Abstract
CLC-5 is a H+/Cl− exchanger that is expressed primarily in endosomes but can traffic to the plasma membrane in overexpression systems. Mutations altering the expression or function of CLC-5 lead to Dent’s disease. Currents mediated by this transporter show extreme outward rectification and are inhibited by acidic extracellular pH. The mechanistic origins of both phenomena are currently not well understood. It has been proposed that rectification arises from the voltage dependence of a H+ transport step, and that inhibition of CLC-5 currents by low extracellular pH is a result of a reduction in the driving force for exchange caused by a pH gradient. We show here that the pH dependence of CLC-5 currents arises from H+ binding to a single site located halfway through the transmembrane electric field and driving the transport cycle in a less permissive direction, rather than a reduction in the driving force. We propose that protons bind to the extracellular gating glutamate E211 in CLC-5. It has been shown that CLC-5 becomes severely uncoupled when SCN− is the main charge carrier: H+ transport is drastically reduced while the rate of anion movement is increased. We found that in these conditions, rectification and pH dependence are unaltered. This implies that H+ translocation is not the main cause of rectification. We propose a simple transport cycle model that qualitatively accounts for these findings.
INTRODUCTION
Members of the CLC protein family are expressed in all phyla, from bacteria to mammals, and play a variety of physiological roles ranging from maintaining the membrane potential, regulating transepithelial salt transport, and controlling intravesicular pH (Jentsch, 2008). The centrality of their role in human physiology is underscored by four hereditary diseases caused by mutations in CLC genes: myotonia congenita, Dent’s disease, Bartter’s syndrome, and osteopetrosis (Jentsch, 2008).
One of the distinguishing characteristics of the CLC family is that it is split in two, equally populated subclasses: Cl−-selective ion channels or transporters that catalyze the stoichiometric exchange of one H+ for two anions, either Cl− or NO3− (Accardi and Miller, 2004; Picollo and Pusch, 2005; Scheel et al., 2005; De Angeli et al., 2006; Graves et al., 2008). The basic structural features are conserved between these two classes: all CLCs are dimers, where each monomer forms a Cl− permeation pathway that is defined by three anionic binding sites (Dutzler et al., 2002, 2003; Lobet and Dutzler, 2006). Solute exchange between the pathway and the extracellular solution is regulated by a highly conserved glutamate residue, Gluex, which simultaneously serves as the outside Cl− gate and extracellular H+ acceptor (Dutzler et al., 2003; Accardi and Miller, 2004; Picollo and Pusch, 2005; Scheel et al., 2005). Access to the intracellular side of the transporters’ Cl− pathway is regulated by a gate formed by conserved tyrosine and serine residues (Accardi and Miller, 2004; Accardi et al., 2006; Jayaram et al., 2008). In the channels, this gate is thought to be absent and thus Cl− transport is regulated only by Gluex. Intracellular protons bind to a second glutamate residue, Gluin, which is strictly conserved only in the transporter subclass (Accardi et al., 2005; Zdebik et al., 2008; Zifarelli et al., 2008) and replaced by non-protonatable residues like valine or leucine in channels. It is not known how protons move from Gluin to Gluex; several mechanisms have been proposed (Accardi et al., 2006; Kuang et al., 2007; Wang and Voth, 2009), but none proven.
