Summary
Fibrinogen is a pleiotropic blood protein that regulates coagulation, inflammation and tissue repair. Fibrinogen extravasates in the nervous system after injury or disease associated with vascular damage or blood-brain barrier (BBB) disruption. Fibrinogen is not merely a marker of BBB disruption, but plays a causative role in neurologic disease as a potent inducer of inflammation and an inhibitor of neurite outgrowth. Fibrinogen mediates functions in the nervous system as a ligand for cell-specific receptors. In microglia, fibrinogen mediates activation of Akt and Rho via the CD11b/CD18 integrin receptor, while in neurons fibrinogen induces phosphorylation of epidermal growth factor (EGF) receptor via the αvβ3 integrin. Pharmacologic targeting of the interactions of fibrinogen with its nervous system receptors could provide novel strategies for therapeutic intervention in neuroinflammatory and neurodegenerative diseases.
Keywords: coagulation, inflammation, integrins, neurodegeneration, microglia, multiple sclerosis
Introduction
Fibrinogen is unique among blood factors due to its multiple non-overlapping binding sites that facilitate specific interactions with a number of both integrin and non-integrin receptors expressed on a wide variety of cells of the hematopoietic, immune and nervous systems. An important step in unveiling the pleiotropic functions of fibrinogen was the determination of its crystal structure, solved in 1997 by Russell Doolittle, that provided a fundamental structural basis for the diverse biological functions of fibrinogen, fibrin and their derivatives [1–3]. In 1995, Jay Degen and Thomas Bugge showed that sustained deposition of fibrin in tissues is sufficient to cause wasting and tissue necrosis in mice genetically deficient for plasminogen (plg−/−), the precursor of the enzyme plasmin, which is responsible for the removal of fibrinogen deposits from tissues [4]. Remarkably, these abnormalities were rescued when the plg−/− mice were crossed with mice deficient for fibrinogen (fib−/−) [4]. Although the mechanism linking fibrinogen with tissue damage was unknown, this work demonstrated that a blood protein could mediate disease not only in the bloodstream, but also within tissues.
After injury or disease, including stroke, multiple sclerosis (MS), Alzheimer’s Disease (AD) and spinal cord injury, rupture of the vasculature allows the entry of blood proteins into the brain and precedes subsequent edema formation and neuronal damage [5]. We have previously reviewed evidence from neuropathology regarding the temporal and spatial correlation of fibrin deposition in human neurologic diseases [6, 7], the receptors and functions of fibrinogen in the nervous system [6, 8] and the drug discovery potential of selective targeting of fibrinogen’s interactions with its receptors [7]. This review will focus on the fibrinogen-induced signal transduction pathways that play a causative role in nervous system pathology.
Fibrinogen and the CD11b integrin receptor – Neuroinflammation
Interactions of fibrinogen with the platelet αIIbβ3 integrin receptor lead to platelet aggregation during blood coagulation [9]. Besides platelets, fibrinogen can bind to integrins expressed on cells of the immune system, such as CD11b/CD18 and CD11c/CD18 [10, 11]. The CD11b/CD18 integrin receptor [αMβ2, Mac-1, complement receptor 3 (CR3)] is a member of the β2 integrin family, which is expressed on monocytes, macrophages and microglia. Fibrinogen recognizes CD11b through the C-terminal γ377–395 sequence [12, 13], which is a cryptic binding epitope of the fibrinogen molecule exposed only after its polymerization to fibrin or its immobilization on a substrate [14]. Binding of fibrinogen to the CD11b/CD18 integrin causes a broad spectrum of cell signaling responses, such as activation of NF-κB and mitogen-activated protein kinase (MAPK) / phosphatidylinositol 3-kinase (PI3K), to mediate adhesion, migration, chemotaxis and phagocytosis [8].
