Abstract
Background
Immune responses are largely regulated by cytokines secreted by activated T cells. Interactions among these cells are complex, and the interaction between two responses may alter the effect of either response alone. It is established that contact sensitization-induced inflammation can reverse hair loss due to alopecia areata. In parallel, the Renbök phenomenon demonstrates how two distinct autoimmune diseases, psoriasis and alopecia areata, interact to result in clinically active psoriasis suppressing alopecia areata.
Observations
We describe a patient with concurrent psoriasis and alopecia areata universalis with terminal hairs within plaques on his extremities, representing the only normal hair growth on his body. Adjacent biopsies confirmed our clinical suspicion of plaque psoriasis with normal hair follicles and alopecia areata universalis with a peribulbar lymphocytic infiltrate. Our patient’s psoriatic plaques cleared rapidly with nbUVB phototherapy but hair growth at the site was maintained. His scalp alopecia responded to squaric acid contact sensitization therapy.
Conclusions
Our patient represents a natural experiment in whom three distinct but overlapping immune responses favored psoriasis or contact dermatitis over alopecia areata. The precise mechanism responsible for these effects remains unclear; however, based on recent reports, we speculate that cytokine cross-regulation plays a role in competition among these distinct immune responses.
Immune responses are complex as they serve widely variable roles in vivo, including infectious defense, tumor protection, transplant rejection as well as autoimmune and allergic disease. These responses vary in triggers and effector functions, and are largely dependant on T cells. T cell responses are composed of individual subsets each characterized by a specific panel of cytokines to provide “help” to the response, permitting the response to escalate or, in some cases, regress. These T cell subsets are unique to the response and tend to polarize and exclude other subsets. How this occurs in vivo is not well understood, but it appears that the cytokines from a particular subset are able to suppress the development of other competing T cell subsets, which results in a unified, single-purpose response 1.