CLC-5 is a CLC-type exchanger that is expressed in the kidneys and prevalently localizes to the membrane of endosomes, where it controls their acidification (Piwon et al., 2000; Hara-Chikuma et al., 2005; Jentsch, 2008). In overexpression systems, a significant fraction of CLC-5 traffics to the plasma membrane, making it amenable to direct electrophysiological scrutiny (Steinmeyer et al., 1995; Picollo and Pusch, 2005; Scheel et al., 2005). Despite this, our understanding of how CLC-5 facilitates endosomal acidification remains limited. It has been proposed that CLC-5 electrically shunts the activity of the endosomal V-type ATPases by importing two Cl− ions at the expense of one H+, dissipating ∼30% of the work done by the ATPases to acidify these compartments (Picollo and Pusch, 2005; Scheel et al., 2005). Furthermore, CLC-5’s activity is inhibited by lowering intravesicular pH (Friedrich et al., 1999; Picollo and Pusch, 2005), further reducing its shunting capability in the pH range, where it is most relevant. Lastly, CLC-5’s currents are extremely outwardly rectifying, nearly exclusively allowing Cl− efflux from and H+ influx into the endosome (Friedrich et al., 1999). Fluxes in the opposite direction are required to charge-neutralize intra-endosomal H+ accumulation. Thus, the biophysical properties of CLC-5 appear hard to reconcile with its proposed role as a shunt conductance for the V-type ATPases in endosomes. This conundrum is further exacerbated by our limited understanding of the mechanistic origin of the pH dependence and rectification of CLC-5. It has been suggested that the inhibition of CLC-5 by low pHex is due to a reduction in the driving force for H+/Cl− exchange rather than to an allosteric modulation of its activity by protons (Picollo and Pusch, 2005). It has been proposed that rectification in CLC-5 arises from a kinetic effect (Zdebik et al., 2008). In this model, a high energy barrier prevents H+ transport. High positive voltages facilitate H+ efflux by lowering the barrier experienced by intracellular H+ binding to Gluin. In contrast, the height of the barrier encountered by extracellular protons binding to Gluex is voltage independent or remains high at all voltages. So, H+ influx is kinetically unfavorable (Zdebik et al., 2008). However, a recent report has shown that H+ binding to Gluin is voltage independent (Zifarelli and Pusch, 2009), suggesting that the voltage dependence might arise from a different step.
The picture emerging from these findings is that both the extracellular pH dependence and the rectification of CLC-5 stem from its function as a H+-coupled Cl− exchanger. If this is the case, alterations in H+ coupling should be accompanied by changes in its pH dependence and rectification. We set out to test this hypothesis by studying in detail the pH dependence of CLC-5 currents in conditions where anion transport is stoichiometrically coupled (in Cl−) or severely uncoupled (in SCN−) to H+ movement. We found that, in both regimes, high concentrations of extracellular H+ reduce CLC-5 activity in a voltage-dependent manner: proton inhibition is partly relieved at high positive voltages and enhanced at lower voltages. The extent of inhibition is well described by a single-site binding curve. When H+ transport is drastically reduced by replacing extracellular Cl− with SCN− (Hebeisen et al., 2003; Zdebik et al., 2008; Alekov and Fahlke, 2009), rectification and pH dependence remain largely unaltered. These results suggest that CLC-5 is directly modulated by H+ex and that rectification does not arise from a H+ transport step. We propose a simplified transport model that qualitatively accounts for the basic transport properties of CLC-5.
MATERIALS AND METHODS
Molecular biology
hCLC-5 and the E211A mutant were cloned in pTLN vector. The E211A mutation was introduced with the Quickchange method (Agilent Technologies). The RNA was synthesized with the SP6 kit (Applied Biosystems), stored at −80°C, and injected in Xenopus oocytes, which were stored at 18°C. Currents were measured 3–4 d after injection.
Electrophysiology
An amplifier (OC-725C; Warner Instruments) was used for voltage clamp, and data were acquired using a custom acquisition program (GePulse). Currents were evoked by stepping the voltage from −140 to 110 mV in 10-mV increments for 100 ms. The extracellular recording solution was 100 mM NaCl, 5 mM MgCl2, and 10 mM buffer to the appropriate pH (glycine at pH 9, HEPES from pH 8.0 to 6.5, Mes at pH 6.0 and 5.5, and glutamate at pH 5.0 and 4.5). In the low Cl− experiments, 70 mM NaCl was replaced with 140 mM sucrose. In the SCN− experiments, 100 mM NaCl was replaced with 100 mM NaSCN. Recording electrodes were filled with 3 M KCl, and their resistance was 0.3–1 MΩ. Ag/AgCl electrodes and 3-M KCl agar bridges were used as a reference and bath electrodes for all experiments.
