Abstract
Alterations in the inhibitory circuitry of the dorsolateral prefrontal cortex (DLPFC) in schizophrenia include reduced expression of the messenger RNA (mRNA) for somatostatin (SST), a neuropeptide present in a subpopulation of γ-aminobutyric acid (GABA) neurons. However, neither the cellular substrate nor the causal mechanisms for decreased SST mRNA levels in schizophrenia are known. We used in situ hybridization to quantify the compartmental, laminar, and cellular levels of SST mRNA expression in the DLPFC of 23 pairs of schizophrenia or schizoaffective disorder and control subjects. We also explored potential causal mechanisms by utilizing similar methods to analyze SST mRNA expression in 2 animal models. The expression of SST mRNA was significantly decreased in layers 2–superficial 6 of subjects with schizophrenia, but not in layer 1, deep 6 or the white matter. At the cellular level, both the density of cortical SST mRNA-positive neurons and the expression of SST mRNA per neuron were reduced in the subjects with schizophrenia. These alterations were not due to potential confounds and appeared to be a downstream consequence of impaired neurotrophin signaling through the trkB receptor. These findings support the hypothesis that a marked reduction in SST mRNA expression in a subset of GABA neurons contributes to DLPFC dysfunction in schizophrenia.
Keywords: BDNF/TrkB, GABA, human, interneurons, NPY
Introduction
Alterations in the inhibitory circuitry of the dorsolateral prefrontal cortex (DLPFC) appear to be a common feature of schizophrenia (Torrey et al. 2005; Akbarian and Huang 2006). For example, reduced levels of the messenger RNA (mRNA) that encodes for the 67-kDa isoform of glutamic acid decarboxylase (GAD67), an enzyme for γ-aminobutyric acid (GABA) synthesis, have been consistently found in the DLPFC of individuals with schizophrenia (Akbarian et al. 1995; Guidotti et al. 2000; Mirnics et al. 2000; Volk et al. 2000; Hashimoto et al. 2005; Straub et al. 2007). Furthermore, this decrease is due to a marked reduction in GAD67 mRNA expression in a minority (~25–35%) of GABA neurons, with apparently normal levels of expression in the remaining neurons (Volk et al. 2000). The affected neurons include the ~25% of GABA neurons that express the calcium-binding protein parvalbumin (PV), whereas the ~50% of GABA neurons that express calretinin (CR) appear to be unaffected (Hashimoto et al. 2003). However, the deficits in PV expression are restricted to layers 3 and 4, whereas the alterations in GAD67 mRNA are found in layers 2–5; these laminar differences suggest that an additional subset of GABA neurons is affected in schizophrenia.
In a recent study utilizing a custom-designed microarray of GABA-related transcripts, the most robust expression difference in the DLPFC of subjects with schizophrenia was a reduction in the levels of somatostatin (SST) mRNA (Hashimoto et al. 2007), which is expressed in a subpopulation of GABA neurons that do not contain either PV or CR (Kubota et al. 1994; González-Albo et al. 2001; Gonchar and Burkhalter 2003; Sugino et al. 2006). The reduction in SST mRNA expression in schizophrenia was confirmed by both real-time qPCR and in situ hybridization in the same subjects (Hashimoto et al. 2007). However, neither the cellular substrate nor the causal mechanisms for decreased SST mRNA expression in schizophrenia have been explored.
SST neurons are present in all layers of the cortex as well as in the underlying white matter. SST neurons in layer 1, deep layer 6, and the white matter are generated early in development and represent residual neurons from the embryonic preplate, whereas those generated later during the development of the cortical plate reside in layers 2–superficial 6 (Kostovic and Rakic 1980; Chun and Shatz 1989b; Bayer and Altman 1990; for review see Allendoerfer and Shatz 1994). Within the gray matter of the adult monkey and human DLPFC, the greatest densities of SST neurons are found in layers 2–superficial 3 and layer 5 (Lewis et al. 1986; Hayes et al. 1991; Da Cunha et al. 1995). In addition, subsets of SST neurons with different membrane properties and morphological features tend to differ in their laminar locations (Kawaguchi and Kubota 1996; Ma et al. 2006). Thus, it is important to determine if the expression deficit in SST mRNA is 1) restricted to the gray matter compartment, 2) pronounced in certain cortical layers, and 3) confined to a subset of SST neurons.
Consequently, in this study we used in situ hybridization and autoradiographic analyses to quantify the compartmental, laminar, and cellular levels of SST mRNA expression in DLPFC area 9 of 23 matched pairs of schizophrenia and control subjects. In addition, we explored potential causal mechanisms of decreased SST mRNA expression in schizophrenia by utilizing similar methods to analyze SST mRNA expression in 2 animal models.
Materials and Methods
Human Subjects
With the consent of the surviving next-of-kin, brain tissue specimens were obtained from the Allegheny County Medical Examiner’s Office at the time of routine autopsy. Twenty-three subjects with schizophrenia (Table 1) were each matched with 1 control subject for sex, and as closely as possible for age, and postmortem interval (PMI). Subject groups did not differ in mean age, PMI, brain pH, RNA integrity number (RIN; as determined from the Agilent Bioanalyzer 2100) or tissue storage time at −80 °C (for all t(22) < 1.84; P > 0.08).
Table 1.
Characteristics of subjects
| Control subjects |
Schizophrenia subjects |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pair | Case | Sex/race | Age | PMIa | RIN | Storage timeb | Cause of deathc | Case | DSM IV diagnosis | Sex/race | Age | PMIa | RIN | Storage timeb | Cause of deathc | Antipsychotic medicationd |
| 1 | 592 | M/B | 41 | 22.1 | 9.0 | 100 | ASCVD | 533 | Chronic undifferentiated schizophrenia | M/W | 40 | 29.1 | 8.4 | 110 | Accidental asphyxiation | Typical |
| 2 | 567 | F/W | 46 | 15.0 | 8.9 | 104 | Mitral valve prolapse | 537 | Schizoaffective disorder | F/W | 37 | 14.5 | 8.6 | 109 | Suicide by hanging | None |
| 3 | 516 | M/B | 20 | 14.0 | 8.4 | 111 | Homicide by gun shot | 547 | Schizoaffective disorder | M/B | 27 | 16.5 | 7.4 | 107 | Heat Stroke | Typical |
| 4 | 630 | M/W | 65 | 21.2 | 9.0 | 94 | ASCVD | 566 | Chronic undifferentiated schizophreniae | M/W | 63 | 18.3 | 8.0 | 104 | ASCVD | Atypical |
| 5 | 604 | M/W | 39 | 19.3 | 8.6 | 97 | Hypoplastic coronary artery | 581 | Chronic paranoid schizophreniaf,g | M/W | 46 | 28.1 | 7.9 | 102 | Accidental combined drug overdose | Typical |
| 6 | 546 | F/W | 37 | 23.5 | 8.6 | 108 | ASCVD | 587 | Chronic undifferentiated schizophreniae | F/B | 38 | 17.8 | 9.0 | 101 | Myocardial hypertrophy | Both |
| 7 | 551 | M/W | 61 | 16.4 | 8.3 | 107 | Cardiac tamponade | 625 | Chronic disorganized schizophreniah | M/B | 49 | 23.5 | 7.6 | 95 | ASCVD | Typical |
| 8 | 685 | M/W | 56 | 14.5 | 8.1 | 87 | Hypoplastic coronary artery | 622 | Chronic undifferentiated schizophrenia | M/W | 58 | 18.9 | 7.4 | 95 | Right middle cerebral artery infarction | None |
| 9 | 681 | M/W | 51 | 11.6 | 8.9 | 87 | Hypertrophic cardio-myopathy | 640 | Chronic paranoid schizophrenia | M/W | 49 | 5.2 | 8.4 | 93 | Pulmonary embolism | Atypical |
| 10 | 806 | M/W | 57 | 24.0 | 7.8 | 66 | Pulmonary thromboembolism | 665 | Chronic paranoid schizophreniaf | M/B | 59 | 28.1 | 9.2 | 90 | Intestinal hemorrhage | Typical |
| 11 | 822 | M/B | 28 | 25.3 | 8.5 | 63 | ASCVD | 787 | Schizoaffective disorderi | M/B | 27 | 19.2 | 8.4 | 70 | Suicide by gun shot | Typical |
