzoyl-β-D-galactofuranosyl-(1,5)-2,3,5,6-tetra-O-acetyl-β-D-galactofuranoside.
1H NMR (CDCl3): δ 8.13–8.03 (m, 6H, Ar); 7.62–7.41 (m, 9H, Ar); 7.30–7.24 (m, 2H, Ar); 6.95–6.87 (m, 3H, Ar); 5.73–5.51 (m, 4H); 5.51 (s, 1H, H-1′); 5.35–5.23 (m, 2H); 5.29 (s, 1H, H-1); 4.99 (dd, J = 5.8, 2.5, 1H); 4.68–4.50 (m, 4H); 4.43 (dd, J = 5.7, 3.9,, 1H); 4.32–4.01 (m, 4H); 3.96–3.91 (m, 2H); 2.03, 2.00, 1.96 (3s, 9H, 3xCH3CO); 1.78–1.70 (m, 5H); 1.44–1.23 (m, 12H).
Sodium methoxide solution (0.43 mL, 0.5 M in MeOH) was added to a stirring solution of the protected lipid-disaccharide (58 mg, 0.054 mmol) in MeOH (2 mL). The reaction was stirred for 2 h at room temperature and neutralized with Amberlite (IR-120 H+) ion exchange resin, filtered, and concentrated under reduced pressure. Purification by flash chromatography [100% CH2Cl2 to 20% (vol/vol) gradient MeOH:CH2Cl2] provided 26 mg (80%) of compound 11 as a colorless oil.
1H NMR (CD3OD): δ 7.27–7.20 (m, 2H, Ar); 6.91–6.85 (m, 3H, Ar); 5.77–5.47 (m, 2H); 5.17 (s, 1H, H-1′); 4.86 (d, J = 1.6, 1H, H-1); 4.21–3.59 (m, 14H); 2.12–2.01 (m, 2H); 1.80–1.71 (m, 2H); 1.52–1.33 (m, 12H). HRESI MS m/z calculated for [M+Na]+ C30H48O12Na: 623.3038. Found: 623.3035.
4.3. 12-d5-Phenoxy-dodec-2-enyl-β-D-galactofuranosyl-(1,6)-β-D-galactofuranoside (12)
11-d5-Phenoxy-1-undecene was prepared according to the previously published procedure for preparation of unlabeled 11-Phenoxy-1-undecene.8 d5-Phenol (1.0 g, 1.0 mmol) was added to a solution of sodium ethoxide (68 mg, 1.0 mmol) in ethanol (0.6 mL), and the reaction was stirred for 30 min at room temperature. 11-Bromo-1-undecene (0.22 mL, 1.0 mmol) was added, and the reaction mixture was stirred at reflux for 14 h. The solution was cooled to room temperature, diluted with H2O (1.5 mL), and concentrated under reduced pressure. The crude reaction mixture was purified by flash chromatography (hexanes) to yield 110 mg (43%) of 11-d5-phenoxy-1-undecene as a colorless liquid.
1H NMR (CDCl3): δ 5.82 (m, 1H); 5.04–4.92 (m, 2H); 3.95 (t, J = 6.5, 2H); 2.04 (m, 2H); 1.78 (m, 2 H); 1.48–1.30 (m, 12H).
A solution of Grubbs 1st generation catalyst (3.7 mg, 0.005 mmol) in CH2Cl2 (0.3 mL) was added to a stirring solution of allyl disaccharide 1 (59 mg, 0.065 mmol) and 11-d5-phenoxy-1-undecene (65 mg, 0.26 mmol) in CH2Cl2 (0.35 mL). The mixture was heated at reflux for 14 h and concentrated under reduced pressure. The crude product was purified by flash chromatography [0–40% (vol/vol) gradient EtOAc:hexanes] to yield 17 mg (24%) of 12-d5-phenoxy-dodec-2-enyl 2,3,5-tri-O-benzoyl-β-D-galactofuranosyl-(1,6)-2,3,5,6-tetra-O-acetyl-β-D-galactofuranoside.
