Abstract
Background
Practice-based research networks (PBRNs) are the preferred research setting for descriptive/epidemiologic studies and studies that explore the effectiveness of treatments for disease that are managed in community settings, away from the rubric of the academic medical center. A PBRN in Otology/Neurotology, established upon a sustainable research infrastructure, addresses the challenges of performing community-based research through enhanced support for data collection and facilitated research regulatory adherence. A strategic alignment of a PBRN with an established research infrastructure allows for successful implementation of a variety of study methodologies and a framework for successful competition for research funding in hearing and balance disorders. Our goal is to develop a centralized, high quality research infrastructure that supports a dynamic research alliance between regional centers for research excellence, community physicians, allied health professionals, and patients.
Objective
We describe herein current plans and progress toward the goal of developing a network of academic and community based research sites to facilitate the conduct of clinical research in hearing and balance disorders. We have formed a PBRN that we call the CHEER Network: Creating Healthcare Excellence through Education and Research. CHEER was proposed in response to a request for applications from the National Institute for Deafness and other Communication Disorders (NIDCD) to further develop clinical research in Otolaryngology, specifically focusing on disorders in hearing and balance.
Conclusion
Our expectation is that a network organized and focused around regional research alliances between academic institutions and community practitioners will have broad appeal to community-based health care professionals and patients, resulting in enhanced communications, interoperability, and success in the conduct of high quality multi-center clinical research in hearing and balance disorders.
Keywords: clinical research, clinical otology, national research network, hearing and balance research
Introduction
Effective and efficient translational research is a major goal of current clinical practice. This goal requires both a cadre of knowledgeable clinical practitioners and an infrastructure that provides streamlined processes for doing research in the setting of a busy clinical practice. An effective research network may collaborate with federal, industrial, academic medical center, and community partners, as well as patient advocacy groups, to develop and implement highly relevant research programs by experienced and committed physicians.
The current priority in translational research is reflected in a major initiative undertaken by the National Institutes of Health (NIH) in the development of the Clinical and Translational Science Awards (CTSA) program.1 This program supports two critical areas of translational research: (1) the process of applying discoveries generated in the basic science laboratory and in preclinical studies to the development of trials and studies in humans; and (2) research aimed at enhancing the adoption of best practices in the community. Development of a network of CTSA programs in academic health centers is one way to engage multidisciplinary research teams to develop programs that benefit patients. Thus, the NIH has demonstrated a strong commitment to translational and clinical research, and affirmed the critical importance of research in the area of evidence-based medicine.
The first step in translational research, from bench to bedside, is critical in terms of identifying new and potentially more effective therapeutic options. We may well be at the threshold of exciting new advances in otologic practice. However, without an established infrastructure available for implementation of clinical trials, the practical application of such discoveries is likely to be significantly delayed. Sound research practices must be implemented to study the efficacy, applicability, and feasibility of potential treatments in large scale community practice.
Equally important, we must take the treatments we already know to be effective and apply these uniformly to clinical practice. One review found that only 14% of research findings are widely applied to clinical practice, with those requiring an average of 17 years before implementation.2 Clinical practice guidelines must be constructed based on the results of clinical trials, assessed for practicality, and then communicated effectively to the practicing community of otolaryngologists with a strategy for how to implement new treatment paradigms. Strong incentives for implementing ‘best practices’ include the application of consistently excellent patient care as well as financial incentives (or lack of dis-incentives) in the upcoming pay-for-performance schemes.3
It has been suggested that one reason for the ‘disconnect’ between research and everyday practice is that most research is performed in academic medical centers, where less than 1% of Americans visiting physicians receive medical care.4 Community-engaged research can take place on many different levels, with varying degrees and types of participation from the community. Engagement of the community otolaryngologist in the planning of research questions and approaches has several potential advantages. Such an approach may help to insure that the topic is relevant to general practice, and therefore applicable to large numbers of patients. This promotes engagement of the community provider as well as future translation into practice. Inclusion of community practices would likely increase diversity (ethnic, minority, socioeconomic, severity of disease) and the number of patients included in a clinical study, which is crucial for appropriate generalizability and translation. Participation of the community provider in the planning and application phase of the study helps research methodologists understand how the study can be practically carried out in a busy community practice that may have little in the way of resources for clinical research. Education of the community practitioner and office personnel in research processes may increase the general knowledge of disease management, ‘best practice’ guidelines, data management, and general research practices. The incorporation of standardized terminology and definitions, achieved through the research collaboration, improves the quality of data recorded in the network and the consistent and reliable reporting of patient care in the medical record.5