Access resistance
Given the large amplitude of the currents measured when using two-electrode voltage clamp, one possible source of error is the presence of a large access resistance. This could lead to an underestimation of the current amplitude at high voltages and an apparent voltage dependence of the inhibition. We controlled for this possibility in four independent ways. (1) In each experiment solution exchanges at the test pHs were alternated with washes in the control solution (at pH 7.5). If we recorded >10% change in the maximal current between two successive control washes, the experiment was terminated. This control serves two purposes. First, a complete recovery indicates that there is no rundown in the current, which would alter our estimates of inhibition. Second, it shows that the access resistance, if it plays a determinant role in our measurements, is constant during the experiment. (2) Experiments spanning the full pH range and experiments spanning only a partial range give indistinguishable results. (3) The current amplitude does not affect the estimate of the pK or of the electrical distance traveled by the H+ (Fig. S1). (4) If larger currents led to invariably higher estimates for zV, we would expect that the estimates in 100 mM SCN− should be systematically higher than in 100 mM Cl−, which in turn should be higher than the estimates in 30 mM Cl−, which is not the case (Figs. 1–3). These observations indicate that in our experimental conditions, access resistance plays a minor role in the evaluation of the apparent pK of inhibition by H+.
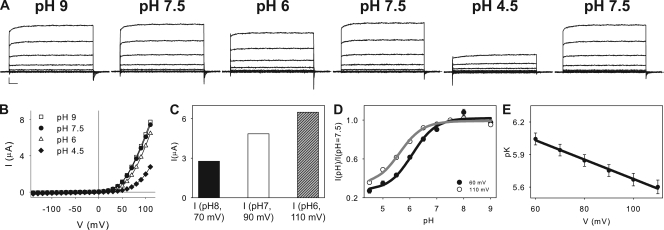
Figure 1.
Inhibition of CLC-5 currents by extracellular H+’s. (A) CLC-5 currents recorded from the same oocyte at various pHex. Scale bars represent 1 µA and 10 ms. (B) Current–voltage plots for the currents shown in A. Squares, pH 9; circles, pH 7.5; triangles, pH 6.0; diamonds, pH 4.5. Solid lines hold no theoretical meaning. (C) Bar representation of the currents recorded at various voltages and pHs: black bar, I(pH 8, +70 mV); white bar, I(pH 7, +90 mV); hatched gray bar, I(pH 6, +110 mV). (D) Plot of normalized currents at +60 (filled circles) and +110 (open circles) mV as a function of pH. Solid lines are fits to Eq. 3 as described in Results, with K = 8.1 × 10−7, Imax = 1.02 and Imin = 0.27 at +60 mV, and K = 2.4 × 10−6, Imax = 0.99 and Imin = 0.33 at +110 mV. (E) Plot of the mean values of the pK obtained as in D, versus voltage. Solid line is a fit to pK(V) = pK(0) + zFV/RT, where pK(V) is the pK at voltage V, pK(0) = 6.6 is the pK extrapolated at V = 0, z = 0.51 is the fraction of the membrane potential acting on the blocking ion, and F, R, and T have their usual meanings.
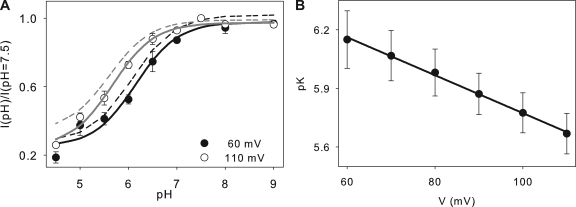
Figure 2.
Proton inhibition of CLC-5 currents in low extracellular Cl−. (A) Plot of normalized currents at +60 (filled circles) and +110 (open circles) mV as a function of pH. Solid lines are fits to Eq. 3 as described in Results, with K = 7.1 × 10−7, Imax = 0.98 and Imin = 0.25 at +60 mV, and K = 2.1 × 10−6, Imax = 0.97 and Imin = 0.24 at +110 mV. Dotted lines represent the fits at +110 (gray) and +60 (black) mV at 100 mM [Cl−]ex. (B) Plot of the mean values of the pK obtained as in A, versus voltage. Solid line is a fit to pK(V) = pK(0) + zFV/RT, where pK(V) is the pK at voltage V, pK(0) = 6.7 is the pK extrapolated at V = 0, z = 0.56 is the fraction of the membrane potential acting on the blocking ion, and F, R, and T have their usual meanings.