1H NMR (CDCl3): δ 8.08–8.04 (m, 4H, Ar); 7.90–7.87 (m, 2H, Ar); 7.60–7.26 (m, 9H, Ar); 5.85–5.54 (m, 4H); 5.47–5.45 (m, 1H); 5.40–5.31 (m, 1H); 5.33 (s, 1H, H-1); 5.10 (s, 1H, H-1′); 5.00–4.94 (m, 2H); 4.63 (dd, J = 5.1, 3.6, 1H); 4.35–4.03 (m, 6H); 3.96–3.90 (m, 3H); 2.10–1.96 (m, 14H), 1.82–1.71 (m, 2H); 1.49–1.25 (m, 12H).
To the deuterium-labeled lipid-disaccharide (16 mg, 0.015 mmol) in MeOH (0.63 mL) was added sodium methoxide solution (0.12 mL, 0.5 M in MeOH). The reaction was stirred for 1.5 h at room temperature and neutralized with Amberlite (IR-120 H+) ion exchange resin, filtered, and concentrated under reduced pressure. Purification by flash chromatography [0–20% (vol/vol) gradient MeOH:CH2Cl2] provided 6 mg (61%) of compound 12 as a white solid.
1H NMR (CD3OD): δ 5.78–5.49 (m, 2H); 4.98 (s, 1H, H-1′); 4.95 (d, J = 1.9, 1H, H-1); 4.23–3.50 (m, 16H); 2.10–2.01 (m, 2H); 1.80–1.71 (m, 2H); 1.52–1.28 (m, 12H). HRESI MS m/z calculated for [M+Na]+ C30H43 D5O12Na: 628.3352. Found: 628.3325.
4.4. Allyl 2,3,5,6-tetra-O-acetyl-β-D-galactofuranoside (16)
Monosaccharide 1523 (700 mg, 1.8 mmol) and allyl alcohol (0.25 mL, 3.6 mmol) were dissolved in CH2Cl2 (9 mL). The flask was placed into an ice bath and stirred for 15 min. BF3•Et2O (0.67 mL, 5.4 mmol) was added dropwise to the stirring solution. After 15 min, the ice bath was removed, and the mixture was stirred for 1 h at room temperature. The reaction mixture was quenched with triethylamine and diluted with CH2Cl2 (20 mL). The organic layer was washed with saturated NaHCO3 solution (2×15 mL) and brine (15 mL) and dried over MgSO4. The solvent was removed, and the crude mixture purified by column chromatography [10–40% (vol/vol) gradient EtOAc:hexanes] to give 566 mg of 16 (81%) as a colorless oil.
1H NMR (CDCl3): δ 5.96–5.83 (m, 1H); 5.42–5.37 (m, 1H); 5.35–5.28 (m, 1H); 5.23–5.19 (m, 1H); 5.09 (s, 1H, H-1); 5.08 (d, J = 0.9, 1H); 5.02 (dd, J = 1.5, 6, 1H); 4.36 (dd, J = 11.8, 4.5, 1H); 4.29–4.16 (m, 4H); 4.07–3.99 (m, 1H); 2.14, 2.11, 2.09, 2.06 (4s, 12H, 4×CH3CO). HRESI MS m/z calculated for [M+Na]+ C17H24O10Na: 411.1262. Found: 411.1270.
4.5. 12-Phenoxy-dodec-2-enyl-2,3,5,6-tetra-O-acetyl-β-D-galactofuranoside (17)
To a stirring solution of 16 (33 mg, 0.085 mmol) and 11-phenoxy-1-undecene (84 mg, 0.34 mmol) in CH2Cl2 (0.85 mL) was added Grubbs 1st generation catalyst (5.0 mg, 0.006 mmol). The mixture was heated at reflux for 14 h and concentrated under reduced pressure. The crude product was purified by flash chromatography [0–30% (vol/vol) gradient EtOAc:hexanes] to yield 39 mg (76%) of 17 as a light brown oil.