| 12 | 727 | M/B | 19 | 7.0 | 9.2 | 80 | Trauma | 829 | Schizoaffective disorderf,j | M/W | 25 | 5.0 | 9.3 | 61 | Suicide by drug overdose | None |
| 13 | 871 | M/W | 28 | 16.5 | 8.5 | 53 | Trauma | 878 | Disorganized schizophreniaf | M/W | 33 | 10.8 | 8.9 | 52 | Myocardial fibrosis | Atypical |
| 14 | 575 | F/B | 55 | 11.3 | 9.6 | 102 | ASCVD | 517 | Chronic disorganized schizophreniaf | F/W | 48 | 3.7 | 9.3 | 111 | Intracerebral hemorrhage | Atypical |
| 15 | 700 | M/W | 42 | 26.1 | 8.7 | 84 | ASCVD | 539 | Schizoaffective disorderk | M/W | 50 | 40.5 | 8.1 | 109 | Suicide by combined drug overdose | Atypical |
| 16 | 988 | M/W | 82 | 22.5 | 8.4 | 31 | Trauma | 621 | Chronic undifferentiated schizophrenia | M/W | 83 | 16.0 | 8.7 | 95 | Accidental asphyxiation | None |
| 17 | 686 | F/W | 52 | 22.6 | 8.5 | 87 | ASCVD | 656 | Schizoaffective disorderf | F/B | 47 | 20.1 | 9.2 | 91 | Suicide by gun shot | Atypical |
| 18 | 634 | M/W | 52 | 16.2 | 8.5 | 93 | ASCVD | 722 | Undifferentiated schizophreniajl | M/B | 45 | 9.1 | 9.2 | 81 | Upper gastrointestinal bleeding | Typical |
| 19 | 852 | M/W | 54 | 8.0 | 9.1 | 56 | Cardiac tamponade | 781 | Schizoaffective disorderk | M/B | 52 | 8.0 | 7.7 | 71 | Peritonitis | Typical |
| 20 | 987m | F/W | 65 | 21.5 | 9.1 | 31 | ASCVD | 802 | Schizoaffective disorderfl | F/W | 63 | 29.0 | 9.2 | 67 | Right ventricular dysplasia | Both |
| 21 | 818 | F/W | 67 | 24.0 | 8.4 | 64 | Anaphylactic reaction | 917 | Chronic undifferentiated schizophrenia | F/W | 71 | 23.8 | 7.0 | 44 | ASCVD | Typical |
| 22 | 857 | M/W | 48 | 16.6 | 8.9 | 55 | ASCVD | 930 | Disorganized schizophreniakj | M/W | 47 | 15.3 | 8.2 | 41 | ASCVD | Typical |
| 23 | 739 | M/W | 40 | 15.8 | 8.4 | 79 | ASCVD | 933 | Disorganized schizophrenia | M/W | 44 | 8.3 | 8.1 | 40 | Myocarditis | Atypical |
| Mean | 48.0 | 18.0 | 8.7 | 80.0 | 47.9 | 17.8 | 8.4 | 84.3 | ||||||||
| SD | 15.5 | 5.5 | 0.4 | 23.6 | 14.1 | 9.3 | 0.7 | 23.7 | ||||||||
PMI indicates postmortem interval in hours.
Storage time (months) at −80 °C.
ASCVD indicates arteriosclerotic cardiovascular disease.
Indicates prescribed antipsychotic medications at time of death.
Alcohol abuse, in remission at time of death.
Alcohol dependence, current at time of death.
Other substance abuse, current at time of death.
Alcohol abuse, current at time of death.
Other substance dependence, current at time of death.
Other substance abuse, in remission at time of death.
Alcohol dependence, in remission at time of death.
Other substance dependence, in remission at time of death.
History of posttraumatic stress disorder, in remission 39 years at time of death.
An independent committee of experienced research clinicians made consensus DSMIV (American Psychiatric Association, 1994) diagnoses based on medical records and structured interviews conducted with family members of the deceased. One control subject (987) had a history of posttraumatic stress disorder that had been in remission for 39 years at the time of death. For the subjects with schizophrenia, the mean (SD) age of illness onset was 25.2 (8.0) years and the mean duration of illness was 23.3 (13.3) years. We define the age of onset as the 1st episode of psychotic symptoms, as indicated by the available information from medical records and the subject’s family. Fifteen subjects with schizophrenia had a history of substance (including alcohol) abuse and/or dependence disorder, although only 8 met criteria for dependence at time of death. Four subjects with schizophrenia (537, 622, 621, and 829) were free of antipsychotic medications at time of death for 9.6 months, 1.2 months, 8.2 years, and unknown length of time, respectively. Toxicology of all subjects detected positive plasma alcohol levels (0.01% and 0.06%) in 2 control subjects (516 and 685) and 1 subject with schizophrenia (0.09%; 656). All procedures were approved by the University of Pittsburgh’s Institutional Review Board for Biomedical Research and Committee for Oversight of Research Involving the Dead.
Tissue Preparation
The right frontal cortex from each brain was blocked coronally, immediately frozen, and stored at −80 °C. Serial sections with a thickness of 20 µm containing the superior frontal gyrus were cut, thaw-mounted onto the middle portion of glass slides, 1 section per slide, and stored at −80 °C until processed. The location of DLPFC area 9 was identified by cytoarchitectonic criteria in Nissl-stained sections as previously described (Glantz et al. 2000; Volk et al. 2000). Three sections per subject, at intervals of approximately 300 µm, were matched for anterior-posterior location within subject pairs, and used to assess SST mRNA expression.
In Situ Hybridization
Templates for the synthesis of the antisense and sense riboprobes for human and mouse SST mRNA were 1st generated by polymerase chain reaction (PCR). The specific primers amplified a 337 and 439 base pair (bp) fragment of human and mouse SST, respectively. These fragments corresponded to bases 112–448 of the human (GenBank NM_001048) and 89–527 of the mouse (GenBank NM_009215) SST gene. Nucleotide sequencing confirmed 100% homology of the amplified fragment to the previously reported sequence. The fragment was then subcloned into a plasmid (pSTBlue-1, Novagen, Madison, WI). The antisense and sense riboprobes were transcribed in the presence of 35S-CTP (Amersham Biosciences, Piscataway, NJ) using T7 and SP6 RNA polymerase, respectively. DNase I was used to digest the DNA template. The riboprobes were purified using RNeasy mini spin columns (Qiagen, Valencia, CA). One section from each pair was processed during a single run with the sections from each pair processed side by side, and with the location of the slides in the hybridization container counterbalanced between diagnostic groups during each run.
Prior to the hybridization reaction, tissue sections were fixed with 4% paraformaldehyde in phosphate-buffered saline solution, acetylated with 0.25% acetic anhydrate in 0.1 M triethanolamine/0.9% NaCl for 10 min, dehydrated with a graded alcohol series, and then defatted in chloroform for 10 min. The sections were then hybridized with 35S-labeledriboprobes(1.0 × 106 cpm/µL) in hybridization buffer at 56 °C for 16 h. The hybridization buffer contained 50% formamide, 0.75 M NaCl, 20 mM 1,4-piperazine diethane sulfonic acid, pH 6.8, 10 mM ethylenediaminetetraacetic acid (EDTA), 10% dextran sulfate, 5× Denhardt’s solution (0.2 mg/mL Ficoll, 0.2 mg/mL polyvinylpyrrolidone, 0.2 mg/mL bovine serum albumin), 50 mM dithiothreitol, 0.2% sodium dodecyl sulfate, and 100 µg/mL yeast transfer RNA. Following the hybridization reaction, sections were washed in a solution of 0.3 M NaCl, 20 mM Tris–HCl, pH 8.0, 1 mM EDTA, pH 8.0, and 50% formamide at 63 °C, treated with RNase A (20 µg/mL) at 37 °C, washed in 0.1 × SSC (1.5 mM NaCl, 150 µM sodium citrate) at 67 °C, dehydrated through a graded ethanol series, and air dried. The sections, as well as carbon-14 radioactive standards, were exposed on the same BioMax MR film (Kodak, Rochester, NY) for 3 days. Afterward, sections were coated with NTB2 emulsion (Kodak) diluted 2:1 with water. The consistency of the thickness of the emulsion was maintained with use of a mechanical dipper (Auto-dip Emulsion Coater, Ted Pella, Redding, CA) at a constant withdrawal speed (64 mm/min) and temperature (43 °C). Utilizing DLPFC sections from control subjects, different emulsion exposure times were systematically evaluated in order to achieve an optimal signal to noise ratio. The emulsion was exposed for 18 days at a constant temperature of 4 °C The slides were developed with D-19 (Kodak) and counterstained with Cresyl violet.