A model that seems well-suited to patient-oriented research conducted in the community practice setting is the “Practical Clinical Trial” (PCT) espoused by Tunis et al.6 and Califf and DeMets,7,8 among others. Citing the prevalence and significance of gaps in knowledge about clinical effectiveness and the poor quality of evidence available to physicians about common clinical problems, Tunis and colleagues made a case for more widespread use of practical (or pragmatic) clinical trials, defined as trials for which the underlying hypothesis and study design are generated based on information necessary to make a clinical decision; this is fundamentally different from the conventional “explanatory clinical trial,” where the goal is to provide an account of how and why an intervention works. Practical clinical trials consider practical questions about the risks, benefits, and costs of an intervention as they would occur in routine clinical practice.9 Their major features are: (1) select clinically relevant interventions to compare (‘clinical effectiveness research,’ or CER; no placebo controls utilized); (2) include a diverse population of study participants; (3) recruit participants from a variety of practice settings; and (4) collect data on a broad range of health outcomes.6
Practice-based research networks (PBRNs) have been utilized most extensively in primary care physician offices -- including family practice, pediatrics, and internal medicine community practices.4,10,11,12 Within these settings, PBRNs have experienced a multitude of demonstrated successes. The primary care physician is involved in the ongoing management of patients throughout his or her community, which provides a heterogeneous subject population. Family practitioners are focused not only on the treatment of disease, but also on preventative medicine. The community-based family practice becomes the ideal setting in which to conduct longitudinal studies and studies to assess new disease management strategies.
The results of studies conducted within PBRNs are often very different from those of randomized clinical trials. The controlled environment of a typical clinical trial cannot accurately account for treatment effects or benefit-to-harm ratios encountered in everyday clinical practice.13 Evidence-based clinical practice guidelines for treatment of upper respiratory tract infections were found to apply to only 13% of patients presenting with upper respiratory symptoms.14 Guidelines developed for treating unstable angina, intended to decrease unnecessary hospitalizations, were found in one study to actually contribute to increased admissions.15 These controlled, randomized trials are often conducted by experts in the field, and their clinical skills may not be available in the community setting.13
In primary care practices, treatment guidelines are implemented within the context of the individual. These subjects are suffering from multiple diseases, and are more than likely being treated with concomitant medications. This setting provides a distinct advantage over randomized clinical trials. A recent study investigated the implementation of the evidence-based Kidney Disease Outcome Quality Improvement Initiative (K/DOQI) released by the National Kidney Foundation. The survey of primary care physicians found a general lack of awareness of the guidelines, but also a strong desire for more guidance on how to treat patients with chronic kidney disease. This dichotomy highlights the importance of PBRNs and their role of bridging the gap between empirical research and clinical practice.
In the surgical PBRN model, several factors uniquely complicate the conduct of surgical trials across multi-sites. In a randomized, controlled trial design, blinding is often impossible because surgeons directly administer the trial intervention to patients. Placebo-control may be ethically unacceptable, and randomization requires clinical equipoise which often conflicts with the surgical culture of decisiveness.16 Additionally, relevant to all study designs, the complexities and stage of disease at which the patients present creates greater heterogeneity than in most non-surgical groups; and while individualization of surgical technique is highly valued, it complicates standardization, quality assurance, and comparisons in surgical studies.16 There are successful surgical research networks that involve private practitioners and the community. The infrastructure and support necessary for conducting successful innovative surgical trials has been established through groups like ACOSOG (American College of Surgeons Oncology Group) and successful surgical PBRN models should incorporate the key components ACOSOG has found to be imperative, including education, steering committee mechanisms to vet and define research studies, and creation of central resources for administrative and statistical support. Importantly, there has been demonstrated success in the recruitment of a diverse pool of clinical investigators as well as patients.17 Finally, surgical PBRNs should incorporate the lessons discussed at the 2007 National Institute for Deafness and other Communication Disorders (NIDCD) Clinical Research Trials in Otology Workshop -- that the increased use of expertise-based design and methodology will enhance the validity, applicability, feasibility, and ethical integrity of randomised controlled trials in surgery, as well as in other areas, and a focus on established surgical interventions rather than new surgical procedures in which clinicians have not established expertise will allow for more generalizable results.18
Implementation of efficient, large scale clinical trials in otology has met with several obstacles in the past. NIH-sponsored multi-center clinical trials require an experienced investigator with access to clinical research infrastructure resources that are based on specific needs, requirements, and guidelines. These resources include teams of professionals with experience in project leadership, data management, data analysis, regulatory requirements, pharmaceutical management, and legal professionals to handle subcontracts. The infrastructure, once assembled, has commonly remained in place only for the duration of a single funded trial. The infrastructure is then often disbanded or reallocated along with the site research collaborations, and the disease-specific knowledge that the team members gained from the conduct of the study is diluted or lost. For instance, the nuances of collecting audiologic and vestibular data in an appropriate and timely manner along with subjective parameters of dizziness or hearing impairment present different challenges than recording ejection fraction and mortality data from a cardiovascular clinical trial. Ideally, the established infrastructure could be utilized for multiple sequential trials, but, given the long NIH review process and the small number of investigators involved, this has not been achieved.