Our group showed that in vitro, the binding of fibrinogen to the CD11b/CD18 integrin receptor in microglia, which are the immune cells of the central nervous system (CNS), induces activation of Akt and Rho signaling, resulting in cytoskeletal rearrangements and increased phagocytosis [15]. Activation of innate immune responses plays a central role in mediating autoimmunity in MS [16]. In active MS plaques, fibrin deposition correlates with activated microglia [17]. Interestingly, early, pre-demyelinating MS lesions are characterized by fibrin deposition and microglia activation that precedes the infiltration of T cells in the brain parenchyma [18]. In addition to fibrinogen, other blood proteins and especially components of the coagulation cascade, such as tissue factor, protein C inhibitor, thrombospondin, fibronectin, and vitronectin have been identified in chronic active MS plaques [19]. Anticoagulants reduced clinical symptoms in experimental autoimmune encephalomyelitis (EAE), an established animal model for MS [15, 19]. Studies of pharmacologic or genetic depletion of fibrinogen showed that fibrinogen plays a causative role in the development of inflammatory demyelination [15, 20]. Disruption of the fibrinogen-CD11b/CD18 interaction, both genetically in fibrinogen knock-in mice expressing a mutant form of fibrinogen that cannot bind to the CD11b/CD18 integrin receptor [21] or pharmacologically using the γ377–395 peptide that inhibits binding of fibrinogen to CD11b [22], reduced inflammatory demyelination and paralysis in mice after induction of EAE [15]. Similarly, fibrinogen knock-in mice showed reduced clinical symptoms and inflammation in an animal model of rheumatoid arthritis [23]. Selective inhibition of fibrinogen binding to CD11b/CD18 integrin did not affect fibrinogen’s clotting functions [7, 15, 21]. Thus, selective pharmacological modulation of the interaction between fibrinogen and its target integrin receptor may be a pertinent strategy for attenuating innate immune responses in chronic inflammatory diseases such as MS without adverse hemorrhagic effects [7, 15].
In addition to inflammatory demyleination, there is a striking correlation between fibrin deposition, CD11b+ microglia and Aβ accumulation as mice age and develop AD-like pathology [24]. Genetic or pharmacologic inhibition of fibrin deposition reduces BBB disruption and inflammatory reactivity [24]. Moreover, intrahippocampal injection of beta-amyloid peptide demonstrates that the reciprocal interactions between microglial-mediated signaling and fibrinogen deposition are associated with neuronal damage [25]. These studies provide further evidence for the contribution of fibrin in neuroinflammation and strongly suggest that activation of microglia by fibrinogen is a general mechanism for the induction of inflammation in the nervous system at sites of vascular damage.
Fibrinogen and EGF receptor transactivation – Neurodegeneration
The interactions between fibrinogen and integrin receptors have been studied mostly in the context of inflammatory responses. Recently, this paradigm was expanded to include neurons as another cell type that is responsive to fibrinogen through an integrin receptor. Our lab demonstrated that fibrinogen can inhibit neurite outgrowth by binding to the β3 integrin, which in turn trans-activates the EGF receptor [26]. Fibrinogen induced phosphorylation of the EGF receptor in primary neurons, and this effect was abolished by a neutralizing antibody for β3 integrin [26]. Moreover, transactivation of the EGF receptor by β3 integrin depends on the Src family kinases. The integrin-mediated transactivation of growth factor receptors is a known mechanism of receptor cross-talk for the coordination of growth responses to extracellular stimuli, suggesting that fibrinogen could serve as the “signal,” and β3 integrin might function as a “sensor” to traumatic changes in the CNS microenvironment [26]. Therefore, upon injury or disease in the CNS that is associated with hemorrhage, fibrinogen can act as a modulator of local neuronal responses by inhibiting neurite outgrowth, which is a central impediment for CNS repair.
A potential role for fibrinogen as an inhibitor of axonal regeneration in the CNS is further strengthened by its abundant deposition after spinal cord injury, which correlates with phosphorylation of EGF receptor in severed axons [26]. Fibrin deposition also correlates with neuronal damage in stroke. Extravascular fibrin in stroke could mediate both neuroinflammatory effects as an activator of microglia and neurodegenerative effects as a modulator of neuronal functions. Indeed, fib−/− mice show reduced reperfusion impediment and subsequent infarction after cerebral ischemia-hypoxia than their heterozygous littermates [27]. These observations show a deleterious role for intravascular fibrin deposition in driving ischemia-hypoxia and a potential role for extravasated fibrinogen in inducing secondary vascular and neuronal injury after an initial stroke insult.