Quantification of mRNA Expression Levels
Each section was randomly coded, so that subject number and diagnosis were unknown to the single rater (H.M.M.). Autoradiographic films were trans-illuminated and captured on video camera under controlled conditions, digitized, and analyzed with a Microcomputer Imaging Device (MCID; Imaging Research, Inc., London, Ontario, Canada). Digitized images of adjacent sections stained with cresyl violet were superimposed onto autoradiographic images to draw contours of the full cortical thickness of the zones of area 9 that were cut perpendicular to the pial surface. Optical density (OD) measures within each sampled area were calibrated to radioactive carbon-14 standards (ARC, Inc., St Louis, MO), exposed on the same autoradiographic film, and expressed as nanocuries per gram (nCi/g) of tissue. The mean (SD) total area of gray matter sampled in each subject was 381 (141) mm2 for control subjects and 353 (101) mm2 for subjects with schizophrenia. OD measures in the superficial white matter were determined in a zone 800 µm below, and with a contour that followed, the layer 6/white matter border of the previously sampled gray matter zones. The mean total areas of sampled superficial white matter per subject were 32 (14) mm2 for control subjects and 30 (10) mm2 for subjects with schizophrenia. Total white matter was determined by outlining the gray matter/white matter border and including all white matter on the section. The mean total areas of the sampled total white matter per subject were 280 (117) mm2 for control subjects and 277 (105) mm2 for subjects with schizophrenia.
SST mRNA expression as a function of cortical layer was determined in a series of cortical traverses (1–2 mm in width) extending from the pial surface to the white matter. Three cortical traverses were sampled for each section (9 traverses per subject) (Fig. 1A). Each traverse was divided into 50 equal bins parallel to the pial surface and the OD was determined for each bin. These bins were then combined into zones that approximated laminar boundaries based on previous studies (Akbarian et al. 1995; Pierri et al. 1999). These zones (i.e., bins 1–5, 6–15, 16–25, 26–30, 31–40, and 41–50) corresponded to layers 1, 2/ superficial 3 (2/3s), deep 3 (3d), 4, 5, and 6, respectively (Fig. 1B). The mean OD was calculated for each zone. Background measures were sampled from deep white matter where no specific expression of SST mRNA was observed. All sampled areas were corrected by subtracting the corresponding background measure from the same slide.
Figure 1.
Schematic representation of the sampling strategy for grain analysis of SST mRNA expression. (A) Camera lucida drawing of the portion of DLPFC containing area 9, with the dotted line indicating the gray/white matter border. Three cortical traverses from the pial surface to white matter were placed in each section. 1(B) Brightfield photomicrograph of a representative traverse from a Nissl-stained section. (B’) Darkfield photomicrograph of an adjacent emulsion-dipped section illustrating silver grain accumulation over neuronal nuclei. Two ROIs (large dashed rectangles) were placed in layers 2/3s and 5. Four sampling frames (smaller dashed rectangles) were placed in each zone. (C) Representative brightfield image in which Nissl-stained neuronal nuclei were identified and sampled based upon unbiased inclusion and exclusion rules. Each 120 × 170-µm counting frame had broken and solid lines indicating inclusion and exclusion boundaries, respectively. Circles with a 22 µm diameter were centered over all neuronal nuclei (solid circles) and 4 glia nuclei (dashed circles) in every counting frame, and the number of grains in each circle was counted in the corresponding darkfield image (C’).
Evaluation of mRNA expression at the cellular level was performed by determining silver grain accumulation on emulsion-dipped, Nissl counterstained sections. Using the MCID system coupled to a microscope equipped with a motor-driven stage, 2 regions of interest (ROIs) (Fig. 1B′) were defined in 1-mm-wide cortical traverses (3/section; 9/subject). The superficial ROI extended from 10% to 30% of the distance from the pial surface to the white matter (corresponding to layer 2–superficial 3), and the deep ROI extended from 60% to 80% (corresponding to layer 5). Four sampling frames (120 × 170 µm) were placed in each ROI such that the edges of the frames were equidistant from the border of the ROI and the edge of the next sampling frame. Within each frame, a circle with a fixed diameter of 22 µm (380 µm2) was placed over each Nissl-stained nucleus under brightfield illumination using an unbiased inclusion and exclusion rule (Fig. 1C), and then the number of grains within the circle were counted in the corresponding darkfield image (Fig. 1C′). Because RNase A treatment during the in situ hybridization procedure degrades Nissl-stainable substances within the cytoplasm, it was not possible to draw contours around the soma of neurons. In a previous study, we determined that the largest cross-sectional area of human DLPFC GABA neurons is ~400 µm2 (Volk et al. 2000). Thus, in order to include the maximal number of grains/neuron, we utilized a fixed 22 µm diameter (380 µm2) circle, which would include most grains from the largest interneurons as previously described (Hashimoto et al. 2003). The use of fixed diameter sampling does not account for potential differences in somal size across subject groups. However, this confound is unlikely because the somal size of GAD67 mRNA-positive neurons has been reported to be unchanged in subjects with schizophrenia (Akbarian et al. 1995; Volk et al. 2000). Background grain density was measured in each sampling frame by using the same sampling circle to count grains over 4 glial nuclei in each sampling frame. The smaller size and intense cresyl violet staining of glial nuclei distinguished them from the larger, more faintly stained neuronal nuclei (Fig. 1C). Total neuron numbers sampled in the superficial ROI were 13 791 and 14 593 for control and subjects with schizophrenia, respectively. Deep ROI total neuron numbers sampled were 13 981 and 14 356 for control and subjects with schizophrenia, respectively.
Grain density per neuron (i.e., number of grains within the 22 µm diameter circle) was calculated for all neurons. Specifically labeled neurons were determined by creating a threshold of grains per neuron. For both subject groups, histograms (natural log transformed) of the grain density of all sampled neurons had a bimodal distribution representing nonspecifically and specifically labeled neuron populations (Gerfen et al. 1991). A point of rarity between these populations was used to calculate the threshold above background needed to distinguish the labeled neuron population. Using this threshold, which was equal to 7× background grain density, histograms (natural log transformed) of the grain density of all sampled neurons appeared unimodal and normal in both subject groups. Therefore, grain densities of ≥ 7× background were considered to be specifically labeled and are referred to as SST mRNA+ cells.
Haloperidol-Exposed Monkeys
To mimic the treatment of schizophrenia with high doses of haloperidol, 4 pairs of young adult, male macaque monkeys (Macaca fascicularis), matched for age and weight, were chronically exposed to haloperidol decanoate (mean [SD] trough plasma level, 4.3 [1.1] ng/mL) and benztropine mesylate (1 mg b.i.d.) to treat extrapyramidal symptoms for 9–12 months, as previously described (Pierri et al. 1999). Processing of monkey brain tissue was conducted as previously described (Hashimoto et al. 2003). Briefly, coronal sections with a thickness of 16 µm were cut from fresh-frozen tissue blocks containing the middle one-third of the principal sulcus. Two serial sections from each animal were processed for SST mRNA expression utilizing the 35S-labeled riboprobe as described above. The OD of the cortex was determined for the full cortical thickness of areas 9 and 46 cut perpendicular to the pial surface similar to the methods in the human study. Background measures were sampled from deep white matter where no specific expression of SST mRNA was observed. All sampled areas were corrected by subtracting corresponding background measures from the same section.