1H NMR (CDCl3): δ 7.23–7.18 (m, 2H, Ar); 6.88–6.81 (m, 3H, Ar); 5.71–5.61 (m, 1H); 5.49–5.40 (m, 1H); 5.35–5.30 (m, 1H); 5.00, 4.99 (2s, 2H, H-1, H-2); 4.94–4.91 (m, 1H); 4.28 (dd, J = 11.7, 4.5, 1H); 4.21–4.03 (m, 3H); 3.92–3.86 (m, 3H); 2.07, 2.03, 2.02, 1.99 (4s, 12H, 3×CH3CO); 2.05–1.94 (m, 2H); 1.75–1.66 (m, 1H); 1.41–1.18 (m, 12H). HRESI MS m/z calculated for [M+Na]+ C32H46O11Na: 629.2933. Found: 629.2921.
4.6. 12-Phenoxy-dodec-2-enyl-β-D-galactofuranoside (13)
To compound 17 (39 mg, 0.064 mmol) in MeOH (0.5 mL) was added sodium methoxide solution (0.3 mL, 0.5 M in MeOH). The reaction was stirred for 3h at room temperature and neutralized with Amberlite (IR-120 H+) ion exchange resin, filtered, and concentrated under reduced pressure. Purification by flash chromatography [0–10% (vol/vol) gradient MeOH:CH2Cl2] provided 21 mg (75%) of compound 13 as a white solid.
1H NMR (CD3OD): δ 7.26–7.20 (m, 2H, Ar); 6.90–6.85 (m, 3H, Ar); 5.76–5.67 (m, 1H); 5.59–5.48 (m, 1H); 4.89 (d, J = 1.8, 1H, H-1); 4.16–4.10 (m, 1H); 4.01–3.90 (m, 5H); 3.73–3.68 (m, 1H); 3.63–3.60 (m, 2H); 2.08–2.01 (m, 2H); 1.80–1.71 (m, 2H); 1.52–1.28 (m, 12H). HRESI MS m/z calculated for [M+Na]+ C24H38O7Na: 461.2510. Found: 461.2524.
4.7. 12-Phenoxy-dodec-2-enyl-β-D-galactopyranoside (14)
Galactopyranose pentaacetate (0.50 g, 1.3 mmol) and allyl alcohol (0.20 mL, 2.6 mmol) were dissolved in CH2Cl2 (6.4 mL). The flask was placed into an ice bath and stirred for 10 min. BF3•Et2O (0.50 mL, 3.8 mmol) was added dropwise to the stirring solution. After 20 min, the ice bath was removed, and the mixture was stirred for 2 h at room temperature. The reaction mixture was quenched with triethylamine and diluted with CH2Cl2 (20 mL). The organic layer was washed with saturated NaHCO3 solution (2×15 mL) and brine (15 mL) and dried over MgSO4. The solvent was removed, and the crude mixture purified by column chromatography [10–40% (vol/vol) gradient EtOAc:hexanes] to give 311 mg (63%) of allyl 2,3,5,6-tetra-O-acetyl-β-D-galactopyranoside as a colorless oil. The characterization of this compound has been previously reported.25
To a stirring solution of allyl 2,3,5,6-tetra-O-acetyl-β-D-galactopyranoside (311 mg, 0.801 mmol) and 11-phenoxy-1-undecene (767 mg, 3.11 mmol) in CH2Cl2 (8.0 mL) was added Grubbs 1st generation catalyst (46 mg, 0.056 mmol). The mixture was heated at reflux for 15 h and concentrated under reduced pressure. The crude product was purified by flash chromatography [0–40% (vol/vol) gradient EtOAc:hexanes] to yield 298 mg (61%) of 12-phenoxy-dodec-2-enyl-2,3,5,6-tetra-O-acetyl-β-D-galactopyranoside as a brown oil.