Two previous reports have summarized a total of five patients with overlapping scalp alopecia areata and scalp sebopsoriasis that resulted in hair growth localized within affected plaques 2,3. This phenomenon was named the Renbök, or reverse Köbner, phenomenon due to the ability of psoriatic inflammation to “normalize” hair growth within patches of alopecia areata 2. Furthermore, the treatment of extensive alopecia areata using contact sensitization has been well-characterized, proving effective in 29-78% of patients, likely depending on the severity of involvement 4.
We report a case that demonstrates three separate immune responses, two spontaneous autoimmune and one through induced contact dermatitis. At sites where there was overlap between two competing processes the result favored psoriasis or contact dermatitis at the expense of alopecia areata, to the clinical benefit of the patient. We believe that this case provides insight into cytokine cross-regulation among competing T cell subsets within a human subject, a patient from our clinic.
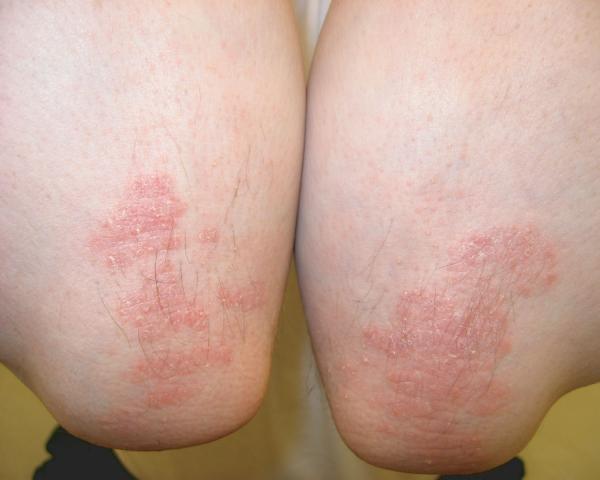
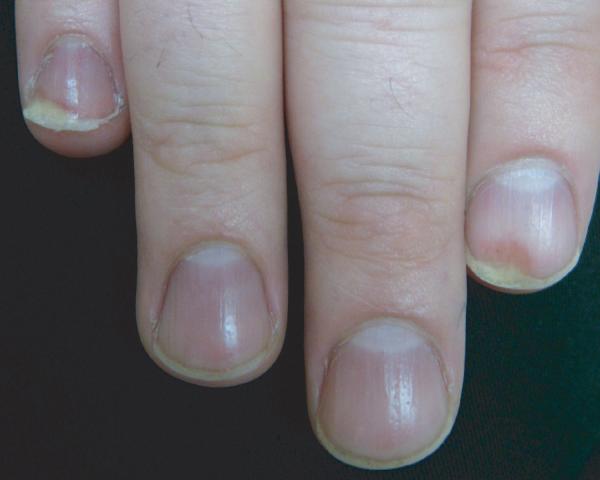
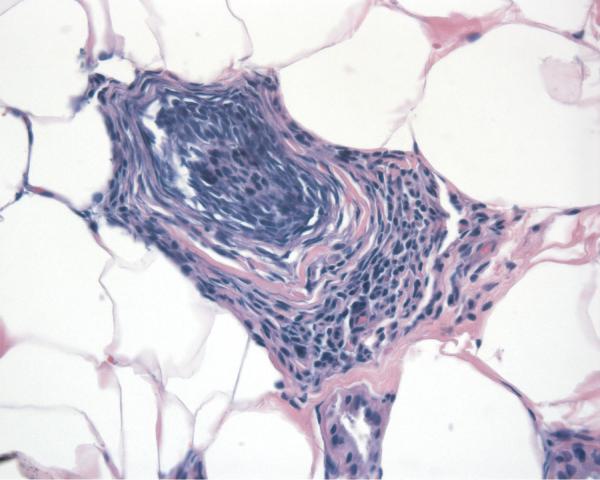
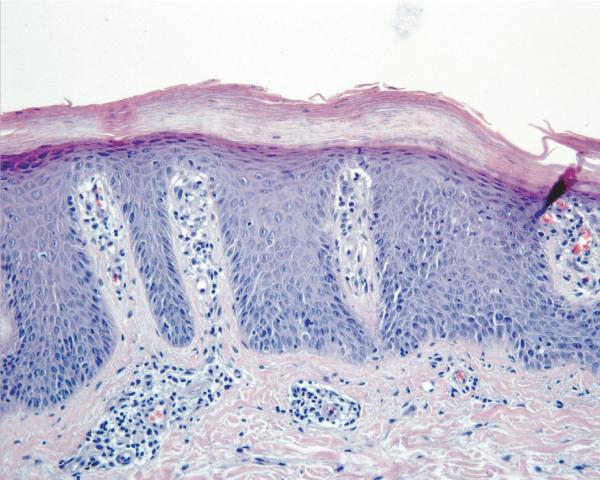
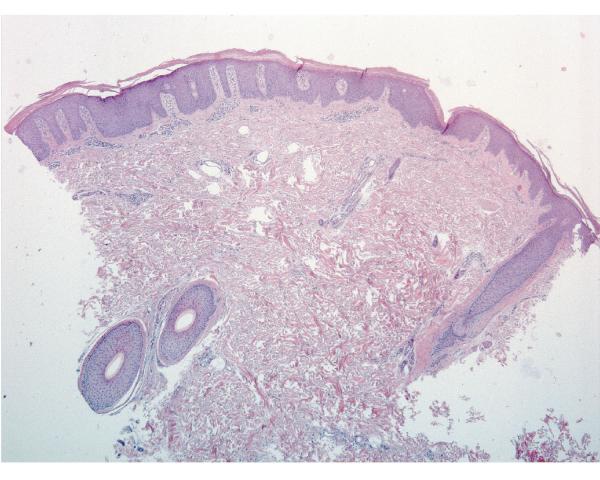
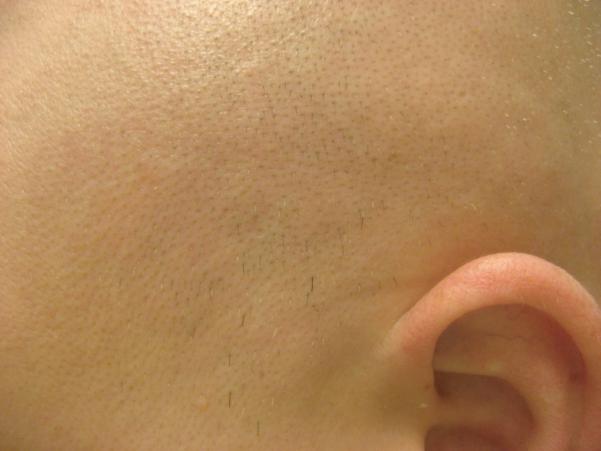
Report of a case: A 33-year-old man presented to our clinic with hair loss that began in a patchy distribution in his beard and became widespread to involve his entire scalp, face, and the rest of his body over a course of about 6 years. He had a past medical history of plaque psoriasis on his extensor extremities for 12 years. He was otherwise well and was not taking any medications. He reported a family history of psoriasis in a distant cousin and an aunt with alopecia areata. On physical exam, he had widespread nonscarring hair loss involving his entire body. On his extensor extremities he had well-defined, scaly red plaques with terminal hair growth within them (Fig 1a), representing the only areas on his body with normal hair growth. His fingernails revealed regular pitting as well as “oil spots” and onycholysis (Fig 1b). Laboratory examination demonstrated normal thyroid function. Two punch biopsy specimens were obtained from adjacent areas. One specimen from a hairless patch revealed a hair follicle with a peribulbar lymphocytic infiltrate (Fig2a). The second specimen from a scaly plaque with normal hair growth showed regular acanthosis, confluent parakeratosis, loss of the granular layer and a perivascular lymphocytic infiltrate (Fig 2b). Anagen hair follicles were identified within this specimen showing normal histology without evidence of peribulbar inflammation (Fig 2c). The patient initiated phototherapy three times weekly with narrow band ultraviolet B, clearing his psoriatic plaques within 8-10 weeks. Hair