Genetically Engineered Mice
To test the effect of decreased expression of the neurotrophin receptor tyrosine kinase B (trkB) on the expression of SST mRNA, we used trkB hypomorphic mice (Xu et al. 2000) in which the 1st coding exon of the trkB gene is replaced with a trkB cDNA unit flanked by 2 loxP sites (fBZ locus). These mice were generated with 129 strain mice-derived embryonic stem cells and C57BL/6 mice-derived blastocytes (Xu et al. 2000) and back-crossed into C57BL/6 mice for at least 5 generations. Wild-type C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were used as control. Homozygous (fBZ/fBZ) and heterozygous (fBZ/+) animals were reported to express ~25% and ~62%, respectively, of the trkB protein levels present in the wild-type animals (Xu et al. 2000; Rohrer 2001; Rico et al. 2002). Animals with the fBZ/fBZ genotype do not express the truncated isoforms of trkB (Xu et al. 2000). Heterozygous and homozygous mice for the fBZ locus and wild-type controls (n = 3 for each group) were euthanized at 8 weeks of age.
The OD from the autoradiographic film was measured for SST mRNA expression of the cortex in the PFC, including the cingulate and prelimbic cortices, as described for the human study. All density measures were corrected by subtracting background measures in the corpus callosum.
Statistical Analyses
Analyses were performed on SPSS (SPSS, Inc., Chicago, IL). Analysis of covariance (ANCOVA) models were used to test differences in SST mRNA expression between control subjects and subjects with schizophrenia. The data were averaged across the 3 sections for each subject before statistical analysis. The 1st ANCOVA model used diagnostic group as the main effect, pair as a blocking effect, and storage time and RIN as covariates. The pair effect reflects the matching of individual subject pairs for sex, age, and PMI. RIN was included as a covariate because it reflects mRNA integrity (Stan et al. 2006). Subject pairing may be considered an attempt to balance the 2 diagnostic groups with regard to the experimental factors instead of a true statistical paired design. Thus, to validate the 1st model, a 2nd ANCOVA model was performed with a main effect of diagnostic group and covariates of sex, age, PMI, RIN, and storage time. Storage time as a covariate was not significant in either model and thus was excluded in the reported analyses. Both models produced comparable results for diagnostic group effect; however, because the effect of age on SST mRNA expression was significant, the results of the 2nd model are reported.
In order to correct for multiple comparisons in the laminar analyses, significance of the diagnostic group effect was determined for individual layers using the Holm simultaneous inference procedure (Holm 1979) as previously described (Volk et al. 2002). To maintain consistency, the reported P-values for each laminar comparison have been adjusted to correspond to the family-wise error rate of 0.05.
The potential influence of sex, diagnosis of schizoaffective disorder, lifetime history of any substance abuse/dependency, diagnosis of alcohol abuse/dependency at time of death, use of antidepressant medication at time of death, use of benzodiazepines/valproate at time of death, or suicide on the within-pair percentage of differences in mRNA expression was assessed by 2-sample t-test analyses. Correlations between age and mRNA expression were assessed by Pearson’s correlation analyses.
For the haloperidol-treated monkeys, paired t-test analyses were used to assess the effects of treatment group on SST mRNA expression.
For the trkB hypomorphic mice, the effects of genotype on SST mRNA expression were determined by a single-factor ANOVA. Tukey’s multiple comparison test was used in post hoc comparisons across genotypes.
Results
Specificity of SST Riboprobe
Several lines of evidence confirm the specificity of the riboprobe for SST mRNA used in this study. First, the distinctive laminar distribution of SST mRNA expression is very similar to that previously reported for both SST-immunoreactive cell bodies in monkey and human prefrontal cortex (Lewis et al. 1986; Hayes et al. 1991) and SST mRNA expression in monkey prefrontal cortex (Da Cunha et al. 1995). Specifically, the density of SST mRNA+ neurons was lowest in layer 1, highest in layers 2 and superficial 3, moderate in deep layer 3 and layer 4, high in layer 5, and moderate in layer 6 (Figs 1B′ and 2A). Second, the presence of intensely SST mRNA+ neurons in the superficial white matter (Figs 1B′ and 2A) is consistent with previous studies of SST-immunoreactive cell bodies in human and monkey prefrontal cortex (Lewis et al. 1986; Hayes et al. 1991) and SST mRNA expression in the monkey prefrontal cortex (Da Cunha et al. 1995). Third, sense riboprobes for SST mRNA showed an absence of signal above background (data not shown).
Figure 2.
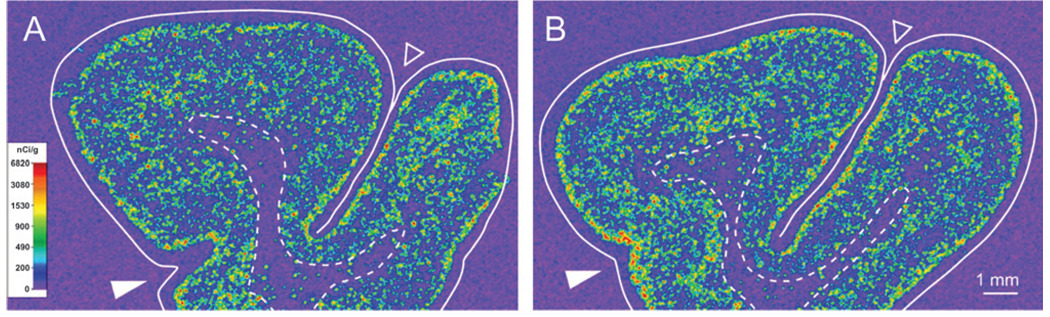
Representative film autoradiograms from 1 pair of subjects illustrating SST mRNA expression in human DLPFC. The densities of hybridization signals are represented in a pseudocolor manner according to the calibration scale (center). The solid and dashed lines indicate the pial surface and the gray/white matter border, respectively. Expression of SST mRNA in the cortical gray matter is reduced in the subject with schizophrenia (B) relative to the matched control subject (A).
Compartmental Expression of SST in DLPFC Area 9 of Control Subjects and Individuals with Schizophrenia
We previously reported reduced SST mRNA expression by microarrary, quantitative PCR (qPCR), and in situ hybridization in the DLPFC of subjects with schizophrenia (Hashimoto et al. 2007). However, whether this expression deficit was specific to the gray matter or also present in the white matter was not examined. Consistent with qualitative impressions (Fig. 2), quantitative measures of SST mRNA expression in the gray matter of DLPFC area 9 confirmed that the mean (±SD) OD of gray matter was significantly (F(1,40) = 18.07; P < 0.001) decreased by 36% in subjects with schizophrenia (55.8 ± 31.9 nCi/g) compared with control subjects (87.5 ± 22.9 nCi/g). OD measurements in the gray matter were lower in the subjects with schizophrenia for 20 of the 23 subject pairs (Fig. 3A). In contrast, mean film OD from the total white matter did not significantly differ (F(1,40) = 0.72; P = 0.40) between control subjects (18.8 ± 5.6 nCi/g) and subjects with schizophrenia (17.0 ± 5.3 nCi/g) (Fig. 3B). Because SST mRNA expression was most dense in the superficial white matter (Fig. 2), the OD was also determined from contours in the 800 µm of white matter immediately below the layer 6–white matter border. These measures also did not differ (F(1,40) = 1.74; P = 0.20) between control subjects (338 ± 10.9 nCi/g) and subjects with schizophrenia (28.3 ± 10.1 nCi/g) (Fig. 3C).
Figure 3.
SST mRNA expression levels assessed by autoradiographic film OD measures in DLPFC area 9 of subjects with schizophrenia (gray circles) and control subjects (black circles). Subjects in each pair are connected by black lines and the mean expression levels for each subject group are represented by a horizontal line. The expression of SST mRNA is significantly reduced in the gray matter (A), but not in the total white matter (B) or the superficial white matter (C) of the schizophrenia subjects.
Examination of Factors that May Affect Cortical Expression of SST mRNA
In the 2nd ANCOVA model, age was a significant (F(1,40) = 19.24; P < 0.0001) determinant of SST mRNA levels in the gray matter. Indeed, OD measures in the gray matter contours were negatively correlated with age in both control subjects (r = −0.74; P < 0.0001) and subjects with schizophrenia (r = −0.51; P = 0.01) (Fig. 4). However, the within-subject pair percent differences in SST mRNA expression in the gray matter did not differ as a function of sex, diagnosis of schizoaffective disorder, lifetime history of any substance abuse or dependency, diagnosis of alcohol abuse or dependency at time of death, use of antidepressant medication at time of death, use of benzodiazepines/valproate at time of death, or suicide (all t(21) < 1.52, all P > 0.14) (Fig. 5).