Fibrinogen and ERK1/2 phosphorylation – Peripheral nerve regeneration
Similar to the CNS, peripheral nerve injury is usually accompanied by disruption of the blood-nerve barrier, fibrinogen leakage and fibrin deposition. We previously showed that a fibrinogen-regulated downstream signaling cascade plays a central role in peripheral nerve regeneration [28]. The extracellular signal-regulated kinase (ERK) 1/2 signal transduction pathway is activated by fibrinogen in Schwann cells and arrests them in a proliferating, non-myelinating state that persists until the fibrinolytic system clears fibrin [28, 29]. Fibrinogen [28] and neuregulin [30] are two major molecular signals identified that drive Schwann cell dedifferentiation and proliferation after nerve injury. Importantly, genetic or pharmacologic depletion of fibrinogen after sciatic nerve crush enhanced the remyelinating activity through a faster transition of the Schwann cells to a myelinating state [28]. In accordance, peripheral nerve injury was exacerbated in tissue plasminogen activator (tPA)-deficient or plg−/− mice [29], which show increased fibrin deposition. These findings identify fibrinogen signaling as a critical regulator of remyelination of peripheral nerves, and provide a mechanism for how a blood-derived protein can be the environmental cue that balances the proliferation versus differentiation of myelinating cells after peripheral nerve injury [28].
Conclusions
There is abundant evidence that fibrinogen is present in the nervous system after traumatic injury or disease associated with vascular rupture or disruption of the blood-brain or the blood-nerve barrier in the CNS and peripheral nervous system, respectively. Fibrinogen’s interactions with receptors on microglia, neurons and Schwann cells can induce cell-specific signaling pathways, and thereby regulate inflammatory, neurodegenerative or tissue repair functions involved in a wide spectrum of nervous system pathologies, such as peripheral neuropathies, MS, AD, spinal cord injury and stroke (Fig. 1). The spatiotemporal regulation of different components of fibrinogen signal transduction, including expression of receptors, availability of co-receptors and intracellular adaptor proteins at different disease stages will determine the contribution of fibrinogen in neurologic disease. Pharmacologic targeting of the interactions of fibrinogen with its receptors may provide a selective therapeutic strategy to regulate disease onset and progression
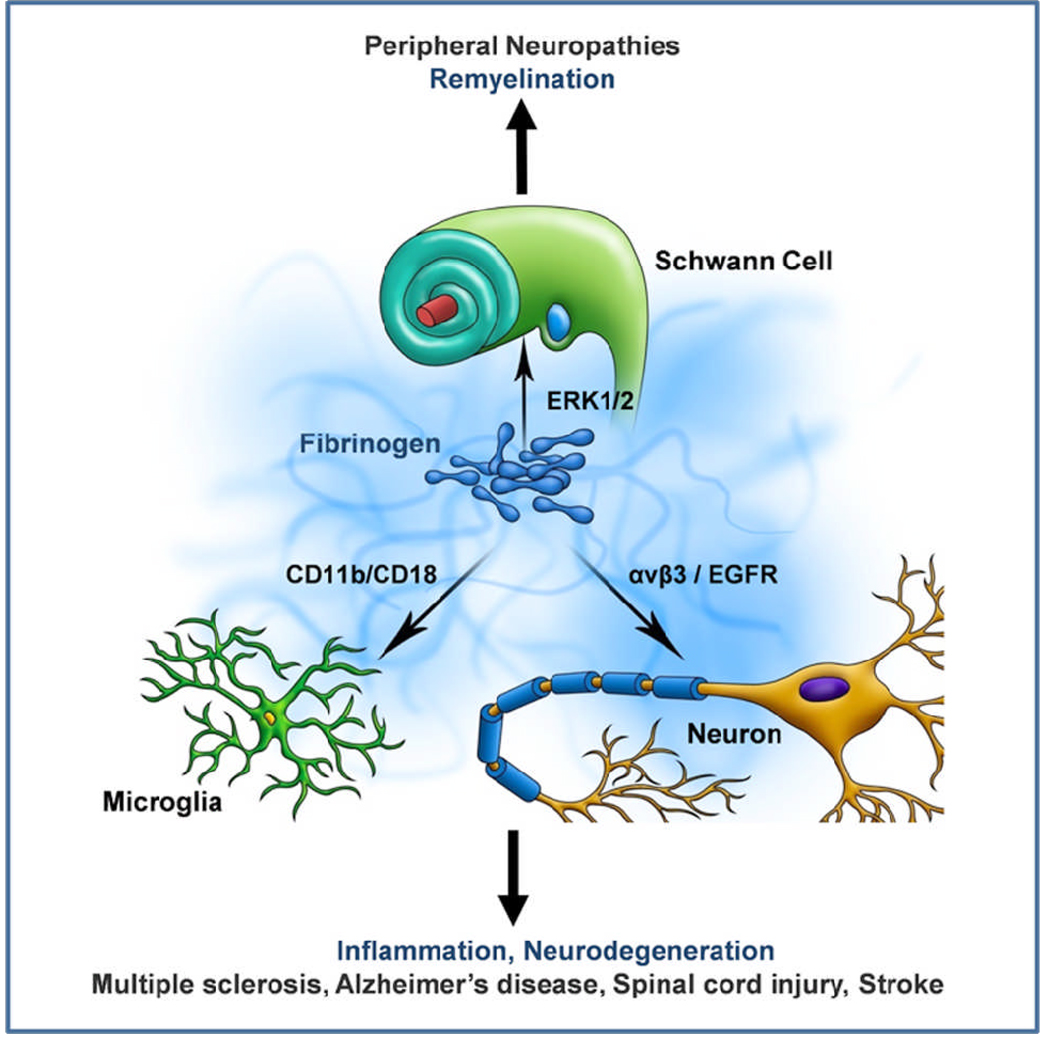
Figure 1. Fibrinogen-induced signal transduction in the nervous system.
