Abstract
Lipoprotein[a] may represent an independent risk factor for peripheral arterial disease of lower limbs (LL-PAD), but prospective data are scant. We estimated the association between baseline lipoprotein[a] with prevalent and incident LL-PAD in older subjects from the InCHIANTI Study.LL-PAD, defined as an ankle-brachial index (ABI)<0.90, was assessed at baseline and over a six-year follow-up in a sample of 1002 Italian subjects aged 60-96 years. Plasma lipoprotein[a] as well as potential traditional and novel cardiovascular risk factors (including a score based on relevant inflammatory markers) were entered in multivariable models to assess their association with prevalent and incident LL-PAD.At baseline, lipoprotein[a] concentration was directly related to the number of elevated inflammatory markers (p< 0.05). There were 125 (12.5%) prevalent cases of LL-PAD and 57 (8.3%) incident cases. After adjustment for potential confounders, participants in the highest quartile of the lipoprotein[a] distribution (≥32.9 mg/dl) were more likely to have LL-PAD compared to those in the lowest quartile (odds ratio, OR=1.83, 95% confidence interval, CI=1.01-3.33). The association was stronger (OR=3.80, 95% CI=1.50-9.61) if LL-PAD was defined by harder criteria, namely ABI <0.70. Compared to subjects in the lowest lipoprotein[a] quartile, those in the highest quartile showed a somewhat increased risk of incident LL-PAD (lowest quartile, 7.7%, highest quartile 10.8%), but the association was not statistically significant (OR 1.52, 95% CI 0.71-3.22). In conclusion, lipoprotein[a] is an independent LL-PAD correlate in the cross-sectional evaluation, but further prospective studies on larger populations, with longer follow-up and more definite LL-PAD ranking might be needed to establish a longitudinal association.
Keywords: atherosclerosis, inflammation, lipids, lipoprotein[a], peripheral vascular disease
Introduction
Several epidemiologic studies suggest that plasma Lp[a] is a strong risk factor for coronary heart disease [1, 2] and stroke [3], while fewer investigations assessed the risk for LL-PAD yielding controversial results [4,5]. Differences in Lp[a] plasma levels between populations [6] suggest ethnic differences in the control of Lp[a] concentration, which may imply potentially different prognostic values; while LL-PAD has been investigated in Italy [7, 8], only one population-based study examined Lp[a] and the risk of LL-PAD [9]. A pathophysiological link between elevated Lp[a] levels and LL-PAD is plausible since this molecule has both atherogenic and thrombogenic properties. Lp[a] contributes to foam cell formation because it is prone to oxidative modification, and when oxidated, it is taken up by the scavenger receptor pathway [10]; besides, apo[a] has structural homology with plasminogen suggesting that Lp[a] may exert anti-fibrinolytic properties [11]. Prevalence of LL-PAD increases with age [8, 12] and older patients with LL-PAD suffer from faster functional decline, greater disability, and higher mortality compared to those without LL-PAD [13]. Identifying risk factors for LL-PAD onset may disclose new opportunities for prevention and cure, therefore contributing to decrease the burden of morbidity and functional consequences of LL-PAD in older persons. Using data from a population-based study of older Italian individuals, we investigated the relation of baseline plasma Lp[a] levels with prevalent and incident LL-PAD.
Methods
The InCHIANTI study is a prospective, population-based study of randomly selected older people living in two cities in the Chianti area, Tuscany, Italy. The study was designed by the Laboratory of Clinical Epidemiology of the Italian Research Council of Aging (Florence, Italy) to identify risk factors for late-life disability, as previously described [14]. Briefly, participants were selected from the city registries of Greve in Chianti and Bagno a Ripoli using a multistage sampling method. In 1998, 1453 persons randomly selected from the population agreed to participate in the project (91.6% response rate). The Italian National Research Council on Aging Ethical Committee ratified the study protocol and participants provided written consent to participate. The present analysis was performed in 1002 persons ≥ 60 years, with an ankle brachial index (ABI) lower than 1.5, and with plasma Lp[a] measured at baseline. Of the original 1453 participants enrolled in the study, 250 were excluded because they were younger than 60, three because of an ABI >1.5, 182 because they had no ABI assessment at baseline, and 16 had missing Lp[a] values. The longitudinal analysis was limited to 686 participants without prevalent LL-PAD at baseline and with at least one valid ABI measure over the follow-up: 125 were excluded because of prevalent LL-PAD, 47 because had died and 144 because they did not have ABI assessment at follow-up. Observation that were lost to follow-up (n=191) were significantly, more likely to be older, male, and have higher levels of inflammatory markers; they had similar baseline levels of Lp[a] and similar ABI score.