Figure 4.
OD measures of SST mRNA expression in human area 9 as a function of age. SST mRNA expression levels are significantly negatively correlated with age in both subject groups. The regression line for subjects with schizophrenia (gray line) is parallel to and shifted downward from that for control subjects (black line), suggesting that the decreased expression of SST mRNA is similar in magnitude across adult life.
Figure 5.
The effects of potential confounding factors on the expression changes in SST mRNA in subjects with schizophrenia. The bars represent the mean (SD) percent differences from control subjects for SST mRNA within-subject pairs, and the numbers within each bar indicate the number of subject pairs. Neither sex, diagnosis of schizoaffective disorder, lifetime history of any substance abuse or dependence, diagnosis of alcohol abuse or dependence at time of death, history of antidepressant medications, the presence of benzodiazepines/valproate at time of death, nor cause of death significantly affected the expression changes in SST mRNA.
In a previous study, the expression of SST mRNA in DLPFC areas 9 and 46 of monkeys did not differ among monkeys chronically exposed to haloperidol, olanzapine, or sham (Hashimoto et al. 2007). However, the steady-state trough plasma levels (~1.5 ng/mL) in the haloperidol-treated monkey group might be considered relatively low compared with levels likely achieved in at least some of the subjects with schizophrenia included in this study. Thus, in order to determine if chronic exposure to higher levels of haloperidol could affect SST mRNA expression, we examined the gray matter of DLPFC areas 9 and 46 from monkeys that received haloperidol decanoate for 1 year with mean trough plasma levels >4 ng/mL (Fig. 6). Mean (SD) SST mRNA expression did not differ (t(3) = 1.08, P = 0.36) between these haloperidol-exposed monkeys (275.3 ± 40.3) and their sex-, age-, and weight-matched controls (238.4 ± 56.1).
Figure 6.
Representative film autoradiograms illustrating the expression of SST mRNA in the dorsal PFC of a control monkey (A) and an age-, sex-, and body weight–matched monkey chronically exposed to haloperidol (B). The densities of the hybridization signal are presented in a pseudocolor manner according to the calibration scale (in A). The signal density appears to be unchanged in the haloperidol-exposed monkey (B) relative to the control monkey (A). Solid and dashed lines indicate the pial surface and the gray/white matter border, respectively. Open and filled arrowheads indicate the principal and cingulate sulci, respectively.
Laminar Expression Patterns of SST mRNA in DLPFC Area 9
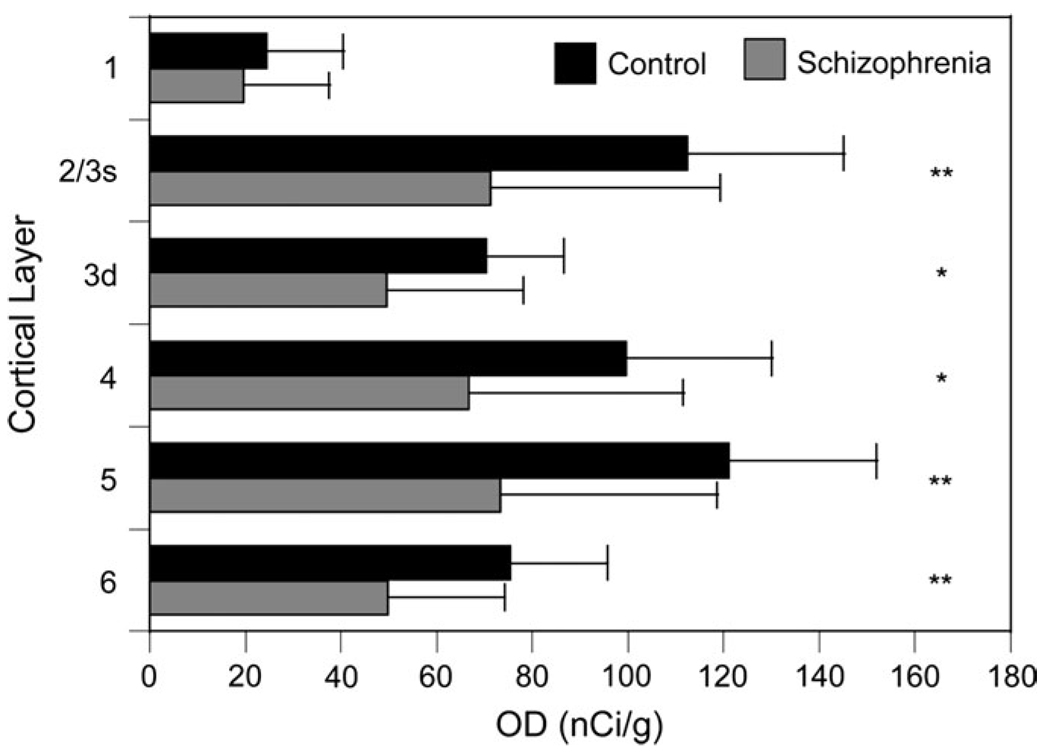
In order to determine if the reduction in SST mRNA expression in schizophrenia was selective for certain cortical layers, we examined the OD measures by layer (Fig. 7). Because of the high density of SST mRNA+ neurons in layers 2–superficial 3 (2/3s), these layers were combined and distinguished from deep layer 3 (3d). SST mRNA expression was significantly decreased in all layers (F(1,40) > 4.34; P < 0.04, for all layers), with the exception of layer 1 (F(1,40) = 0.64; P = 0.23). Of the layers with a significant diagnostic effect, the largest percentage difference was in layer 2/3s (36.6%) and the smallest difference in layer 3d (29.6%).
Figure 7.
Mean (SD) expression levels of SST mRNA by layer as assessed by film autoradiography OD measures in each cortical layer. *P < 0.05, after Holm’s correction. **P < 0.01, after Holm’s correction.
Cellular Levels of SST mRNA Expression
In order to determine if all or a subset of SST cortical neurons were affected, we determined the expression level per neuron by counting the silver grains deposited over Nissl-stained neurons in layers 2/3s and 5. The mean (±SD) number of grains per positive neuron in layers 2/3s was significantly (F(1,40) = 972, P = 0.003) 31% lower in the subjects with schizophrenia (104.4 ± 49.1) than in the control subjects (150.4 ± 53.4). Similarly, the mean number of grains per positive neuron in layer 5 was significantly (F(1,40) = 6.96, P = 0.012) decreased by 25% in subjects with schizophrenia (121.5 ± 67.3) compared with controls (162.7 ± 45.7) (Fig. 8A,B). Furthermore, the mean density of SST mRNA+ neurons in layers 2/3s was significantly (F(1,40) = 10.79, P = 0.002) decreased by 26% in subjects with schizophrenia (63.3 ± 237 neurons/mm2) relative to control subjects (85.3 ± 16.2 neurons/mm2), and in layer 5 the 23% decrease in mean density of SST mRNA+ neurons in the subjects with schizophrenia (52.5 ± 27.2 neurons/mm2) compared with control subjects (67.7 ± 15.4 neurons/mm2) showed a trend (F(1,40) = 3.93, P = 0.054) toward statistical significance (Fig. 8C,D).
Figure 8.
Cellular SST mRNA measurements in the DLPFC area 9 of subjects with schizophrenia (gray circles) and control subjects (black circles) as expressed by grains per positive neuron (A, B) in layer 2/3s (A) and layer 5 (B) and positive neuron density (C, D) in layer 2/3s (C) and layer 5 (D). Subjects in each pair are connected by black lines and the mean values for each subject group are indicated by a horizontal line.