1H NMR (CDCl3): δ 7.30–7.25 (m, 2H, Ar); 6.95–6.88 (m, 3H, Ar); 5.74–5.59 (m, 1H); 5.52–5.43 (m, 1H); 5.38 (d, J = 3.3, 1H); 5.22 (ddd, J = 10.6, 7.9, 2.6, 1H); 5.02 (dd, J = 10.4, 3.5, 1H); 4.51 (dd, J = 7.8, 1.2, 1H, H-1); 4.31–4.02 (m, 3H); 3.95 (t, J = 6.6, 2H); 3.88 (m, 1H); 2.15 (s, 3H, CH3CO); 2.05–1.98 (m, 11H, 3xCH3CO); 1.82–1.73 (m, 2H); 1.48–1.28 (m, 12H).
Sodium methoxide solution (2.0 mL, 0.5 M in MeOH) was added to the acetate-protected lipid-galactopyranose (298 mg, 0.491 mmol). The reaction was stirred for 2 h at room temperature and neutralized with Amberlite (IR-120 H+) ion exchange resin, filtered, and concentrated under reduced pressure. Purification by flash chromatography [10% (vol/vol) MeOH:CH2Cl2] provided 184 mg (85%) of compound 14 as a colorless oil.
1H NMR (CD3OD): δ 7.27–7.20 (m, 2H, Ar); 6.90–6.85 (m, 3H, Ar); 5.79–5.53 (m, 2H); 4.37–4.27 (m, 1H); 4.24 (d, J = 7.3, 1H, H-1); 4.08 (ddd, J = 11.9, 6.8, 1, 1H); 3.94 (t, J = 6.4, 2H); 3.82–3.69 (m, 3H); 3.54–3.40 (m, 3H); 2.09–2.01 (m, 2H); 1.80–1.70 (m, 2H); 1.49–1.33 (m, 12H). HRESI MS m/z calculated for [M+Na]+ C24H38O7Na: 461.2510. Found: 461.2510.
4.8. Procedure for MALDI MS analysis of GlfT2 reaction products
Reactions consisted of 120 mL total volume containing final concentrations of 0.2 mM His6-GlfT2, 50–200 mM acceptor, 150–1250 mM UDP-Galf in 50 mM Hepes, pH 7.0, 25 mM MgCl2, and 100 mM NaCl. Reactions were incubated at room temperature for 18 h, then quenched with 120 mL of a 1:1 mixture of CHCl3:MeOH. Quenched reaction mixtures were evaporated to dryness under vacuum in a SpeedVac SC100 (Varian) then resuspended in 50 mL 50% MeCN for MALDI MS analysis. Samples for MALDI MS analysis were spotted as a 1:3 mixture with α-cyano-4-hydroxycinnamic acid matrix and spectra were recorded in positive linear mode using a Bruker Ultraflex III mass spectrometer.
Acknowledgments
This paper is dedicated to Peter H. Seeberger on the occasion of his receipt of the Tetrahedron Young Investigator Award. This research was supported by National Institutes of Health Grant AI063596. R.A.S. thanks the American Chemical Society Division of Medicinal Chemistry for a graduate fellowship. We acknowledge M. R. Levengood and J. F. May for MALDI-TOF MS experiments with compounds 13 and 14 and C. Brotschi for her preliminary synthetic studies. MALDI-TOF MS and HRESI MS data were obtained at the University of Wisconsin Madison Chemistry Instrument Center Mass Spectrometry Facility supported by NIH grant number NIH NCRR 1S10RR024601-01 (MALDI-TOF MS) and NSF grant number NSF CHE-9974839 (HRESI MS). The NMR facility is supported in part by the NSF (CHE-9208463).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weigel PH, DeAngelis PL. J Biol Chem. 2007;282:36777. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 2.Whitfield C. Annu Rev Biochem. 2006;75:39. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 3.Stubbe J, Tian J, He A, Sinskey AJ, Lawrence AG, Liu P. Annu Rev Biochem. 2005;74:433. doi: 10.1146/annurev.biochem.74.082803.133013. [DOI] [PubMed] [Google Scholar]