The ABI was measured during the clinical test session with a handheld Doppler stethoscope (Parks model 41-A; Parks Medical Electronics, Inc, Aloha, Ore). As previously described [15], systolic pressures were measured twice in the right brachial artery and twice in each posterior tibial artery. The highest pressure in each set of measurements was used to calculate the ABI score by dividing the lowest of the two systolic pressures for each leg by the brachial artery pressure. The ABI was measured at baseline and LL-PAD was defined as an ABI less than 0.90 and absence of LL-PAD as an ABI between 0.90 and 1.50. Individuals were excluded if their ABI >1.50, indicating poorly compressible leg arteries and inability to gauge arterial perfusion accurately. An ABI <0.90 is consistent with LL-PAD [16]. Participants were revaluated for ABI over the follow-up at three and six years. Incident LL-PAD cases were defined as an ABI <0.90 at any follow-up visit among those without prevalent LL-PAD (ABI <0.90 at baseline).
Blood samples were obtained from participants after a 12-hour fast. Aliquots of serum and plasma were stored at −80°C and were not thawed until analyzed. Lp[a] concentration was evaluated by measuring apo[a] from frozen plasma by an ELISA test (Mercodia, Uppsala, Sweden) [17] where 1 U apo[a] is approximately equal to 0.7 mg Lp[a] (Mercodia Manual); the results are expressed in mg/dl. This assay is very sensitive and highly specific and gives no measurable cross-reactivity with plasminogen and apolipoprotein B; besides it minimizes the possible interference of heterogeneity in apolipoprotein(a) isoforms with the results; the detection limit is 0.0035 mg/dl; the overall coefficient of variation for Lp[a] measurements in this study was 6.6%. High-density lipoprotein (HDL-C) cholesterol, total cholesterol and triglycerides were determined using commercial enzymatic tests (Roche Diagnostics, Mannheim, Germany). We used the Friedwald equation to calculate low density lipoprotein cholesterol (LDL-C) concentration. Oxidized LDL were measured by ELISA (Mercodia, Uppsala, Sweden).
Cigarette-smoking behavior was assessed through survey questions. Daily alcohol (g/day) and total energy intake (Kcal/day/Kg) were estimated by the European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire [18]. Weight and height were measured using objective standard techniques and used to calculate body mass index (BMI, Kg/m2). Physical activity during the year prior to the interview was assessed through an interviewer-administered questionnaire as previously described [19]. Responses were coded on an ordinal scale ranging from 1 to 7. A score of 1 indicated no physical activity (bed rest) and a score of 6 indicated intense physical activity (ie, a sport activity) performed several times per week. A score of 7 denoted participants who engaged in intensive and prolonged physical activity (at least 5 km of walking per day occurring at least 5 times per week) consistently over the past 5 years.
The presence of specific medical conditions was established using standardized criteria that combined information from self-reported history, medical records, and a clinical medical examination. Participants were also asked to report any medication taken in the last 2 weeks. Diagnostic algorithms were modified versions of those created for the Women's Health and Aging Study [20]. The following diseases were assessed: coronary heart disease (angina and acute myocardial infarction), stroke (and/or transient ischemic attack), hypertension, and diabetes. Serum creatinine and urinary creatinine from the 24-hour urine collection were measured using a modified Jaffe method and used to calculate creatinine clearance (CrCL) as a measure of glomerular filtration rate (GFR)
Based on the results of previous work of this group, 4 inflammatory markers were considered for this project: interleukin (IL)-6, IL-1 receptor antagonist (IL-1ra), fibrinogen and C-reactive protein (CRP) [22]. IL-6 and IL-1ra were measured in duplicate by high-sensitivity enzyme-linked immunoabsorbent assays (BIOSOURCE, Camarillo, Calif). The CVs were 4.5% for IL-1ra and 7% for IL-6; CRP was measured in duplicate using an enzyme-linked immunoabsorbent assay and colorimetric competitive immunoassay (interassay CV: 5%). Plasma fibrinogen levels were automatically determined using a commercially available STA fibrinogen assay (Diagnostic Stago, Roche Diagnostics, GmbH, Mannheim, Germany) according to the Clauss method using a Stago Boerhinger Mannheim Analyzer. The reference range used by this laboratory is 150 to 400 mg/dL.