Correlation of Altered Expression of SST mRNA with Changes in Other Transcripts
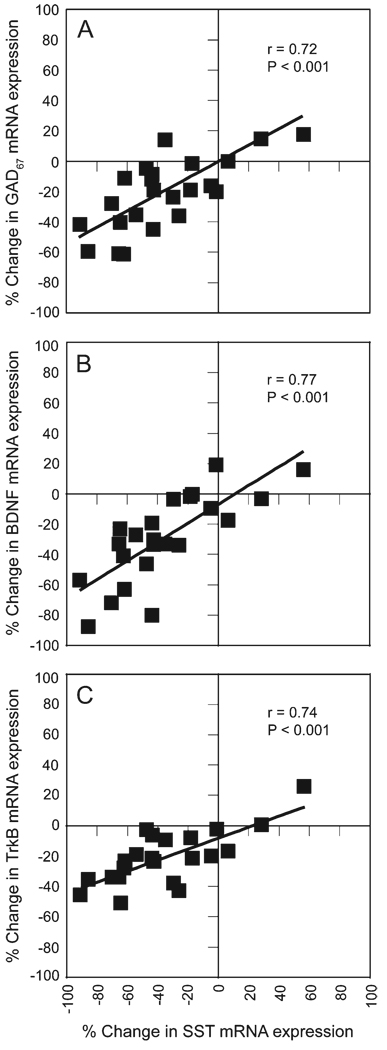
Within-pair percentage differences in SST mRNA expression were significantly correlated (r = 0.72, P < 0.001; Fig. 9A) with those found for GAD67 mRNA expression in a previous study of the same subjects (Hashimoto et al. 2005). These reductions in GAD67 mRNA expression in schizophrenia were also strongly correlated with changes in the expression of receptor trkB mRNA, the principal receptor for brain-derived neurotrophic factor (BDNF), and to a lesser extent with those in BDNF mRNA expression (Hashimoto et al. 2005). Thus, because trkB is expressed in ~50% of SST neurons (Gorba and Wahle 1999), we examined whether reduced BDNF-trkB signaling might be associated with the reduced SST mRNA expression in schizophrenia. The within-pair percentage differences in SST mRNA were significantly correlated with within-pair percentage differences in both BDNF mRNA expression (r = 0.77, P < 0.001; Fig. 9B), and trkB mRNA expression (r = 0.74, P < 0.001; Fig. 9C).
Figure 9.
Correlations of within-subject pair percent differences in SST mRNA expression with GAD67 (A), BDNF (B), and TrkB (C) mRNA expression.
SST mRNA Expression in Genetically Engineered Mice
We have previously shown that mice with reduced expression of BDNF mRNA exhibit decreased SST mRNA expression (Glorioso et al. 2006), suggesting that the correlations observed in the human subjects might represent cause and effect. In order to determine if the level of trkB mRNA expression also regulates SST mRNA expression, we examined SST mRNA expression in the PFC of trkB hypomorphic mice (Xu et al. 2000). Levels of trkB mRNA in the frontal cortex of fBZ/+ and fBZ/fBZ genotype mice were decreased by 42% and 75%, respectively, as compared with wild-type animals (Hashimoto et al. 2005). TrkB genotype was significantly (F(2,6) = 6.03, P = 0.037) related to the expression level of SST mRNA (Fig. 10). SST mRNA levels in wild-type mice (596.9 ± 31.4 nCi/g) were significantly greater (P = 0.045) than in the fBZ/fBZ genotype (406.3 ± 55.6 nCi/g), and SST mRNA expression in the fBZ/+ genotype (426.6 ± 110.5 nCi/g) was intermediate, showing a trend to lower values as compared with wild-type mice (P = 0.068).
Figure 10.
Representative film autoradiograms illustrating SST mRNA expression in a wild-type mouse (A), a mouse heterozygous for the fBZ locus (fBZ/+) (B), and a mouse homozygous for the fBZ locus (fBZ/fBZ) (C). The arrowheads (apply to all sections) in A indicate the quantified regions in the frontal cortex. The densities of hybridization signals are presented in a pseudocolor manner according to the calibration scale (below C). (D) Bar graph representing mean ± SD of SST mRNA in the PFC in each genotype. Bars not sharing the same letter are significantly different (P < 0.05; post hoc Tukey’s multiple comparison).
Discussion
The expression levels of SST mRNA were significantly decreased in layers 2–6 of DLPFC area 9 in subjects with schizophrenia compared with matched controls. This decrease was evident in 20 of the 23 subject pairs studied; determining whether this heterogeneity reflects interindividual variability in SST mRNA expression or diversity in the underlying disease process (i.e., reduced SST mRNA expression identifies a subtype of schizophrenia) will require studies in larger cohorts of subjects. In contrast, SST mRNA expression in layer 1 and the white matter did not differ between subject groups. At the cellular level, both the density of cortical SST mRNA+ neurons and the expression of SST mRNA per neuron were reduced in the subjects with schizophrenia. These alterations appear to reflect the disease process of schizophrenia and not to be the consequence of potential confounds. Observations in both humans and genetically engineered mice suggest that these changes are a downstream consequence of impaired neuro-trophin signaling in schizophrenia. Together, these findings indicate that reduced SST mRNA expression in the DLPFC of subjects with schizophrenia is restricted to the gray matter, confined to a subset of SST neurons, and the consequence of a plausible pathogenetic mechanism.
Altered SST mRNA Expression in Schizophrenia is not due to Confounds
Several lines of evidence indicate that the reduction in SST mRNA expression is due to the disease process of schizophrenia, and is not the consequence of the methods employed or the result of other factors commonly associated with the illness. First, in a subset of the subject pairs included in this study, SST mRNA was found to be significantly decreased in the subjects with schizophrenia using DNA microarray and realtime quantitative PCR with probes that recognize different sequences of SST mRNA than the probe used in the present study (Hashimoto et al. 2007). In addition, the within-subject pair differences in SST mRNA expression as determined by the in situ hybridization probe used in the present study were significantly correlated with those determined by microarray (r = 0.78; P < 0.002) and real-time qPCR (r = 0.91; P < 0.001). Second, all 4 subjects with schizophrenia off antipsychotic medications at the time of death demonstrated a decrease in SST mRNA expression relative to their matched controls. Third, SST mRNA expression was unaltered in the DLPFC of monkeys chronically exposed to either haloperidol or olanzapine with mean trough plasma levels in the therapeutic range (~1.5 and ~15 ng/mL, respectively) for humans (Hashimoto et al. 2007). Fourth, in the present study the gray matter expression of SST mRNA was not altered in monkeys chronically exposed to higher mean trough plasma levels of haloperidol (4.3 ng/mL) that produced marked extrapyramidal symptoms requiring treatment with benztropine mesylate, a manner of treatment likely achieved in at least some of our human subjects. Fifth, neither SST mRNA (Marcus et al. 1997) nor protein (Sakai et al. 1995) levels were decreased in the frontal cortex of rats exposed to haloperidol. Sixth, neither sex, lifetime history of substance abuse or dependency, diagnosis of alcohol abuse or dependency at time of death, treatment with antidepressant medications or benzodiazepines/valproate, nor suicide accounted for the decreased expression of SST mRNA in the subjects with schizophrenia (see Fig. 5). However, it should be noted that the within-subject pair differences in SST mRNA expression did not differ between subjects with schizophrenia or schizoaffective disorder, leaving open the possibility that this reduction is common to psychotic illnesses.
A significant negative correlation between age and SST mRNA gray matter expression was seen in both controls and individuals with schizophrenia, consistent with previous findings of an inverse relationship between age and SST mRNA or protein levels in rat hippocampus and monkey frontal cortex (Hayashi et al. 1997; Vela et al. 2003). The regression line for the subjects with schizophrenia was parallel to and shifted downward from that of the control subjects (Fig. 4), indicating that the decreased expression of SST mRNA in the subjects with schizophrenia was present across adult life and is thus unlikely to be a consequence of illness chronicity. Furthermore, this observation suggests that the SST mRNA expression deficit is present early in the course of the illness and thus could contribute to the pathophysiology underlying the clinical features of the illness.