Subject accrual has presented a challenge to the principal investigators (PIs) for some clinical studies, particularly those that have been conducted only within academic health centers (AHC). Patients with common otologic conditions that are often the subject of clinical trials are generally seen in largest numbers in community practices, and community practitioners may be reluctant to send patients away from their practices for care as part of a research protocol at the AHC.
As noted above, one of the greatest limitations of the current model of clinical research in the United States is the lack of sustaining infrastructure, which includes shared resources, common data standards, and effective use of information technology among researchers, as well a suitable forum to learn from one another’s mistakes and successes. Through CHEER we aim to create a practice-based clinical research network that facilitates an effective, robust, and dynamic research alliance between regional centers for research excellence, community physicians, and allied health professionals and patients.
CHEER -- a network for research in hearing and balance disorders
The research network was proposed in response to a request for applications from the NIDCD for proposals to further develop clinical research in Otolaryngology. The program is funded through an R21/R33 mechanism from the NIH/NIDCD. The structure of the proposed network is shown in Figure 1. We have selected thought leaders in five research-intensive national medical centers as our initial collaborators in forming our partnership between academic health centers and community physicians. These PIs serve as a core team for development of the network infrastructure and later development of research protocols. Each AHC (PI and staff) will develop a collaborative group of community-based otolaryngologists with whom they will work closely in network and protocol development.
Figure 1.
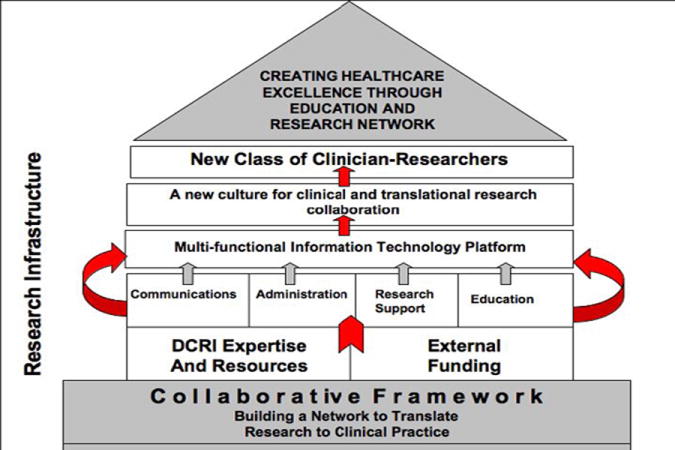
Hierarchy structure of CHEER network.
Methods: Phase 1 (R21)
Development of the CHEER network
The R21/R33 mechanism provides for two phases of development, including a one year, R21 planning phase, followed by a four year, R33 implementation phase. One of the most important tasks accomplished in the R21 phase, completed in 2007, was to identify the attributes of existing, successful PBRNs that should be considered in establishing the infrastructure for CHEER. This information was used to create our CHEER infrastructure and to develop a business plan to guide future program development.
Seven domains have been identified by the IECRN (Inventory and Evaluation of Clinical Research Networks19) as important in development and maintenance of a successful research network. These domains include: leadership structure, information technology (IT) systems, subject recruitment and retention, network administration, education and training, data management, financial policies, and sustainability. These were reviewed and summarized for application to the CHEER network. This analysis was used to develop a business plan for the CHEER network, which has since been review and approved by our academic center Co-PIs.
Results: Phase 1
Results of our analysis are summarized briefly below, by domain.