Variables are reported as mean values ± standard deviation, median and interquartile range (Q1-Q3), or percentages. Comparisons across Lp[a] quartiles were performed using ANOVA, Chi-square test, or Kruskal-Wallis nonparametric test for variables with skewed distribution [21]. An ordinal score was created that summarizes the intensity of the proinflammatory state across multiple markers. The score (range 0-4) consisted of the number of inflammatory markers in the upper tertiles, among the markers previously identified as independent correlates of ABI, namely, CRP (>4.2 mg/dL), IL-6 (>1.8 pg/mL), IL-1ra (>162.7 pg/mL), fibrinogen (>376 mg/dL) [22]. Multivariable logistic regression analysis was then used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the cross-sectional association between Lp[a] concentration and LL-PAD (ABI <0.9) or LL-PAD defined by more stringent criteria (ABI <0.7) approximating the median ABI value (0.72) among participants with ABI <0.9. Covariates hypothesized as potential confounders of the association between plasma Lp[a] and LL-PAD were progressively added to the initial model. The longitudinal analysis was limited to 686 participants without prevalent LL-PAD at baseline and with at least one valid ABI measure over the follow-up. Incident LL-PAD was defined as ABI value <0.9 assessed at either the year-3 or year-6 clinic visit, in participants with ABI value ≥0.9 at baseline. We used logistic multivariable analyses to estimate the strength of the association between baseline Lp[a] categories and the likelihood of incident LL-PAD, adjusting for potential confounders; baseline ABI was included in the model to account for regression to the mean. To investigate selective loss to follow-up, we performed a sensitivity analysis in which we included also those who were lost to follow-up (n=191). Two converse options were examined, assuming that either those lost to follow-up would all have incident LL-PAD, or that none of them would have such condition. All analyses were performed using STATA (StataCorp. 2005. Stata Statistical Software: release 9. College Station, TX: StataCorp LP).
Results
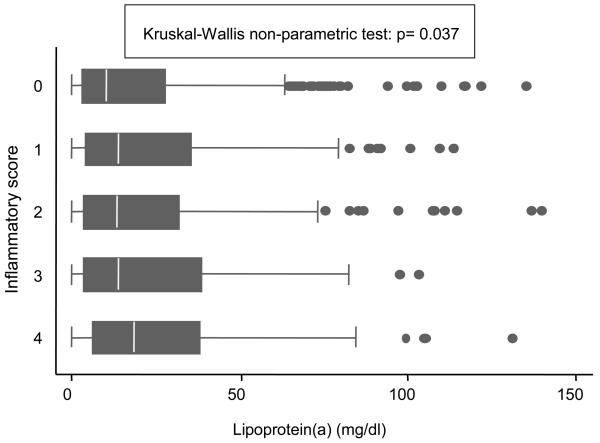
The mean age of participants was 73.7 years (range 60-95 years); 44.0% were men. Median Lp[a] concentration was 12.4 mg/dl (IQ range 3.5-32.8). No differences in Lp[a] concentration were found according to age and gender. Prevalence of coronary heart disease and use of lipid lowering medications were greater among those with the highest Lp[a] concentration. Participants in the highest Lp[a] quartile had the highest levels of total cholesterol, LDL-C and HDL-C. No significant differences were found in triglycerides and oxidized LDL-C according to Lp[a] quartiles (Table 1). Of the inflammatory markers considered, only fibrinogen was significantly associated to Lp[a] concentration in the univariate analysis; nevertheless, we found a direct significant association between Lp[a] plasma levels and the ordinal score summarizing the intensity of the proinflammatory state (Figure 1).
Table 1.
Selected general and clinical characteristics of the study sample according to Lipoprotein[a] quartiles (N=1002).