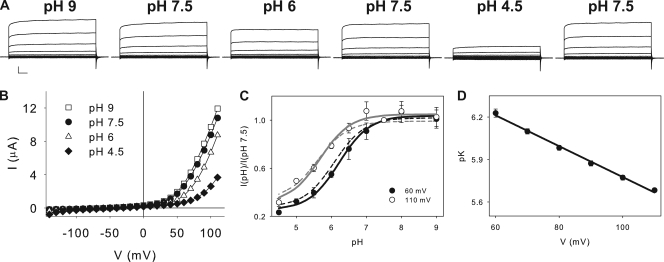
Figure 3.
Inhibition of CLC-5 currents in SCN−. (A) CLC-5 currents recorded from the same oocyte in various pHex. Scale bars represent 2 µA and 10 ms. (B) Current–voltage plots for the currents shown in A. Squares, pH 9; circles, pH 7.5; triangles, pH 6.0; diamonds, pH 4.5. Solid lines hold no theoretical meaning. (C) Plot of normalized currents at +60 (filled circles) and +110 (open circles) mV as a function of pH. Solid lines are fits to Eq. 3 as described in Results, with K = 6.0 × 10−7, Imax = 1.04 and Imin = 0.25 at +60 mV, and K = 2.1 × 10−6, Imax = 1.05 and Imin = 0.31 at +110 mV. Dotted lines represent the fits at +110 (gray) and +60 (black) mV at 100 mM [Cl−]ex. (D) Plot of the mean values of the pK obtained as in C, versus voltage. Solid line is a fit to pK(V) = pK(0) + zFV/RT, where pK(V) is the pK at voltage V, pK(0) = 6.9 is the pK extrapolated at V = 0, z = 0.63 is the fraction of the membrane potential acting on the blocking ion, and F, R, and T have their usual meanings.
Analysis
Electrophysiological data were analyzed using Ana software (M. Pusch; http://www.ge.cnr.it/ICB/conti_moran_pusch/programs-pusch/programs-mik.htm) and Sigmaplot (Systat Software).
Online supplemental material
Fig. S1 shows that the current amplitude does not affect the estimate of the pK or of the electrical distance traveled by protons. This strongly argues that access resistance does not measurably affect our conclusions. Fig. S1 is available at http://www.jgp.org/cgi/content/full/jgp.201010428/DC1.
RESULTS
Extracellular pH dependence of CLC-5
We tested the effects of a wide range of extracellular pHs on the currents mediated by CLC-5. In agreement with previous reports (Friedrich et al., 1999; Picollo and Pusch, 2005), we found that CLC-5 is inhibited by extracellular acidification (Fig. 1). When pHex is reduced from 7.5 to 6, the currents are reduced by ∼20%, and further lowering pHex to 4.5 leads to ∼65% inhibition (Fig. 1, A and B). The total electromotive force driving H+/Cl− exchange by CLC-5 is the sum of three components
| (1) |
where V(amp) is the voltage applied through the amplifier, V(ΔpH) and V(ΔCl) are, respectively, the voltage due to a pH and Cl gradient, which for a two Cl−/one H+ exchanger are
| (2a) |
| (2b) |
where r is the stoichiometric exchange ratio, and EH and ECl are the ideal Nernst potentials for H+ and Cl−, respectively. Thus, if the inhibition reflects a reduction in Vtot, an ∼20-mV increase in the applied voltage should compensate 1 U of pH gradient. In other words, the currents at pH 8.0 and +70 mV should be equal to those measured at pH 7.0 and +90 mV, or at pH 6.0 and +110 mV. This is not the case (Fig. 1 C). Furthermore, increasing pHex from 7.5 to 9 has no effect on the currents at all voltages, whereas the H+ electromotive force is increased by ∼30 mV (Fig. 1, A and B). These results suggest that a reduction in the driving force is not the sole reason for the inhibition of CLC-5 by pHex and raise the possibility that protons, in addition to being a catalytic substrate for transport, also act as regulators of activity. To quantify the pH dependence of this regulation, we plotted the normalized current, Inorm = I(pH)/I(pH = 7.5), as a function of pH (Fig. 1 D). For voltages between +60 and +110 mV, Inorm is well described by a simple 1:1 binding curve of the form
| (3) |
where [H]ex is the free extracellular proton concentration, K is the apparent binding constant (pK = −logK), and Imax and Imin are the maximal and minimal values, respectively, of the normalized currents recorded at any given voltage. H+ inhibition appears to be voltage dependent, it is less pronounced at +110 mV (Fig. 1 D, open circles) than at +60 mV (filled circles), and the apparent pK of inhibition varies almost linearly from ∼6.1 at +60 mV to ∼5.6 at +110 mV (Fig. 1 E). Because inhibition of CLC-5 currents can be fit by a single-site binding model and the apparent pK of inhibition depends linearly on the applied voltage, we used the Woodhull formalism (Woodhull, 1973) to estimate that H+’s travel through ∼51% of the transmembrane electric field to reach their inhibitory binding site. Recent reports have shown that extracellular protons travel through similar portions of the electric field when they bind to Gluex in the CLC-0 and CLC-2 channels (Engh et al., 2007; Niemeyer et al., 2009). Consistent with this hypothesis, charge-neutralizing mutations at Gluex abolish H+ transport and pH dependence in CLC-5 and other CLC-type transporters (Picollo and Pusch, 2005; Scheel et al., 2005) not depicted.