- 4.Perlstein DL, Wang TSA, Doud EH, Kahne D, Walker S. J Am Chem Soc. 2010;132:48. doi: 10.1021/ja909325m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan F, Jackson M, Ma YF, McNeil MR. J Bacteriol. 2001;183:3991. doi: 10.1128/JB.183.13.3991-3998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sassetti CM, Boyd DH, Rubin EJ. Mol Microbiol. 2003;48:77. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 7.Besra GS, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan PJ. Biochemistry. 1995;34:4257. doi: 10.1021/bi00013a015. [DOI] [PubMed] [Google Scholar]
- 8.May JF, Splain RA, Brotschi C, Kiessling LL. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11851. doi: 10.1073/pnas.0901407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan PJ, Nikaido H. Annu Rev Biochem. 1995;64:29. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 10.Mikusova K, Yagi T, Stern R, McNeil MR, Besra GS, Crick DC, Brennan PJ. J Biol Chem. 2000;275:33890. doi: 10.1074/jbc.M006875200. [DOI] [PubMed] [Google Scholar]
- 11.Belanova M, Dianiskova P, Brennan PJ, Completo GC, Rose NL, Lowary TL, Mikusova K. J Bacteriol. 2008;190:1141. doi: 10.1128/JB.01326-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikusova K, Belanova M, Kordulakova J, Honda K, McNeil MR, Mahapatra S, Crick DC, Brennan PJ. J Bacteriol. 2006;188:6592. doi: 10.1128/JB.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremer L, Dover LG, Morehouse C, Hitchin P, Everett M, Morris HR, Dell A, Brennan PJ, McNeil MR, Flaherty C, Duncan K, Besra GS. J Biol Chem. 2001;276:26430. doi: 10.1074/jbc.M102022200. [DOI] [PubMed] [Google Scholar]
- 14.Rose NL, Completo GC, Lin SJ, McNeil M, Palcic MM, Lowary TL. J Am Chem Soc. 2006;128:6721. doi: 10.1021/ja058254d. [DOI] [PubMed] [Google Scholar]
- 15.Lovering AL, de Castro LH, Lim D, Strynadka NCJ. Science. 2007;315:1402. doi: 10.1126/science.1136611. [DOI] [PubMed] [Google Scholar]
- 16.Roy R, Dominique R, Das SK. J Org Chem. 1999;64:5408. doi: 10.1021/jo9900984. [DOI] [PubMed] [Google Scholar]
- 17.Leeuwenburgh MA, van der Marel GA, Overkleeft HS. Curr Opin Chem Biol. 2003;7:757. doi: 10.1016/j.cbpa.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Plettenburg O, Mui C, Bodmer-Narkevitch V, Wong CH. Adv Synth Catal. 2002;344:622. [Google Scholar]
- 19.Adams JH, Cook RM, Hudson D, Jammalamadaka V, Lyttle MH, Songster MF. J Org Chem. 1998;63:3706. [Google Scholar]
- 20.Choudhury AK, Roy N. Carbohydr Res. 1998;308:207. [Google Scholar]
- 21.Sarkar SK, Choudhury AK, Mukhopadhyay B, Roy N. J Carbohydr Chem. 1999;18:1121. [Google Scholar]
- 22.Pathak AK, Pathak V, Seitz L, Maddry JA, Gurcha SS, Besra GS, Suling WJ, Reynolds RC. Biorg Med Chem. 2001;9:3129. doi: 10.1016/s0968-0896(01)00179-1. [DOI] [PubMed] [Google Scholar]
- 23.Chittenden GJF. Carbohydr Res. 1972;25:35. [Google Scholar]
- 24.Gelin M, Ferrieres V, Plusquellec D. Eur J Org Chem. 2000;2000:1423. [Google Scholar]
- 25.Pastore A, Adinolfi M, Iadonisi A. Eur J Org Chem. 2008;2008:6206. doi: 10.1021/jo8003953. [DOI] [PubMed] [Google Scholar]