| Characteristics | Lipoprotein[a] quartiles (range, mg/dl) | ||||
|---|---|---|---|---|---|
| Variables | I (0-3.5) (N=251) |
II (3.6-12.3) (N=250) |
III (12.4-32.8) (N=249) |
IV (32.9-175.9) (N=252) |
P* |
| Age (mean±SD) (Years) | 74.0±7.3 | 74.1±7.6 | 73.3±6.7 | 74.3±7.2 | .432 |
| Men | 43.8% | 44.8% | 43.0% | 44.4% | .978 |
| Smokers | |||||
| Former | 30.5% | 27.1% | 24.5% | 26.2% | |
| Current | 15.1% | 13.2% | 15.7% | 13.1% | .787 |
| Alcohol consumption (g/day) | |||||
| 0-20 | 47.0% | 44.0% | 47.0% | 46.0% | |
| >20 | 24.7% | 28.8% | 25.4% | 25.2% | .972 |
| Physical activity level scale [1-7, 7=best], (mean±SD) |
3.2 ±0.98 | 3.2±0.95 | 3.3±0.88 | 3.2±1.02 | .516 |
| Body Mass Index (Kg/m2,mean±SD ) | 27.2±4.0 | 27.8±4.0 | 27.4±4.2 | 27.6±3.8 | .293 |
| Hypertension | 62.2% | 60.0% | 62.7% | 62.3% | .928 |
| Positive Rose Questionnaire | 14.4% | 24.4% | 28.9% | 32.2% | .080 |
| Diabetes mellitus | 13.9% | 11.6% | 12.1% | 9.9% | .578 |
| Coronary Heart Disease (Angina or myocardial infarction) |
8.0% | 8.8% | 10.8% | 15.1% | .046 |
| Stroke or Transient Ischemic Attack (%) | 7.2% | 4.8% | 6.4% | 7.9% | .533 |
| Statins therapy | 0.4% | 2.0% | 3.6% | 7.9% | <.001 |
| Total Cholesterol (mg/dl, mean±SD) | 215.3±41.4 | 215.3±38.4 | 217.6±36.6 | 227.0±38.2 | .0015 |
| Low-density lipoprotein-cholesterol (mg/dl, mean±SD) |
132.6±36.7 | 135.0±33.2 | 137.3±31.3 | 144.2±32.7 | <.001 |
| High-density lipoprotein-cholesterol (mg/dl, mean±SD) |
56.3±16.7 | 54.1±14.2 | 55.0±14.4 | 57.8±14.3 | .029 |
| Oxidized low-density lipoprotein-cholesterol (U/l, mean±SD) |
41.4±13.3 | 42.9±12.6 | 42.7±11.8 | 42.9±12.9 | .435 |
| Triglycerides (mg/dl, median-iqr) | 105(81-161) | 117(84-161) | 112(85-148) | 108(88-146) | .709 |
| Creatinine clearance (mL/min) | 77.5±27.1 | 78.8±25.8 | 76.9±25.2 | 75.2±24.9 | .487 |
| High sensitivity-C-reactive protein (μg/ml, median- iqr) |
2.44(1.11-4.92) | 2.36 (1.27-5.51) | 2.82 (1.36-5.83) | 2.99 (1.53-5.57) | .151 |
| Interleukin-6 (pg/ml, median-iqr) | 1.38 (0.78-2.01) | 1.47 (0.88-2.36) | 1.44 (0.84-2.09) | 1.38 (0.86-2.27) | .629 |
| Fibrinogen (mg/dl, median-iqr) | 345 (308-390) | 340 (302-390) | 345 (312-395) | 360 (318-413) | .007 |
| Interleukin-1 receptor antagonist (pg/ml, median- iqr) |
127(95-175) | 136 (101-192) | 137 (93-190) | 134 (95-180) | .472 |
| Homocysteine (μmol/L, media-iqr) | 14.8 (12.2-17.5) | 14.6 (12.0-17.3) | 14.4 (11.9-17.9) | 13.5 (11.7-16.5) | .176 |
| Estimated creatinine clearance (ml/min, mean±SD) | 64.9 ±20.8 | 65.6 ±19.1 | 64.4 ±18.4 | 63.9 ±18.3 | .784 |
P value from ANOVA, Chi-square test, or Kruskal-Wallis nonparametric test for variables with skewed distribution
Figure 1.
Box-plots showing unadjusted baseline lipoprotein[a] concentration according to inflammatory score.
Footnote. The central box extends from the 25th to the 75th percentile; the line within the box represents the median value; points outside the lines are the “outliers.” P from Kruskal-Wallis nonparametric test for variables with skewed distribution. Inflammatory markers considered in the inflammatory score are: CRP (>4.2 mg/dL), IL-6 (>1.8 pg/mL), IL-1ra (>162.7 pg/mL), fibrinogen (>376 mg/dL).