pH Dependence of CLC-5 in low extracellular Cl−
To test whether Gluex, E211 in CLC-5, is a viable candidate H+ acceptor site, we decided to investigate the effects of lowering [Cl−]ex on the pH dependence of CLC-5 currents. Structural and functional work has shown that Cl− ions and the Gluex side chain compete for occupancy of the extracellular binding site, and that protonation of the latter promotes Cl− occupancy (Chen and Chen, 2001; Dutzler et al., 2003; Accardi and Miller, 2004). Thus, if E211 is the inhibitory binding site for extracellular H+, then lowering [Cl−]ex should potentiate the inhibitory effect. This is indeed the case (Fig. 2). When we reduce [Cl−]ex from 100 to 30 mM, there is a small right shift in the pK of inhibition, ∼0.2 pH units at all voltages (Fig. 2, A and B), indicating that an approximate threefold reduction in [Cl−]ex promotes H+ inhibition. The site of inhibition appears to be the same. H+ traverse ∼56% of the transmembrane electric field to reach their single binding site (Fig. 2 C). We could not test whether a further reduction in [Cl−]ex would lead to larger shifts because the currents become too small to be reliably measured, especially at low pH and low voltages.
Although consistent with our hypothesis, these results do not demonstrate that Gluex is the H+ acceptor site in CLC-5. Protons may bind at a different residue that influences the extracellular gate through a voltage-dependent allosteric step, which could give rise to the apparent voltage dependence of inhibition. In this case, Gluex mutations could just remove the actuator of the gating process and mask the true site of inhibition.
Impairing H+ transport does not affect the pH dependence of CLC-5
Our data suggest that protons directly modulate CLC-5 activity by binding at a single site located ∼50% into the transmembrane electric field, possibly E211. To determine whether a H+ gradient opposing H+ efflux affects the transport rate of CLC-5, we investigated the effects of pHex on CLC-5 currents when extracellular Cl− is replaced by SCN−, a maneuver that drastically reduces H+ transport (Nguitragool and Miller, 2006; Zdebik et al., 2008; Alekov and Fahlke, 2009). This allows us to dissect the effects of proton binding to CLC-5 from those due to a reduction in the driving force for transport. We found that the modulation of CLC-5 currents by pHex is nearly identical in coupled and uncoupled conditions. Lowering pHex to 4.5 leads to an ∼70% current reduction, whereas increasing it to 9 leaves the currents largely unchanged (Fig. 3, A–C). Inhibition is well described by a 1:1 binding curve, with pKs ranging from ∼6.2 at +60 mV to ∼5.7 at +110 mV (Fig. 3 D), and protons traveling through ∼63% of the transmembrane electric field to get to their binding site. Overall, these results show that protons inhibit CLC-5 in a very similar manner, regardless of whether they are the substrate of a tightly coupled or a severely uncoupled exchanger. This implies that the H+ electromotive force plays only a minor role in determining the shape and pH dependence of the current–voltage relationship of CLC-5. Thus, transport is likely to be rate limited by other voltage-dependent steps, such as conformational changes in the protein and/or Cl− binding and translocation. Lastly, it is worth noting that despite an ∼2.5-fold increase in the current magnitude at positive potentials, outward rectification is preserved in SCN− (Fig. 3, A and B). This implies that rectification does not arise from H+ transport, but rather that it arises from another step, possibly a voltage-dependent conformational change in the protein or from Cl− binding and translocation.