1. Leadership Structure
Management and governance for a PBRN should be organized to maximize participation and input from co-investigators and other participants, but must be clear to provide a cohesive structure for the network. Therefore, written policies and procedures for network guidance and administration must be implemented. The leadership structure includes key representation from community sites. Collaborators with expertise in biostatistics and methodology are included to ensure that research questions are appropriately developed, and issues such as heterogeneity of patients and skill level of surgeon are accounted for in study design.
2 & 3. Information technology and data management
IT platforms and data management underlie network communications. Whether communication of an idea or a data point, the IT platform must relay the information in an accessible yet secure manner, utilizing an non-arbitrary format that has review and audit functions and is not dependent upon specific hardware for day-to-day information exchange. The IT system must have flexibility using a proven platform that can be adopted and managed by all centers and sites without significant cost.
4. Subject recruitment and retention
This has been a challenge for most clinical research projects and networks, and there appears to be no clear successful strategy to apply across all studies. Important factors that impact subject recruitment include study scope, disease epidemiology, complexity of the intervention, and methods of data collection. Resources for site investigators, project coordinators, and community leaders must be considered early in study development and design to provide adequate support to carry out the study, whether observational or interventional. Inclusion of community practitioners in the early stages of study design may be particularly helpful in this domain. Additionally, the importance of recruiting both a diverse pool of clinical investigators as well as patients has been shown to be successful in terms of patient accrual.17
5. Network administration
Administration should be based upon policies and procedures that have been reviewed, documented, and agreed upon by all levels of personnel involved in study conduct. Clear roles, responsibility and accountability must be articulated and documented so that interrelationships can grow securely and communication can be enhanced.
6. Education and training
Education and training must be consistently applied to all members of the research network. Lack of knowledge of important principles and protocols creates significant potential for regulatory mistakes and possible patient subject harm. Best practice networks must employ educational standards and develop a “learning community” through sharing of educational resources. Centralized educational resources with adoption of a core curriculum that has oversight and integrated learning evaluation methods will facilitate network success.
7. Financial policies and sustainability
Most networks surveyed by the IECRN are supported in substantial part by federal funding, but are also engaged in business development activities that reach out to different NIH institutes, foundations, and private sponsors. Strategies to sustain financial health include efforts to secure a diverse base of funding, increase work efficiency, and provide aggressive management of subject recruitment and retention. Some networks also provide incentives for leadership to prospectively manage the financial health of the network.
Methods, Phase 2: Plan for Years 2 – 5: R33 phase
The plan for Years 2 through 5 is to employ the groundwork and strategic plan developed in Year 1 to guide a systematic, stepwise implementation of systems, programs, and IT platforms that will develop and sustainable PBRN focused on research in hearing and balance disorders. Specific activities and goals are listed in Table 1.
Table 1.
| Activity | Years of grant |
|---|---|
| 1) Establish and implement the CHEER network administrative infrastructure at the Duke Clinical Research Institute (DCRI). This will serve as the coordinating center for research activities. | Years 2-3 |
| 2) Adapt our IT platform at the DCRI to facilitate communication, administration, research, and education in our regional academic centers and community-based practices. | Years 2-3 |
| 3) Implement programs and tools focused on building research capabilities through innovative educational programs. | Year 3 |
| 4) Explore and facilitate the use of common data standards for hearing and balance that are consistent with the principles of Health Level 7 (HL-7) and the Clinical Data Interchange Standards Consortium (CDISC). | Year 3 |
Discussion: Challenges identified
The CHEER network has great strengths, but there are weaknesses and challenges that must be addressed for the network to become successful in a way that significantly enhances patient care. First, we acknowledge that unanticipated challenges will surface as we proceed with protocol development and research implementation. We have tried to anticipate what most challenges will be, based on an extensive review of existing networks (above) and an exploration of challenges specific to surgical networks and trials.
In the months ahead, we will use the experience gained in the first two years of the grant to build a sustainable clinical research network that is responsive to the needs of the patients we serve as well as academic and community practitioners engaged in their care and in the practice of innovative clinical research. Through our experiences with the SOURCE research collaborative and the BEST-ENT research network (recent studies include TO TREAT1 and SLEEP2) we recognize that there is a great deal of interest among community otolaryngologists in participating in clinical research. IT platforms and educational systems will enable practitioners and identified research coordinators to receive the training they need as well as the support to participate. In addition, our goal is to engage these physicians in protocol development so that they are invested in the research protocol, and have the opportunity to tailor the required activities to fit into their practice. AHC PIs may recruit practitioners associated with their medical centers in the community. This may include those well known to the centers from residency training or other professional relationships. However, community practitioners do not have to be closely associated geographically with a given center to participate, and academic otolaryngologists in any institution may be included in the network. We also plan to actively recruit motivated practitioners through the BEST-ENT network and through dissemination of information about CHEER at the annual American Academy of Otolaryngology - Head and Neck Surgery (AAO-HNS) meeting, and meetings of the senior societies, such as the American Neurotology Society and the American Otological Society.