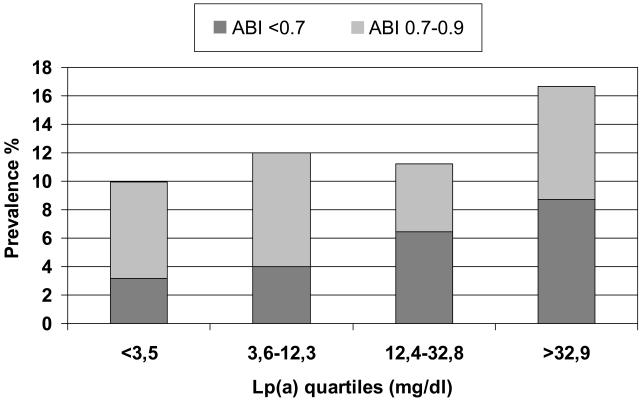
As depicted in figure 2, there was a stepwise increase in the likelihood of prevalent LL-PAD across quartiles of Lp[a] distribution (p for linear trend = 0.038). The association was stronger when the analysis was limited to participants with ABI <0.7, a proxy for more severe disease (n=66, p for linear trend = 0.004), who had higher Lp[a] plasma levels (median 25.7 ml/dl) than those with ABI 0.7-<0.9 and ABI ≥0.9 (median Lp[a] =11.8 and 12.1 mg/dl, respectively; Kruskal-wallis test: p =0.005). These findings were confirmed by multivariable logistic regression analysis estimating the likelihood of prevalent LL-PAD as a function of Lp[a] distribution (Table 2). After adjustment for age, gender, smoking, and alcohol intake (Model 1), subjects in the highest quartile of the Lp[a] distribution had a two-fold higher probability of being affected by LL-PAD compared to those in the lowest quartile. Further adjustment for BMI, LDL-C, HDL-C, diabetes, and hypertension did not modify the association (Model 2), whereas a mild reduction was observed after adjusting for inflammation score (Model 3), but the strength of the association still remained statistically significant. In the analysis limited to ABI< 0.70, there was a clear dose-response relation across quartiles of Lp[a]. After full adjustment for potential confounders and compared to subjects in the lowest quartile (model 3), those in the third quartile were more than twice as likely to have LL-PAD, and participants in the highest quartile had almost a four-fold probability of LL-PAD (OR 3.8 95% CI: 1.5-9.6, p=0.005). Further adjustment for statin use and estimated creatinine clearance did not modify the results. No significant interactions between Lp[a] concentration and other cardiovascular risk factors were observed.
Figure 2.
Crude proportion of participants with prevalent peripheral arterial disease according to baseline lipoprotein[a] distribution and Ankle-Brachial Index score.
Table 2.
Adjusted Odds Ratio and 95% Confidence Intervals for prevalent Lower-limb Peripheral Artery Disease by lipoprotein[a] quartiles according to Ankle Brachial Index score
|
ABI<0.90 (n. 125) |
ABI<0.70 (n. 66) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) |
Odds Ratio (95% CI) |
||||||||
| Lp[a] quartiles: (range, mg/dl) |
N | n | Model 1 | Model 2 | Model 3 | n | Model 1 | Model 2 | Model 3 |
| 0-3.5 | 251 | 25 | 1 | 1 | 1 | 8 | 1 | 1 | 1 |
| 3.6-12.3 | 250 | 30 | 1.33 (0.73-2.41) |
1.32 (0.71-2.44) |
1.27 (0.68-2.38) |
16 | 1.39 (0.52-3.71) |
1.65 (0.60-4.57) |
1.53 (0.55-4.30) |
| 12.4-32.8 | 249 | 28 | 1.34 (0.73-2.46) |
1.33 (0.72-2.47) |
1.25 (0.67-2.34) |
20 | 2.47 (1.00-6.12) |
2.64 (1.04-6.71) |
2.41 (0.94-6.21) |
| 32.9-175.9 | 252 | 42 | 2.05 (1.16-3.62) |
2.00 (1.11-3.61) |
1.83 (1.01-3.33) |
22 | 3.39 (1.42-8.09) |
4.11 (1.65-10.3) |
3.80 (1.50-9.64) |
| P for trend | 0.016 | 0.024 | 0.055 | 0.002 | 0.001 | 0.002 | |||
Model 1: adjusted for age, gender, smoking, and alcohol intake
Model 2: additional adjustment for BMI, lipid parameters (LDL-C, HDL-C), diabetes, and hypertension
Model 3: additional adjustment for inflammation score
Table 3 summarizes the results of the longitudinal analysis. Over the six-year follow-up there were 57 new cases of LL-PAD (cumulative incidence 8.3%). Compared to subjects in the lowest Lp[a] quartile, those in the highest quartile showed an increased, but not statistically significant, risk of incident LL-PAD (10.8% vs. 7.7 %); after adjustment for potential confounders and baseline ABI score, the strength of the association was further attenuated.
Table 3.