DISCUSSION
We set out to investigate the inhibition of CLC-5 currents on extracellular H+ concentration, and we found that protons seem to bind to a single site located halfway through the transmembrane electric field, and that neither rectification nor the pH dependence of CLC-5’s currents depends on H+ transport. Lowering [Cl−]ex potentiates the inhibitory effect of extracellular protons, suggesting that, in analogy to the recent results on CLC-0 and CLC-2, protons bind to Gluex (E211).
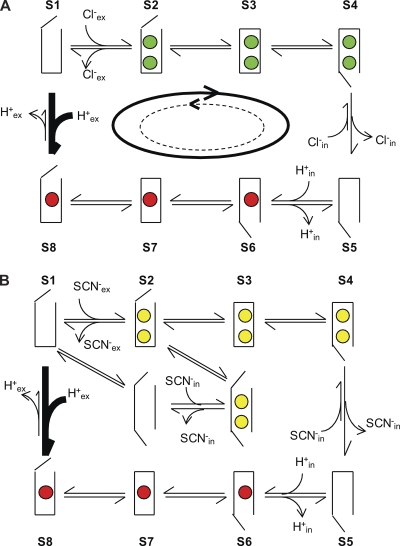
Currently, there are no working models to quantitatively describe CLC function, and the proposed qualitative models are incomplete and in need of refinement (Miller and Nguitragool, 2009). For these reasons, we use a simple alternating access transport model to describe a two Cl−/one H+ exchanger (Fig. 4 A). Although the CLC’s exchange cycle will likely be more complex, this model captures the essential features of an exchanger. The transporter can exist in three basic states, outwardly or inwardly open or occluded, and alternatively binds Cl− ions (green spheres) or H+ (red spheres). CLC-5’s outward rectification implies that at positive voltages, the transporter preferentially cycles clockwise (solid arrow), and that at least one of the rates in the counterclockwise cycle (dashed arrow) is small and rate limiting. It is worth noting that at equilibrium (0 applied voltage and symmetrical solutions) transport must happen at the same rate in both directions. Thus, the asymmetry seen in the transport cycle at positive potentials arises from the steep voltage dependence of one or more rates. Inhibition of CLC-5 by extracellular H+’s is easily accounted for by this simplified scheme: an increase in [H+]ex drives the transporter counterclockwise (from S1 to S8) in the less permissive direction and eventually into a state with a low forward escape rate. Thus, most of the time, the transporter will undergo only a partial cycle and will have to backtrack through the states in reverse order. This leads to a reduction in the net transport rate. Lowering [Cl−]ex reduces the rate from S1 to S2, favoring counterclockwise transport, which leads to a right shift in the apparent pK of inhibition for extracellular H+. Conversely, increasing [H+]in drives transport in the permissive direction and enhances transport, as has been recently reported (Zifarelli and Pusch, 2009).
Figure 4.
Simplified transport model for CLC-5. (A) Coupled transport in Cl−. (B) Uncoupled transport mode in SCN−. Green circles, Cl− ions; red circles, H+; yellow circles, SCN− ions. The model is described in the Discussion.