growth was maintained within previously affected areas despite resolution of his psoriatic plaques. For his alopecia, the patient was first sensitized topically to squaric acid dibutylester (SADBE) and then subsequently treated topically once weekly with 0.2% SADBE in acetone. Within 1-2 months he noted significant regrowth of his hair despite the prior presence of alopecia universalis for 3-4 years (Fig 3).
Figure 1.
Clinical exam showing scaly red plaques on the extremities with terminal hair growth despite surrounding alopecia (A); fingernails reveal regular pitting as well as “oil spots” and onycholysis (B).
Figure 2.
Skin biopsies from adjacent affected areas. A, Specimen from a hairless patch showing a peribulbar lymphocytic infiltrate (hematoxylin-eosin, original magnification 200X). B, Specimen from a scaly plaque showing regular acanthosis, confluent parakeratosis, loss of the granular layer and a perivascular lymphocytic infiltrate (hematoxylin-eosin, original magnification 400X). C, Anagen hair follicles identified within this specimen of a scaly plaque showing normal histology without evidence of peribulbar inflammation (hematoxylin-eosin, original magnification 50X).
Figure 3.
Rapid clinical response of the scalp to weekly treatment with squaric acid dibutylester contact sensitization.
Comment
We report a case of psoriasis and alopecia areata universalis overlapping on the extremities, resulting in terminal hair growth limited to plaques of psoriasis. The Renbök phenomenon was first reported by Happle and his colleagues in 1991 who described four patients with extensive scalp alopecia areata with hair growth within plaques of sebopsoriasis 2. While the Köbner phenomenon describes an escalation of psoriatic inflammation by trauma or other inflammatory response, the Renbök phenomenon describes the opposite, an inhibition of a particular inflammatory response by psoriasis. An additional case was reported in 2001 3. The term was later extended to include patients with mosaic phenomena, one with alopecia areata that spared a nevus flammeus and another a congenital nevus 5,6. The protective mechanism in mosaic skin might be through altered expression of immunomodulatory proteins within the protected skin and therefore may be distinct from that in overlapping inflammatory diseases. The mechanism of antagonism between separate inflammatory conditions is not well understood, however the ability of T cell subsets to suppress competing responses through cytokine secretion has been documented in vitro, in mice, and in human subjects.