Adjusted Odds Ratio and 95% Confidence Intervals for incident Lower limb Peripheral Artery Disease, according to lipoprotein[a] quartiles in 686 people with Ankle-brachial index ≥0.9 at baseline and at least 1 ankle-brachial index assessment over the six years of follow-up
| Peripheral Arterial Disease (ABI<0.90) |
||||
|---|---|---|---|---|
| Lp[a] quartiles: (range, mg/dl) | Odds Ratio (95% CI) |
|||
| Model 1 | Model 2 | |||
| Number of patients |
||||
| (0-3.5) | 181 | 14 (7.7%) | 1 | 1 |
| (3.6-12.3) | 170 | 13 (7.7%) | 0.98 (0.44-2.22) | 0.89 (0.38-2.10) |
| (12.4-32.8) | 169 | 12 (7.1%) | 0.95 (0.43-2.17) | 0.75 (0.31-1.80) |
| (32.9-175.9) | 166 | 18 (10.8%) | 1.52 (0.71-3.22) | 1.22 (0.54-2.74) |
Model 1: adjusted for age, gender, smoking, and alcohol intake
Model 2: additional adjustment for BMI, LDL-C, HDL-C, diabetes, hypertension, inflammation score, and ABI score at baseline.
To investigate whether the longitudinal analysis was affected by selective loss to follow-up, we performed a sensitivity analysis including the sample of those who did not have ABI reevaluation over the follow-up. When we classified these latter (n =191) as having no incident LL-PAD, the OR for the highest compared to the lowest Lp[a] quartile was 1.33 (95% CI 0.63-2.82), after adjustment for age, gender, smoking, alcohol intake, BMI, and ABI score at baseline. If subjects lost to follow-up were classified in the group with incident LL-PAD the adjusted OR was 1.21 (95% CI 0.75-1.95).
Discussion
Our findings, in a sample of randomly selected Italian older men and women, showed a significant cross-sectional association between higher plasma Lp[a] and LL-PAD. In addition, we found a graded relationbetween Lp[a] concentration and ABI score, suggesting a potential dose-response relationship with LL-PAD severity. Several traditional cardiovascular risk factors were associated with Lp[a] levels at baseline; nevertheless, the multivariate analyses including other lipid parameters, diabetes, hypertension and inflammatory markers, confirmed the independent association between Lp[a] with prevalent LL-PAD. Conversely, the longitudinal association between Lp[a] concentration and the risk of incident LL-PAD over the six-year follow-up was not statistically significant.
Findings from most, but not all [23], previously published cross-sectional reports suggest a direct correlation between Lp[a] plasma levels and LL-PAD prevalence in different populations, including African-Americans, Asians and Caucasians [24, 25]. Our results extend these findings, demonstrating a graded association between the level of Lp[a] and the ABI score, a marker of the vascular disease severity, independent of traditional cardiovascular risk factors and pro-inflammatory markers, an association rarely addressed in previous studies. In contrast, the longitudinal analyses did not point out a significant association between baseline Lp[a] concentration and incident LL-PAD over a 6-year follow-up. Four longitudinal studies investigated the role of novel risk factors as predictors of LL-PAD and reported conflicting findings on the role of Lp[a] as a potential risk factor. In a case-control analysis nested in the Physicians' Health Study, baseline Lp[a] level was not associated with incident symptomatic LL-PAD during an average 9-year follow-up [4]. Another prospective investigation on 403 patients, showed only a moderate association between Lp[a] and the progression of large-vessel LL-PAD after adjustment for traditional risk factors and CRP [5]. On the other hand, recent reports from the Brunek Study and the Women's Health Study showed a borderline relationship over a 5 and 12.3 year median follow-up, respectively [9,26]. In our study, which was characterized by a shorter follow-up period, participants in the highest Lp[a] quartile had a 50% increased risk of incident LL-PAD compared to those in the lowest quartile, after adjustment for demographic characteristics. However, adjustment for other risk factors and baseline ABI score further reduced the strength of this association. Although the interpretation of this finding is difficult, our longitudinal data are in agreement with the results of previous prospective studies. Overall, our results are inconclusive and suggest that the relationship between Lp[a] levels and incident LL-PAD should be further investigated.
Despite the fact that our cross-sectional findings were not confirmed in the prospective study, we believe that a causal relationship between Lp[a] plasma levels and ABI score, a marker of systemic atherosclerosis, should not be ruled out. Plasma Lp[a] is associated with incident CHD [1, 2] and stroke [Error! Bookmark not defined., 3]. Indeed, different biological mechanisms, including both atherogenic and thrombogenic activities, support a role of Lp[a] in the development of atherosclerosis and its clinical complications. Since, Lp[a] levels are fully expressed in the first year of life, have a strong genetic control, do not vary with age and, unlike other lipoproteins, are almost unaltered by environmental factors, it seems unlikely that the robust cross-sectional association identified in the study could be explained by reverse causality. On the other hand, it is possible that after the exclusion of people with prevalent LL-PAD, which included a consistent proportion of those with the highest long-life Lp[a] concentration, a longer follow-up time and a higher incidence of cases might be needed to detect a significant prospective association with LL-PAD.