The mechanism of H+ uncoupling of CLC transporters by extracellular SCN− is not understood. Although SCN− appears to completely abolish H+ transport by CLC-ec1 (Nguitragool and Miller, 2006), some residual H+ transport could be detected in CLC-4 and CLC-5 (Zdebik et al., 2008; Alekov and Fahlke, 2009). In the framework of our model, uncoupling by SCN− (Fig. 4 B, yellow spheres) can be achieved by shunting the upper half of the cycle back to S1, either through a channel-like intermediate state with both gates open (Fig. 4 B) or through an occluded empty state. In this model, the extent of uncoupling is dictated by the relative weight of the rate constants connecting the normal and “shunted” states. Thus, the diverse uncoupling observed between CLC-ec1 and CLC-4/-5 in SCN− could reflect differences in their rates rather than a mechanistic divergence. The persistence of the outward rectification in SCN− implies that this phenomenon does not arise from one of the H+-coupled steps, but rather from one of the anion translocation steps or from a conformational change occurring in the protein (states S1 through S4). Our simplified model also predicts that the inhibition of CLC-5 currents by extracellular H+ should remain similar in SCN− and Cl−, as the binding of extracellular H+’s to the transporter will still drive the transport cycle into the less permissive direction.
We found that extracellular protons are both substrate for and voltage-dependent inhibitors of transport mediated by CLC-5. This surprising result is highly reminiscent of the voltage-dependent inhibition of the Na-K ATPase by extracellular Na+ (Gadsby and Nakao, 1989; Nakao and Gadsby, 1989). This reminiscence and the ability of a simple alternating access model to account for many of the properties of CLC-5–mediated transport (Fig. 4) suggest that, despite the many unique characteristics of CLC-mediated exchange (Accardi et al., 2005), several similarities with classical transport mechanisms exist, and that CLCs might not be as fundamentally different from canonical transporters as previously expected.
Acknowledgments
The authors wish to thank Olaf Andersen, Crina Nimigean, and John Lueck for helpful discussion and comments on the manuscript.
This work was supported by National Institutes of Health (grant GM-085232-01 to A. Accardi).
Christopher Miller served as editor.
References
- Accardi A., Miller C. 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 427:803–807 10.1038/nature02314 [DOI] [PubMed] [Google Scholar]
- Accardi A., Walden M., Nguitragool W., Jayaram H., Williams C., Miller C. 2005. Separate ion pathways in a Cl−/H+ exchanger. J. Gen. Physiol. 126:563–570 10.1085/jgp.200509417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accardi A., Lobet S., Williams C., Miller C., Dutzler R. 2006. Synergism between halide binding and proton transport in a CLC-type exchanger. J. Mol. Biol. 362:691–699 10.1016/j.jmb.2006.07.081 [DOI] [PubMed] [Google Scholar]
- Alekov A.K., Fahlke C. 2009. Channel-like slippage modes in the human anion/proton exchanger ClC-4. J. Gen. Physiol. 133:485–496 10.1085/jgp.200810155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.F., Chen T.Y. 2001. Different fast-gate regulation by external Cl− and H+ of the muscle-type ClC chloride channels. J. Gen. Physiol. 118:23–32 10.1085/jgp.118.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angeli A., Monachello D., Ephritikhine G., Frachisse J.M., Thomine S., Gambale F., Barbier-Brygoo H. 2006. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 442:939–942 10.1038/nature05013 [DOI] [PubMed] [Google Scholar]
- Dutzler R., Campbell E.B., Cadene M., Chait B.T., MacKinnon R. 2002. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 415:287–294 10.1038/415287a [DOI] [PubMed] [Google Scholar]
- Dutzler R., Campbell E.B., MacKinnon R. 2003. Gating the selectivity filter in ClC chloride channels. Science. 300:108–112 10.1126/science.1082708 [DOI] [PubMed] [Google Scholar]
- Engh A.M., Faraldo-Gómez J.D., Maduke M. 2007. The mechanism of fast-gate opening in ClC-0. J. Gen. Physiol. 130:335–349 10.1085/jgp.200709759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T., Breiderhoff T., Jentsch T.J. 1999. Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J. Biol. Chem. 274:896–902 10.1074/jbc.274.2.896 [DOI] [PubMed] [Google Scholar]