The best-characterized T helper inflammatory subsets include T helper (Th) 1, Th2, Th17, and T regulatory cells. Classically, autoimmune responses resulting in a cytotoxic T cell response were classified as Th1-mediated and antibody-mediated responses as Th2. Th1 cells are induced by IL-12 and IFNγ and secrete IFNγ while Th2 cells are primarily induced by IL-4 and secrete IL-4 and IL-10 1. Recently, multiple autoimmune diseases initially thought to be Th1-mediated have been reclassified as Th17 7,8, which are induced by TGFβ, IL-21, IL-23 and IL-6 and secrete IL-17, IL-21, and IL-22. In contrast, T regulatory (Treg) cells are immunosuppressive, capable of potently limiting a wide range of inflammatory responses 1. Each of the inflammatory subsets (Th1, Th2 and Th17) enhances its own response through positive cytokine feedback while antagonizing alternative, competing responses, serving to polarize and recruit T cells to a singular purpose. Therefore, individual responses are mutually exclusive when overlapping within tissue. For example, Th1 cells can suppress Th2 and Th17 responses through IFNγ while Th2 responses suppress Th1 and Th17 responses through IL-4 secretion 1. While it has been speculated that Th17 cells can suppress Th1 and Th2 responses (possibly through TGFβ) 9, this has not been directly shown in vitro or in vivo.
Alopecia areata in both mice and humans has been characterized as a Th1-mediated disease, with secretion of IFNγ, IL-1β, and IL-2 10 and recently psoriasis was reported to be mediated by Th17 inflammatory cytokines 11. Contact sensitization of patients with alopecia areata with diphenylcyclopropenone revealed a decrease in IFNγ with a concomitant increase in IL-2, IL-8, IL-10, and TNFα 12. While it is still unknown what effect individual cytokines play in this reversal, IL-10 is a Th2 cytokine shown to inhibit Th1 responses 13 and TNFα plays a role in Th17 responses in general 11 and psoriasis in particular 14. Interestingly, the local injection of IL-4, a Th2 cytokine, can reverse alopecia areata in a mouse model as well as psoriasis in human patients 15,16.
These findings illustrate how opposing inflammatory responses can manifest clinically. First, in our patient the Th17 psoriatic inflammation antagonized the Th1 alopecia areata inflammatory response, presumably through cytokines central to this response. Why the Th17 response was dominant is not clear, nor is whether it would dominate every time this overlap occurred. An alternative explanation has been proposed – that the Renbök phenomenon occurs because hair growth is necessary for psoriatic inflammation to occur, and so hair growth is a “fertile” medium for psoriatic involvement 2. However, we think this is unlikely in our patient due to the fact that the psoriasis was present before the alopecia, the alopecia was universal, and that the overlap occurred in a pattern characteristic of psoriasis, suggesting that it was the psoriasis that drove this phenomenon. A case report in 2007 described the opposite history – their patient had long-standing alopecia areata and new onset scalp psoriasis that induced the Renbök phenomenon 3. Another case of overlapping scalp psoriasis and alopecia areata was described in a patient with Turner’s syndrome in which the alopecia may have prevented the psoriasis 17. Therefore, it is unclear whether one subset is inherently dominant.
Second, our patient’s alopecia areata responded to contact sensitization with SADBE. The consistent ability to generate a contact dermatitis in areas of alopecia areata to grow hair may be due to the fact that the concentration of allergen is increased until a response is obtained, rather than an inherent dominance of the contact dermatitis. Multiple mechanisms have been proposed for the clinical effects of contact sensitization on alopecia areata, including T cell apoptosis, reduction of homing markers on inflammatory T cells, and a reduction in antigen presenting cell numbers or function 18. These hypotheses are not mutually exclusive to the hypothesis of cross-regulation, and may all be affected through the action of cytokines.