The population-based sample, the assessment of several markers of inflammation, and use of the ABI to measure incident LL-PAD are major strengths of our study. Some evidence suggests that Lp[a] may act as an acute phase reactant and that Lp[a] plasma levels, like traditional acute phase proteins, increase after acute clinical events, including myocardial infarction and surgical operations [27]. Clinical studies reported an association between Lp[a] and inflammatory biomarkers such as CRP, TNFα, TGF-β, IL-6, and monocyte chemoattractant protein 1 [4, 5, 12, 22, 26]. Furthermore IL-6 may up-regulate the expression of apo[a] gene with consequent increase of Lp[a] particles concentration [28]. Our findings of a significant association between plasma Lp[a] concentrations and the number of elevated inflammatory markers, including CRP, IL-6, fibrinogen, and IL-1 receptor antagonist, are in agreement with these previous results and suggest that inflammation may represent a partial unifying mechanism; however multivariable analyses emphasizes a biological link between Lp[a] concentration and LL-PAD largely independent of inflammation.
Despite these strengths, several limitations deserve consideration. First, 22% of individuals without prevalent LL-PAD at baseline were not included in the longitudinal analyses because of missing ABI or because they died over the follow-up time. Given the results of our sensitivity analysis, a bias due to selective loss to follow-up is unlikely. Nevertheless, the exclusion of 191 individuals, along with a relatively short follow-up time, certainly reduced the statistical power of the study and the likelihood of finding a significant association between baseline Lp[a] and the risk of incident LL-PAD. Second, we did not assess apo[a] isoforms. Apo[a] size isoforms are inversely correlated with Lp[a] concentration[29] and low molecular weight apo[a] patterns have been reported as independent risk factors for atherosclerosis [Error! Bookmark not defined.]. Therefore, evaluation of apo[a] genotype would have allowed a better understanding of the biological relationship of Lp[a] and LL-PAD. Finally, we analyzed the association between Lp[a] and LL-PAD incidence using a single assessment of Lp[a] at baseline, which may lead to misclassification of the usual plasma Lp[a] of some participants, although the primarily genetic influence on Lp[a] levels makes its determination rather stable over time.
Acknowledgments
Funding Sources
The InCHIANTI study baseline (1998-2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001-2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-ups 2 and 3 studies (2004-2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002); supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no conflicts of interest to disclose
References
- 1.Danesh J, Collins R, Peto R. Lipoprotein[a] and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102:1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 2.Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, Rumley A, Lowe GD, Danesh J, Gudnason V. Lipoprotein[a] levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168:598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 3.Smolders B, Lemmens R, Thijs V. Lipoprotein [a] and stroke: a meta-analysis of observational studies. Stroke. 2007;38:1959–1966. doi: 10.1161/STROKEAHA.106.480657. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein[a], and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 5.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623–2629. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 6.Sandholzer C, Hallman DM, Saha N, Sigurdsson G, Lackner C, Csaszar A, Boerwinkle E, Utermann G. Effects of the apolipoprotein[a] size polymorphism on the lipoprotein[a] concentration in 7 ethnic groups. Human Genetics. 1991;86:607–614. doi: 10.1007/BF00201550. [DOI] [PubMed] [Google Scholar]
- 7.Vigna GB, Bolzan M, Romagnoni F, Valerio G, Vitale E, Zuliani G, Fellin R. Lipids and other risk factors selected by discriminant analysis in symptomatic patients with supra-aortic and peripheral atherosclerosis. Circulation. 1992;85:2205–2211. doi: 10.1161/01.cir.85.6.2205. [DOI] [PubMed] [Google Scholar]