We intend to put in place quality control systems to insure high quality data collection. We will define and monitor standards of care as related to the studies conducted, monitor compliance with educational and other requirements, and provide support as needed to achieve quality data collection. We will develop common data standards for disease manifestations and objective and subjective data, as needed for each protocol, and educate research investigators in these standards. Some studies cannot be done in community practices, due to the need for special equipment and expertise only found in major academic medical centers. We will identify those research questions that are best answered in community settings.
Much of the success of our strategy will rely on continuous education and communication, ensuring there is not a lapse in either in order to promote a sustainable network. Motivation is another key element, and we assume that practitioners who agree to participate are motivated to do so. We hope to facilitate a relationship and two-way commitment between the academic site PIs and coordinators and the community physicians and their staff. The academic physicians will contribute to the education of the community participants, and the community physicians will devote the time and resources necessary to become clinical investigators.
Conclusion
The new NIH Roadmap initiative was undertaken to bridge the gap that exists between basic biomedical research and the application of such research to direct patient care. Translating new research into therapies and treatments implemented in clinical practice can often take years. Large, controlled clinical studies, with strict inclusion and exclusion criteria and restrictions, often do not mirror the environment of the clinical practice. Treatment management strategies and clinical guidelines researched in such a controlled way may not be efficacious where it really matters – the office of the medical practitioner. PBRNs can transform the findings of clinical research into effective methods and treatments administered directly to the patient. Many challenges have been encountered with the advent of PBRNs, including lack of infrastructure, selection of feasible studies, recruitment, and the composition of qualified, trained study teams.
It is our proposal that a network organized and focused on regional dynamic research alliances will have increased appeal to community-based health care professionals and patients, resulting in enhanced communications, interoperability, and success in the conduct of multi-center clinical research. Once the developmental phase of this new network is complete, we anticipate that clinical research in Otology and Neurotology will become sustainable at both the regional and national level, with a subsequent increase in the level of evidence available to support the effectiveness of diagnostic and therapeutic interventions related to disorders in hearing and balance.
Figure 2.
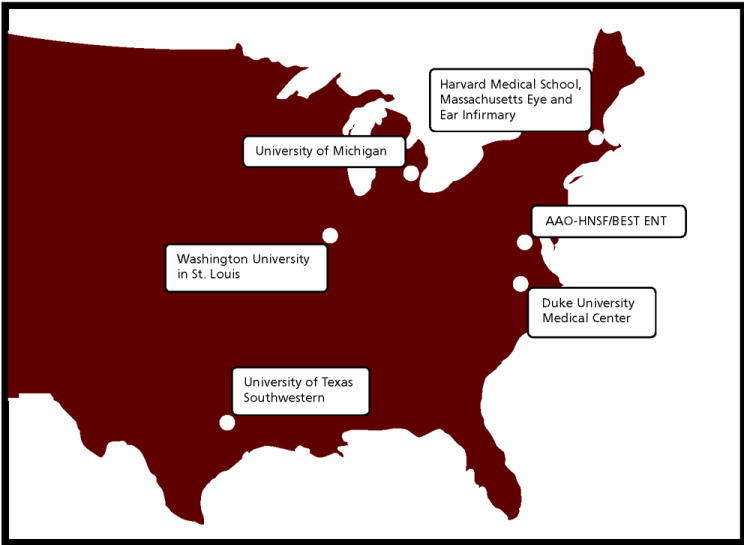
Academic medical center ‘hubs’ in CHEER research network, including: Duke University, Massachusetts Eye and Ear Infirmary, University of Michigan, Washington University, University of Texas Southwestern. Web address of CHEER network: cheerresearch.org
Acknowledgments
We acknowledge the contributions of our academic site co-investigators, Drs. Jay Piccirillo, Steven Rauch, Peter Roland, and Steven Telian, and thank Dr. Maureen Hannley for her contributions to the success of this endeavor. Excellent editorial assistance was provided by Dr. Nancy Gasper Smith. This work is funded by the NIH, NIDCD (R33DC008632).