A Subset of SST Neurons is Affected in Schizophrenia
Our results demonstrate that SST mRNA expression in layers 2–6, but not in layer 1 or the white matter, was reduced in subjects with schizophrenia. During development of the cerebral cortex, early germinal zones proliferate over successive rounds of cell division and give rise to postmitotic migratory neurons. 3H-thymidine birth-dating and autoradiographic analyses have demonstrated that the earliest generated cells comprise the preplate which, later in development, is split into the marginal zone (adult layer 1) and the subplate (adult deep layer 6 and superficial white matter) by the later born neurons of the cortical plate (adult layers 2–superficial 6)(Kostovic and Rakic 1980; Luskin and Shatz 1985; Chun and Shatz 1989a; Bayer and Altman 1990). The combination of birth-dating techniques and immunohistochemistry revealed that a subpopulation of the early generated preplate neurons expresses SST (Chun and Shatz 1989a). Our data suggest that the early generated SST mRNA+ neurons which reside in layer 1 and the superficial white matter are not affected in schizophrenia, whereas the later developing SST mRNA+ neurons which migrate to the cortical plate are affected. Additional analyses support this interpretation. For example, the rat cortex sublayer 6b also contains residual neurons of the embryonic preplate (Valverde et al. 1989), suggesting that SST+ neurons in layer 6b might be less affected in schizophrenia than those present in the more superficial layer 6a. Consistent with this prediction, the mean decrease in OD measures of SST mRNA in subjects with schizophrenia was significantly greater (t(22) = −5.08; P < 0.0001) in the superficial (−37.4 nCi/g) than in the deep (−14.0 nCi/g) half of layer 6. Given that the cortical plate forms during the 2nd trimester of gestation, these findings raise the possibility that the alterations in SST neurons reflect the effect of adverse environmental events during that time frame (e.g., maternal influenza; Brown 2006) that have been associated with an increased risk for schizophrenia. However, given how common the alterations in SST neurons appear to be from the present study (i.e., 20/23 pairs), other causal factors must also be contributory. For example, both SST and PV-containing cortical GABA neurons are affected in schizophrenia, whereas those that contain CR appear to be unaffected. Thus, factors shared by SST and PV neurons that differ from CR neurons (e.g., place and timing of neuron birth, transcription factors regulating cell fate, etc.; see Wonders and Anderson 2006, for review) might also contribute to cell typespecific vulnerability. Of course, other features intrinsic to, or associated with the connectivity of, adult SST neurons in layers 2–superficial 6 might contribute to their greater vulnerability relative to other SST neurons.
In layer 2–superficial 3, we observed a significant reduction in the mean density of SST mRNA+ neurons, but only a trend toward a decrease in layer 5, raising the possibility that the severe reduction in SST mRNA expression within individual neurons is restricted to layer 2/3s. The mean expression level of SST mRNA per neuron was significantly reduced. In subjects with schizophrenia, total neuron number in the frontal lobe is unchanged (Thune et al. 2001) and the density of nonpyramidal neurons in the DLPFC is slightly increased (Selemon et al. 1995) or unchanged (Akbarian et al. 1995). Thus, it appears that SST neurons are still present in the DLPFC of subjects with schizophrenia, but that the expression of SST mRNA per neuron is reduced, with the reduction so great in some neurons that SST mRNA levels fall below the threshold of detection. This pattern of change in SST mRNA expression contrasts with the alterations in GAD67 and PV mRNA expression in the DLPFC of subjects with schizophrenia. The expression levels of GAD67 mRNA were reduced below detectable levels in ~25–35% of GABA neurons, whereas the remaining GABA neurons expressed normal levels of GAD67 mRNA, suggesting that a subpopulation of GABA neurons is affected in the illness (Volk et al. 2000). The reduced expression of PV mRNA was found to be due to a decrease in expression level per neuron rather than to a decrease in the density of PV mRNA+ neurons suggesting that most PV mRNA+ neurons are affected (Hashimoto et al. 2003). Because the reduction in SST mRNA expression was due to both a decrease in positive neuron density and in expression per neuron, these findings suggest that a majority of SST neurons express reduced levels of SST mRNA and that a subset of severely affected SST neurons express undetectable levels of SST mRNA.
The severely affected neurons might include the approximately 50% of SST+ neurons that express trkB (Gorba and Wahle 1999), the principal receptor of BDNF. Consistent with this interpretation, mice with genetically engineered reductions in the expression of BDNF mRNA have significantly lower levels of cortical SST mRNA and protein (Grosse et al. 2005; Glorioso et al. 2006). In addition, the significant reduction in the expression of SST mRNA in the PFC of homozygote trkB hypomorphic mice indicates that signaling via trkB is also involved in regulating the expression of SST mRNA, even in the face of conserved levels of BDNF. Given that the expression levels of BDNF (Weickert et al. 2003; Hashimoto et al. 2005) and trkB mRNAs (Hashimoto et al. 2005; Weickert et al. 2005) are reduced in the DLPFC of subjects with schizophrenia, our findings imply that reduced BDNF-trkB signaling in schizophrenia is an “upstream” event that contributes to reduced SST mRNA expression. This interpretation is consistent with studies demonstrating that the addition of BDNF to cultured cortical cells or the intraventricular administration of BDNF in rats increased SST mRNA and protein levels (Nawa et al. 1994; Villuendas et al. 2001). Also, previous studies have reported that BDNF can influence the expression of GAD67 (Yamada et al. 2002; Cotrufo et al. 2003; Wirth et al. 2003; Patz et al. 2004; Palizvan et al. 2004). Therefore, altered BDNF–trkB signaling in schizophrenia may be a conserved mechanism driving the highly correlated reductions in SST and GAD67 mRNA expression in schizophrenia. However, the present study cannot exclude the possibilities that reduced SST mRNA expression is due to other unknown upstream mechanisms or is a secondary consequence of cortical dysfunction.
Functional Implications of Alterations in SST Neurons in Schizophrenia
In rat and monkey frontal cortex, ~40% of SST neurons also express neuropeptide Y (NPY) (Hendry et al. 1984; Kubota et al. 1994). Interestingly, expression deficits in SST and NPY mRNAs are strongly correlated in schizophrenia (r = 0.81; P < 0.001) (Hashimoto et al. 2007) and both are similarly reduced in BDNF knockout mice (Glorioso et al. 2006), suggesting that the NPY-containing subclass of SST neurons are affected in schizophrenia. Interneurons that contain both SST and NPY include the Martinotti cells (Kawaguchi and Kubota 1997; Reyes et al. 1998; Gibson et al. 1999; Ma et al. 2006). The axons of Martinotti cells project to layer 1 where they synapse on the apical dendrites of pyramidal neurons, and in rodent and monkey neocortex, SST interneurons predominately innervate the dendrites of pyramidal neurons (Hendry et al. 1984; DeLima and Morrison 1989; Kawaguchi and Kubota 1996; Melchitzky and Lewis 2005). Thus, disturbances in the SST/NPY-containing Martinotti class of GABA neurons could contribute to the dysfunction of DLPFC circuitry associated with working memory impairments in schizophrenia (Weinberger et al. 1986; Goldman-Rakic 1994; Cannon et al. 2005; Tan et al. 2005). How the observed alterations in SST-containing GABA neurons could contribute to cognitive dysfunction in schizophrenia requires further study, but the existing literature suggests the following mechanistic hypotheses.
First, in individuals with schizophrenia, disturbances in sensory-gating have been correlated with reductions in working memory performance (Silver and Feldman 2005), suggesting that the inability to filter distracting stimuli disrupts working memory. Interestingly, in computational modeling of cortical microcircuits and working memory, inhibitory interneurons that target the dendritic domain of pyramidal neurons provide resistance against distracting stimuli by sending enhanced inhibition to dendrites of nearby pyramidal neurons that are selective for other stimuli (Wang et al. 2004). Furthermore, in rats, high frequency trains from pyramidal neurons produce facilitating excitatory inputs to Martinotti cells that, via synapses onto the dendrites of neighboring pyramidal neurons, cause disynaptic inhibition (Silberberg and Markram 2007). Therefore, Martinotti interneurons, by mediating the disynaptic inhibition of neighboring pyramidal neurons selective for other stimuli, may filter distracting stimuli during working memory tasks. Thus, alterations in SST/NPY-containing Martinotti cells in schizophrenia may contribute to altered working memory performance.
Second, Martinotti interneurons also exhibit low threshold-spiking membrane properties (Kawaguchi and Kubota 1997; Reyes et al. 1998; Gibson et al. 1999; Ma et al. 2006). These low threshold-spiking, SST-containing interneurons are extensively electrically coupled into networks that robustly synchronize their spiking activity (Gibson et al. 1999, 2005) in the theta range (4–7 Hz) producing synchronized inhibitory postsynaptic potentials in neighboring pyramidal neurons (Beierlein et al. 2000). Although a direct connection between the synchronized theta spiking frequency in these neuronal networks and theta band electroencephalography (EEG) oscillations has not been demonstrated, EEG studies in humans and monkeys have demonstrated that theta band EEG oscillations increase in power during working memory tasks (Krause et al. 2000; Raghavachari et al. 2001; Lee et al. 2005). Furthermore, subjects with schizophrenia demonstrate altered frontal theta oscillations during working memory tasks (Schmiedt et al. 2005). Thus, disturbances in SST/NPY-containing, low threshold-spiking Martinotti interneurons might contribute to alterations in theta oscillations and ultimately to impaired working memory performance in subjects with schizophrenia.