The response of our patient’s psoriatic plaques to nbUVB was not unexpected, but the persistence of hair growth within previously affected areas due to the Renbök phenomenon was interesting. It is possible that the dermal portion of the psoriatic infiltrate continued to secrete antagonistic cytokines while the more superficial epidermal changes resolved. Alternatively, the protective effects of the Renbök phenomenon may be long-lasting, and only continued observation will determine whether the alopecia will return. Incidentally, we subsequently transitioned our patient to topical calcipotriene/betamethasone ointment and he remains clear of psoriasis with continued hair growth.
In summary, the clinical, histopathologic, and therapeutic observations in our patient with psoriasis, alopecia areata universalis, and contact dermatitis are consistent with cytokine cross-regulation previously characterized in vitro and in mouse models and reported in a small number of human studies. Our case represents a natural experiment that provides insight into the complex inflammatory interactions that are otherwise difficult to investigate in human subjects.
Footnotes
Financial disclosures: None reported.
Additional contributions: None.
Funding/Support, Sponsors: None.
Previous Publication: A portion of this report was orally presented in abstract form at the American Society of Dermatopathology 2008 Annual Meeting and then covered also in abstract form as a Case of the Month in Skin and Allergy News February 2009.
References
- 1.Zhu J, Paul WE. CD4 T cells: Fates, functions, and faults. Blood. 2008;112(5):1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Happle R, Van Der Steen PHM, Perret CM. The Renbök phenomenon: an inverse Köbner reaction observed in alopecia areata. Eur J Dermatol. 1991;1:228–30. [Google Scholar]
- 3.Criado PR, Valente NY, Michalany NS, et al. An unusual association between scalp psoriasis and ophiasic alopecia areata: The renbök phenomenon. Clin Exp Dermatol. 2007;32(3):320–1. doi: 10.1111/j.1365-2230.2006.02351.x. [DOI] [PubMed] [Google Scholar]
- 4.Freyschmidt-Paul P, Happle R, McElwee KJ, Hoffmann R. Alopecia areata: Treatment of today and tomorrow. J Investig Dermatol Symp Proc. 2003;8(1):12–7. doi: 10.1046/j.1523-1747.2003.12165.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen W. Alopecia areata universalis sparing nevus flammeus. Dermatology. 2005;210(3):227–8. doi: 10.1159/000083515. [DOI] [PubMed] [Google Scholar]
- 6.Bon AM, Happle R, Itin PH. Renbök phenomenon in alopecia areata. Dermatology. 2000;201(1):49–50. doi: 10.1159/000018430. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 9.Reiner SL. Development in motion: Helper T cells at work. Cell. 2007;129(1):33–6. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117(8):2019–27. doi: 10.1172/JCI31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boniface K, Blom B, Liu YJ, de Waal Malefyt R. From interleukin-23 to T-helper 17 cells: Human T-helper cell differentiation revisited. Immunol Rev. 2008;226:132–46. doi: 10.1111/j.1600-065X.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann R, Wenzel E, Huth A, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103(4):530–3. doi: 10.1111/1523-1747.ep12395722. [DOI] [PubMed] [Google Scholar]
- 13.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–87. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura M, Jo J, Tabata Y, Ishikawa O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (alopecia areata) in a C3H/HeJ mouse model. Am J Pathol. 2008;172(3):650–8. doi: 10.2353/ajpath.2008.061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoreschi K, Thomas P, Breit S, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9(1):40–6. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 17.Rosina P, Segalla G, Magnanini M, Chieregato C, Barba A. Turner’s syndrome associated with psoriasis and alopecia areata. J Eur Acad Dermatol Venereol. 2003;17(1):50–2. doi: 10.1046/j.1468-3083.2003.t01-1-00502.x. [DOI] [PubMed] [Google Scholar]
- 18.Herbst V, Zoller M, Kissling S, Wenzel E, Stutz N, Freyschmidt-Paul P. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16(5):537–42. [PubMed] [Google Scholar]