Footnotes
Tonsillitis Outcomes: Toward Reaching Evidence in Adults and Tots
Studying Life Effects and Effectiveness of Palatopharyngoplasty
References
- 1.Zerhouni EA. Translational research: moving discovery to practice. Clin Pharmacol Ther. 2007;81:126–28. doi: 10.1038/sj.clpt.6100029. [DOI] [PubMed] [Google Scholar]
- 2.Balas EA, Boren SA. Yearbook of Medical Informatics: Managing Clinical Knowledge for Health Care Improvement. Stuttgart, Germany: Schattauer Verlagsgesellschaft mbH; 2000. [PubMed] [Google Scholar]
- 3.Califf RM. Clinical trials bureaucracy: unintended consequences of well-intentioned policy. Clin Trials. 2006;3:496–502. doi: 10.1177/1740774506073173. [DOI] [PubMed] [Google Scholar]
- 4.Tierney WM, Oppenheimer CC, Hudson BL, Benz J, Finn A, Hickner JM, et al. A national survey of primary care practice-based research networks. Ann Fam Med. 2007;5:242–50. doi: 10.1370/afm.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Weel C, de Grauw W. Family practices registration networks contributed to primary care research. J Clin Epidemiol. 2006;59:779–83. doi: 10.1016/j.jclinepi.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–32. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 7.Califf RM, DeMets DL. Principles from clinical trials relevant to clinical practice: Part I. Circulation. 2002;106:1015–21. doi: 10.1161/01.cir.0000023260.78078.bb. [DOI] [PubMed] [Google Scholar]
- 8.Califf RM, DeMets DL. Principles from clinical trials relevant to clinical practice: Part II. Circulation. 2002;106:1172–5. doi: 10.1161/01.cir.0000023218.39412.32. [DOI] [PubMed] [Google Scholar]
- 9.Roland M, Torgerson DJ. What are pragmatic trials? BMJ. 1998;316:285. doi: 10.1136/bmj.316.7127.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham DG, Spano MS, Stewart TV, Staton EW, Meers A, Pace WD. Strategies for planning and launching PBRN research studies: a project of the academy of family physicians national research network (AAFP NRN) J Am Board Fam Med. 2007;20:220–28. doi: 10.3122/jabfm.2007.02.060103. [DOI] [PubMed] [Google Scholar]
- 11.Westfall JM, VanVorst RF, Main DS, Herbert C. Community-based participatory research in practice-based research networks. Ann Fam Med. 2006;4:8–14. doi: 10.1370/afm.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green LA, White LL, Barry HC, Nease DE, Hudson BL. Infrastructure requirements for practice-based research networks. Ann Fam Med. 2005;3(Suppl 1):S5–11. doi: 10.1370/afm.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westfall JM, Mold J, Fagnan L. Practice-based research – “ blue highways” on the NIH roadmap. JAMA. 2007;297:403–6. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor PJ, Amundson G, Christianson J. Performance failure of an evidence-based upper respiratory infection clinical guideline. J Fam Pract. 1999;48:690–7. [PubMed] [Google Scholar]
- 15.Katz DA. Barriers between guidelines and improved patient care: an analysis of AHCPR’s Unstable Angina Clinical Practice Guideline. Health Serv Res. 1999;34(1 pt 2):377–89. [PMC free article] [PubMed] [Google Scholar]
- 16.You YN, Wells SA. Clinical trials in surgery: the role of the American College of Surgeons Oncology Group. World J Surg. 2006;30:1147–51. doi: 10.1007/s00268-006-0076-7. [DOI] [PubMed] [Google Scholar]
- 17.Newman LA, Hurd T, Leitch M, Kuerer HM, Diehl K, Lucci A, et al. A report on accrual rates for elderly and minority-ethnicity cancer patients to clinical trials of the American College of Surgeons Oncology Group. J Am Coll Surg. 2004;199(4):644–51. doi: 10.1016/j.jamcollsurg.2004.05.282. [DOI] [PubMed] [Google Scholar]
- 18.Devereaux PJ, Bhandari M, Clarke M, Montori VM, Cook DJ, Yusuf S, et al. Need for expertise based randomised controlled trials. BMJ. 2005;330(7482):88. doi: 10.1136/bmj.330.7482.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IECRN/Networks for Clinical Research. [30 March 2009]; Available at: https://www.clinicalresearchnetworks.org/5c.asp#IECRN.