- Gadsby D.C., Nakao M. 1989. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J. Gen. Physiol. 94:511–537 10.1085/jgp.94.3.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves A.R., Curran P.K., Smith C.L., Mindell J.A. 2008. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 453:788–792 10.1038/nature06907 [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M., Wang Y., Guggino S.E., Guggino W.B., Verkman A.S. 2005. Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem. Biophys. Res. Commun. 329:941–946 10.1016/j.bbrc.2005.02.060 [DOI] [PubMed] [Google Scholar]
- Hebeisen S., Heidtmann H., Cosmelli D., Gonzalez C., Poser B., Latorre R., Alvarez O., Fahlke C. 2003. Anion permeation in human ClC-4 channels. Biophys. J. 84:2306–2318 10.1016/S0006-3495(03)75036-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H., Accardi A., Wu F., Williams C., Miller C. 2008. Ion permeation through a Cl-selective channel designed from a CLC Cl−/H+ exchanger. Proc. Natl. Acad. Sci. USA. 105:11194–11199 10.1073/pnas.0804503105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T.J. 2008. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 43:3–36 10.1080/10409230701829110 [DOI] [PubMed] [Google Scholar]
- Kuang Z., Mahankali U., Beck T.L. 2007. Proton pathways and H+/Cl− stoichiometry in bacterial chloride transporters. Proteins. 68:26–33 10.1002/prot.21441 [DOI] [PubMed] [Google Scholar]
- Lobet S., Dutzler R. 2006. Ion-binding properties of the ClC chloride selectivity filter. EMBO J. 25:24–33 10.1038/sj.emboj.7600909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Nguitragool W. 2009. A provisional transport mechanism for a chloride channel-type Cl−/H+ exchanger. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364:175–180 10.1098/rstb.2008.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Gadsby D.C. 1989. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J. Gen. Physiol. 94:539–565 10.1085/jgp.94.3.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguitragool W., Miller C. 2006. Uncoupling of a CLC Cl−/H+ exchange transporter by polyatomic anions. J. Mol. Biol. 362:682–690 10.1016/j.jmb.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Niemeyer M.I., Cid L.P., Yusef Y.R., Briones R., Sepúlveda F.V. 2009. Voltage-dependent and -independent titration of specific residues accounts for complex gating of a ClC chloride channel by extracellular protons. J. Physiol. 587:1387–1400 10.1113/jphysiol.2008.167353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picollo A., Pusch M. 2005. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 436:420–423 10.1038/nature03720 [DOI] [PubMed] [Google Scholar]
- Piwon N., Günther W., Schwake M., Bösl M.R., Jentsch T.J. 2000. ClC-5 Cl−-channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature. 408:369–373 10.1038/35042597 [DOI] [PubMed] [Google Scholar]
- Scheel O., Zdebik A.A., Lourdel S., Jentsch T.J. 2005. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 436:424–427 10.1038/nature03860 [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Schwappach B., Bens M., Vandewalle A., Jentsch T.J. 1995. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J. Biol. Chem. 270:31172–31177 10.1074/jbc.270.52.31172 [DOI] [PubMed] [Google Scholar]
- Wang D., Voth G.A. 2009. Proton transport pathway in the ClC Cl−/H+ antiporter. Biophys. J. 97:121–131 10.1016/j.bpj.2009.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A.M. 1973. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 61:687–708 10.1085/jgp.61.6.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdebik A.A., Zifarelli G., Bergsdorf E.Y., Soliani P., Scheel O., Jentsch T.J., Pusch M. 2008. Determinants of anion-proton coupling in mammalian endosomal CLC proteins. J. Biol. Chem. 283:4219–4227 10.1074/jbc.M708368200 [DOI] [PubMed] [Google Scholar]
- Zifarelli G., Pusch M. 2009. Conversion of the 2 Cl(−)/1 H+ antiporter ClC-5 in a NO3(−)/H+ antiporter by a single point mutation. EMBO J. 28:175–182 10.1038/emboj.2008.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifarelli G., Murgia A.R., Soliani P., Pusch M. 2008. Intracellular proton regulation of ClC-0. J. Gen. Physiol. 132:185–198 10.1085/jgp.200809999 [DOI] [PMC free article] [PubMed] [Google Scholar]