- 8.Brevetti G, Oliva G, Silvestro A, Scopacasa F, Chiariello M. Prevalence, risk factors and cardiovascular comorbidity of symptomatic peripheral arterial disease in Italy. Atherosclerosis. 2004;175:131–138. doi: 10.1016/j.atherosclerosis.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, Kronenberg F, Xu Q, Bergmark C, Weger S, Oberhollenzer F, Witztum JL. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. 2006;6(47):2219–2228. doi: 10.1016/j.jacc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Tsimikas S, Tsironis LD, Tselepis AD. New insights into the role of lipoprotein[a]-associated lipoprotein-associated phospholipase A2 in atherosclerosis and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2094–2099. doi: 10.1161/01.ATV.0000280571.28102.d4. [DOI] [PubMed] [Google Scholar]
- 11.Koschinsky ML, Marcovina SM. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr Opin Lipidol. 2004;15:167–174. doi: 10.1097/00041433-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Liu K, Guralnik JM, Ferrucci L, Green D, Greenland P, Tian L, Criqui MH, Lo C, Rifai N, Ridker PM, Zheng J, Pearce W. Functional decline in patients with and without peripheral arterial disease: predictive value of annual changes in levels of C-reactive protein and Ddimer. J Gerontol A Biol Sci Med Sci. 2006;61:374–379. doi: 10.1093/gerona/61.4.374. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc. 2004;52:405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. peripheral arterial disease is independently associated with impaired lower extremity functioning: the women's health and aging study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 17.Marz W, Siekmeier R, Kostner GM. Determination of lipoprotein(a): evaluation of three methods. Eur J Clin Chem Clin Biochem. 1993;31:295–301. doi: 10.1515/cclm.1993.31.5.295. [DOI] [PubMed] [Google Scholar]
- 18.Kaaks R, Riboli E. Validation and calibration of dietary intake measurements in the EPIC project: methodological considerations. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26:S15–S25. doi: 10.1093/ije/26.suppl_1.s15. [DOI] [PubMed] [Google Scholar]
- 19.Patel KV, Coppin AK, Manini TM, Lauretani F, Bandinelli S, Ferrucci L, Guralnik JM. Midlife physical activity and mobility in older age: The InCHIANTI Study. Am J Prev Med. 2006;31:217–224. doi: 10.1016/j.amepre.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LP, Kasper JD, Guralnik JM, Simonsick EM. Introduction. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women's Health and Aging Study: Health and social characteristics of older women with disability. National Institute on Aging; Bethesda, MD: 1995. pp. 1–8. (NIH Pub. No. 95-4009). [Google Scholar]
- 21.Krsukal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Statl Assoc. 1952;47:583–621. [Google Scholar]
- 22.McDermott MM, Guralnik JM, Corsi A, Albay M, Macchi C, Bandinelli S, Ferrucci L. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI Study. Am Heart J. 2005;150:276–281. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 23.O'Neal DN, Lewicki J, Ansari MZ, Matthews PG, Best JD. Lipid levels and peripheral vascular disease in diabetic and non-diabetic subjects. Atherosclerosis. 1998;136:1–8. doi: 10.1016/s0021-9150(97)00175-5. [DOI] [PubMed] [Google Scholar]
- 24.Khawaja FJ, Bailey KR, Turner ST, Kardia SL, Mosley TH, jr, Kullo IJ. Association of novel risk factors with the ankle brachial index in African American and non-Hispanic white populations. Mayo Clin Proc. 2007;82:709–716. doi: 10.4065/82.6.709. [DOI] [PubMed] [Google Scholar]
- 25.Tseng CH. Lipoprotein[a] is an independent risk factor for peripheral arterial disease in Chinese type 2 diabetic patients in Taiwan. Diabetes Care. 2004;27:517–521. doi: 10.2337/diacare.27.2.517. [DOI] [PubMed] [Google Scholar]
- 26.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117:823–831. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 27.Maeda S, Abe A, Seishima M, Makino K, Noma A, Kawade M. Transient changes of serum lipoprotein[a] as an acute phase protein. Atherosclerosis. 1989;78:145–150. doi: 10.1016/0021-9150(89)90218-9. [DOI] [PubMed] [Google Scholar]
- 28.Ramharack R, Barkalow D, Spahr MA. Dominant negative effect of TGF-beta1 and TNF-alpha on basal and IL-6-induced lipoprotein[a] and apolipoprotein[a] mRNA expression in primary monkey hepatocyte cultures. Arterioscler Thromb Vasc Biol. 1998;18:984–990. doi: 10.1161/01.atv.18.6.984. [DOI] [PubMed] [Google Scholar]
- 29.Carantoni M, Zuliani G, Bader G, Palmieri E, Volpato S, Passaro A, Imbastaro T, Mezzetti A, Fellin R. Low density lipoprotein cholesterol, lipoprotein[a], and apo[a] isoforms in the elderly: relationship to fasting insulin. Nutr Metab Cardiovasc Dis. 1999;9:228–233. [PubMed] [Google Scholar]