Fibrinogen elicits diverse biological responses by inducing distinct signal transduction pathways in nervous system cells. Fibrinogen induces ERK 1/2 phosphorylation in Schwann cells and inhibits remyelination [28], activates the CD11b/CD18 integrin receptor in microglia and induces phagocytosis [15] and phosphorylates the EGF receptor in neurons causing inhibition of neurite outgrowth [26]. Fibrinogen signal transduction can potentially modulate inflammatory, neurodegenerative and repair processes in a variety of diseases associated with BBB disruption and vascular damage, such as MS, AD, spinal cord injury, stroke and peripheral neuropathies.
Acknowledgements
This work is supported by grants from NIH/NINDS NS051470 and NS052189, the National Multiple Sclerosis Society (NMSS) RG3782 and the Dana Foundation to KA. DD is a NMSS postdoctoral fellow. We thank John Lewis for expert medical illustration and design.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.
References
- 1.Spraggon G, Everse SJ, Doolittle RF. Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature. 1997;389:455–462. doi: 10.1038/38947. [DOI] [PubMed] [Google Scholar]
- 2.Brown JH, Volkmann N, Jun G, Henschen-Edman AH, Cohen C. The crystal structure of modified bovine fibrinogen. Proc Natl Acad Sci U S A. 2000;97:85–90. doi: 10.1073/pnas.97.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Mochalkin I, Veerapandian L, Riley M, Doolittle RF. Crystal structure of native chicken fibrinogen at 5.5-A resolution. Proc Natl Acad Sci U S A. 2000;97:3907–3912. doi: 10.1073/pnas.080065697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 5.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 6.Adams RA, Passino M, Sachs BD, Nuriel T, Akassoglou K. Fibrin mechanisms and functions in nervous system pathology. Mol Interv. 2004;4:163–176. doi: 10.1124/mi.4.3.6. [DOI] [PubMed] [Google Scholar]
- 7.Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: Lessons from Multiple Sclerosis. Curr Med Chem. 2007;14:2925–2936. doi: 10.2174/092986707782360015. [DOI] [PubMed] [Google Scholar]
- 8.Akassoglou K, Strickland S. Nervous system pathology: the fibrin perspective. Biol Chem. 2002;383:37–45. doi: 10.1515/BC.2002.004. [DOI] [PubMed] [Google Scholar]
- 9.Phillips DR, Charo IF, Parise LV, Fitzgerald LA. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988;71:831–843. [PubMed] [Google Scholar]
- 10.Rubel C, Fernandez GC, Rosa FA, Gomez S, Bompadre MB, Coso OA, et al. Soluble fibrinogen modulates neutrophil functionality through the activation of an extracellular signal-regulated kinase-dependent pathway. J Immunol. 2002;168:3527–3535. doi: 10.4049/jimmunol.168.7.3527. [DOI] [PubMed] [Google Scholar]
- 11.Ugarova TP, Yakubenko VP. Recognition of fibrinogen by leukocyte integrins. Ann N Y Acad Sci. 2001;936:368–385. doi: 10.1111/j.1749-6632.2001.tb03523.x. [DOI] [PubMed] [Google Scholar]
- 12.Ugarova TP, Solovjov DA, Zhang L, Loukinov DI, Yee VC, Medved LV, et al. Identification of a novel recognition sequence for integrin alphaM beta2 within the gamma-chain of fibrinogen. J Biol Chem. 1998;273:22519–22527. doi: 10.1074/jbc.273.35.22519. [DOI] [PubMed] [Google Scholar]