Acknowledgments
Funding
National Institute of Health grants (MH043784 and MH045156).
Footnotes
Conflict of Interest: D.A.L. currently receives research support from the BMS Foundation, Merck and Pfizer and in 2007–2007 served as a consultant to Bristol-Meyer Squibb, Pfizer, Roche, Sepracor and Wyeth.
References
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: Its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Exp Neurol. 1990;107:48–62. doi: 10.1016/0014-4886(90)90062-w. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Chun JJM, Shatz CJ. Interstitial cells of the adult neocortical white matter are the remnant of the early generated subplate neuron population. J Comp Neurol. 1989a;282:555–569. doi: 10.1002/cne.902820407. [DOI] [PubMed] [Google Scholar]
- Chun JJM, Shatz CJ. The earliest-generated neurons of the cat cerebral cortex: Characterization by MAP2 and neurotransmitter immunohistochemistry during fetal life. J Neurosci. 1989b;9:1648–1657. doi: 10.1523/JNEUROSCI.09-05-01648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrufo T, Viegi A, Berardi N, Bozzi Y, Mascia L, Maffei L. Effects of neurotrophins on synaptic protein expression in the visual cortex of dark-reared rats. J Neurosci. 2003;23:3566–3571. doi: 10.1523/JNEUROSCI.23-09-03566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha A, Rausch DM, Eiden LE. An early increase in somatostatin mRNA expression in the frontal cortex of rhesus monkeys infected with simian immunodeficiency virus. Proc Natl Acad Sci USA. 1995;92:1371–1375. doi: 10.1073/pnas.92.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLima AD, Morrison JH. Ultrastructural analysis of somatostatinimmunoreactive neurons and synapses in the temporal and occipital cortex of the macaque monkey. J Comp Neurol. 1989;283:212–227. doi: 10.1002/cne.902830205. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, McGinty JF, Young WS. Dopamine differentially regulates dynorphin, substance P, and enkaphalin expression in striatal neurons: in situ hybridization histochemical analysis. J Neurosci. 1991;11:1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 2005;93:467–480. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Austin MC, Lewis DA. Normal cellular levels of synaptophysin mRNA expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2000;48:389–397. doi: 10.1016/s0006-3223(00)00923-9. [DOI] [PubMed] [Google Scholar]
- Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA, Mirnics K. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry. 2006;11:633–648. doi: 10.1038/sj.mp.4001835. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Distinct GABAergic targets of feedforward and feedback connections between lower and higher areas of rat visual cortex. J Neurosci. 2003;23:10904–10912. doi: 10.1523/JNEUROSCI.23-34-10904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Albo MC, Elston GN, DeFelipe J. The human temporal cortex: Characterization of neurons expressing nitric oxide synthase, neuropeptides and calcium-binding proteins, and their glutamate receptor subunit profiles. Cereb Cortex. 2001;11:1170–1181. doi: 10.1093/cercor/11.12.1170. [DOI] [PubMed] [Google Scholar]
- Gorba T, Wahle P. Expression of trkB and trkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- Grosse G, Djalali S, Deng DR, Holtje M, Hinz B, Schwartzkopff K, Cygon M, Rothe T, Stroh T, Hellweg R, Ahnert-Hilger G, et al. Area-specific effects of brain-derived neurotrophic factor (BDNF) genetic ablation on various neuronal subtypes of the mouse brain. Brain Res Dev Brain Res. 2005;156:111–126. doi: 10.1016/j.devbrainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Yamashita A, Shimizu K. Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNAs during aging. Brain Res. 1997;749:283–289. doi: 10.1016/S0006-8993(96)01317-0. [DOI] [PubMed] [Google Scholar]
- Hayes TL, Cameron JL, Fernstrom JD, Lewis DA. A comparative analysis of the distribution of prosomatostatin-derived peptides in human and monkey neocortex. J Comp Neurol. 1991;303:584–599. doi: 10.1002/cne.903030406. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Jones EG, Emson PC. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4:2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Cytology and the time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Krause CM, Sillanmaki L, Koivisto M, Saarela C, Haggqvist A, Laine M, Hamalainen H. The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol. 2000;111:2071–2078. doi: 10.1016/s1388-2457(00)00429-6. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45:147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Morrison JH. An immunohistochemical characterization of somatostatin-28 and somatostatin-28 (1–12) in monkey prefrontal cortex. J Comp Neurol. 1986;248:1–18. doi: 10.1002/cne.902480102. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Studies of the earliest generated cells of the cat’s visual cortex: Cogeneration of the subplate and marginal zones. J Neurosci. 1985;5:1062–1075. doi: 10.1523/JNEUROSCI.05-04-01062.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus MM, Nomikos GG, Malmerfelt A, Zachrisson O, Lindefors N, Svensson TH. Effect of chronic antipsychotic drug treatment on preprosomatostatin and preprotachykinin A mRNA levels in the medial prefrontal cortex, the nucleus accumbens and the caudate putamen of the rat. Brain Res Mol Brain Res. 1997;45:275–282. doi: 10.1016/s0169-328x(96)00263-x. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Synaptic targets of somatostatin-labeled axon terminals in monkey prefrontal cortex. Soc Neurosci. 2005 doi: 10.1016/j.neuroscience.2004.08.046. [Abstr 675.6] [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Nawa H, Pelleymounter MA, Carnahan J. Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci. 1994;14:3751–3765. doi: 10.1523/JNEUROSCI.14-06-03751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palizvan MR, Sohya K, Kohara K, Maruyama A, Yasuda H, Kimura F, Tsumoto T. Brain-derived neurotrophic factor increases inhibitory synapses, revealed in solitary neurons cultured from rat visual cortex. Neuroscience. 2004;126:955–966. doi: 10.1016/j.neuroscience.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Patz S, Grabert J, Gorba T, Wirth MJ, Wahle P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an Early period of postnatal development. Cereb Cortex. 2004;14:342–351. doi: 10.1093/cercor/bhg132. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo T-U, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujar R, Rozov BN, Somogyi P, Sakman B. Target-cellspecific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B. Gene dosage effect of the TrkB receptor on rod physiology and biochemistry in juvenile mouse retina. Mol Vis. 2001;7:288–296. [PubMed] [Google Scholar]
- Sakai K, Maeda K, Chihara K, Kaneda H. Increases in cortical neuropeptide Y and somatostatin concentrations following haloperidol-depot treatment in rats. Neuropeptides. 1995;29:157–161. doi: 10.1016/0143-4179(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci. 2005;17:391–398. doi: 10.1176/jnp.17.3.391. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: What quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicot JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry. 2005;162:1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- Thune JJ, Uylings HBM, Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res. 2001;35:15–21. doi: 10.1016/s0022-3956(00)00043-1. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Valverde F, Facal-Valverde MV, Santacana M, Heredia M. Development and differentiation of early generated cells of sublayer VIb in the somatosensory cortex of the rat: a correlated Golgi and autoradiographic study. J Comp Neurol. 1989;290:118–140. doi: 10.1002/cne.902900108. [DOI] [PubMed] [Google Scholar]
- Vela J, Gutierrez A, Vitorica J, Ruano D. Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J Neurochem. 2003;85:368–377. doi: 10.1046/j.1471-4159.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- Villuendas G, Sanchez-Franco F, Palacios N, Fernandez M, Cacicedo L. Involvement of VIP on BDNF-induced somatostatin gene expression in cultured fetal rat cerebral cortical cells. Brain Res Mol Brain Res. 2001;94:59–66. doi: 10.1016/s0169-328x(01)00177-2. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy J-M, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA. 2004;101:1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–650. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wirth MJ, Brun A, Grabert J, Patz S, Wahle P. Accelerated dendritic development of rat cortical pyramidal cells and in-terneurons after biolistic transfection with BDNF and NT4/5. Development. 2003;130:5827–5838. doi: 10.1242/dev.00826. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: Modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]