- 13.Altieri DC, Agbanyo FR, Plescia J, Ginsberg MH, Edgington TS, Plow EF. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac-1 (CD11b/CD18) J Biol Chem. 1990;265:12119–12122. [PubMed] [Google Scholar]
- 14.Lishko VK, Kudryk B, Yakubenko VP, Yee VC, Ugarova TP. Regulated unmasking of the cryptic binding site for integrin alpha M beta 2 in the gamma C-domain of fibrinogen. Biochemistry. 2002;41:12942–12951. doi: 10.1021/bi026324c. [DOI] [PubMed] [Google Scholar]
- 15.Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, et al. The fibrin-derived gamma377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204:571–582. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prod'homme T, Zamvil SS. Bench to bedside: tempering antigen-presenting cells in multiple sclerosis. Nat Med. 2008;14:614–615. doi: 10.1038/nm0608-614. [DOI] [PubMed] [Google Scholar]
- 17.Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain. 1997;120:1461–1483. doi: 10.1093/brain/120.8.1461. [DOI] [PubMed] [Google Scholar]
- 18.Marik C, Felts PA, Bauer J, Lassmann H, Smith KJ. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130:2800–2815. doi: 10.1093/brain/awm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han MH, Hwang SI, Roy DB, Lundgren DH, Price JV, Ousman SS, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 20.Akassoglou K, Adams RA, Bauer J, Tseveleki V, Mercado P, Lassmann H, et al. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugarova TP, Lishko VK, Podolnikova NP, Okumura N, Merkulov SM, Yakubenko VP, et al. Sequence gamma 377–395(P2), but not gamma 190–202(P1), is the binding site for the alpha MI-domain of integrin alpha M beta 2 in the gamma C-domain of fibrinogen. Biochemistry. 2003;42:9365–9373. doi: 10.1021/bi034057k. [DOI] [PubMed] [Google Scholar]
- 23.Flick MJ, LaJeunesse CM, Talmage KE, Witte DP, Palumbo JS, Pinkerton MD, et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117:3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, et al. Fibrinogen inhibits neurite outgrowth via beta3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci U S A. 2007;104:11814–11819. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, et al. Cerebral Ischemia-Hypoxia Induces Intravascular Coagulation and Autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akassoglou K, Yu W-M, Akpinar P, Strickland S. Fibrin inhibits peripheral nerve regeneration by arresting Schwann cell differentiation. Neuron. 2002;33:861–875. doi: 10.1016/s0896-6273(02)00617-7. [DOI] [PubMed] [Google Scholar]
- 29.Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–1166. doi: 10.1083/jcb.149.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D, Marchionni MA, et al. Glial growth factor/neuregulin inhibits schwann cell myelination and induces demyelination. J Cell Biol. 2001;152:1289–1300. doi: 10.1083/jcb.152.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]