Abstract
Vagal sensory neurons are dependent on neurotrophins for survival during development. Here, the contribution of brain-derived neurotrophic factor (BDNF) to survival and other aspects of gastric vagal afferent development was investigated. Postmortem anterograde tracing with 1, 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbo-cyanine perchlorate (DiI) was used to selectively label vagal projections to the stomach on postnatal day (P)0, 3, 4, and 6 in wild types and heterozygous or homozygous BDNF mutants. Sampling sites distributed throughout the ventral stomach wall were scanned with a confocal microscope and vagal axon bundles, single axons, putative mechanoreceptor precursors (intraganglionic laminar endings, IGLEs; intramuscular arrays, IMAs) and efferent terminals were quantified. Also, myenteric neurons, which are innervated by IGLEs, were stained with cuprolinic blue and counted. Quantitative comparisons across wild-type stomach compartments demonstrated that the adult distribution of IMAs was not present at P0, but began to form by P3-6. Of all the quantified elements, at P0, only IGLE density was significantly different in homozygous mutants as compared to wild types, exhibiting a 50% reduction. Also, antrum innervation appeared disorganized, and some putative IMA precursors had truncated telodendria. At P3-6, the effect on IGLEs had recovered, the disorganization of antrum innervation had partially recovered, and some IMA telodendria were still truncated. The present results suggest gastric IGLEs are among the vagal sensory neurons dependent on BDNF for survival, or axon guidance. Alternatively, BDNF deficiency may delay gastric IGLE development. Also, BDNF may contribute to IMA differentiation, and patterning of antral vagal innervation.
Indexing terms: autonomic nervous system, gastrointestinal tract, vagus nerve, visceral afferents
INTRODUCTION
Innervation of the stomach by the vagus, or Xth cranial nerve is important for control of digestion, food intake, and emesis, contributing both afferent and efferent innervation. In terms of sensory innervation, two vagal mechanoreceptor classes predominate in the stomach muscle wall: intraganglionic laminar endings (IGLEs) and intramuscular arrays (IMAs). Intraganglionic laminar endings are densely distributed within all major stomach compartments, the forestomach, corpus and antrum (Berthoud et al., 1997a; Fox et al., 2000; Wang and Powley, 2000). These endings appear to transduce shearing forces due to tension and stretch of the muscle wall (Neuhuber and Clerc, 1990; Yang et al., 2006; Zagorodnyuk et al., 2001). Intramuscular arrays, on the other hand, are concentrated in the forestomach (Fox et al., 2000; Phillips et al., 1997; Wang and Powley, 2000) and are hypothesized to detect stretch of the muscle wall caused by stomach dilation (Wang and Powley, 2000; but see Zagorodnyuk et al., 2001). Additionally, vagal sensory neurons supply chemoreceptors, mechanoreceptors and polymodal receptors to the gastric submucosa/mucosa (e.g., Harding and Leek, 1972; Iggo, 1957). These receptor’s nerve terminals approach, but do not contact the basal poles of epithelial cells, and they detect the presence of some GI hormones as well as several properties of the luminal contents (e.g., Page et al., 2002).
Surprisingly little is known about how the development of the complex organization of vagal sensory innervation of the stomach is orchestrated. Recently, netrin expressed in the fetal gut (Jiang et al., 2003) was found to act as a chemoattractant for vagal sensory axons that express the netrin receptor (deleted in colorectal cancer; DCC; Keino-Masu et al., 1996), as they grow toward the stomach and intestine (Ratcliffe et al., 2006). Further, laminin-111, which is also expressed in the developing gut (Ratcliffe et al., 2008; Simon-Assmann et al., 1998) is capable of terminating this attraction (Ratcliffe et al., 2008).
Another molecule that may contribute to regulation of the development of vagal gastric afferents is brain-derived neurotrophic factor (BDNF). BDNF, through activation of its high affinity receptor, trkB (Barbacid, 1994; Rodriguez-Tebar et al., 1992), is essential for the survival of a large proportion of vagal sensory neurons during development. BDNF and trkB homozygous mutants have up to 66% and 94% loss of neurons from the nodose-petrosal ganglion complex, respectively (Conover et al., 1995; Erickson et al., 1996; Ernfors et al., 1994; Jones et al., 1994; Luikart et al., 2003). In the intervening fifteen years since these findings, a GI target organ/tissue innervated by BDNF-dependent vagal sensory neurons has yet to be identified. The available evidence, described below could imply that BDNF supports survival of stomach innervation. Virtually all vagal sensory neurons (97%) die during development in BDNF, neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4) triple knockout mice (Liu and Jaenisch, 2000), which could imply that at a minimum one of these neurotrophins must support innervation of the stomach. Moreover, Fox et al. (2001b) found that most IGLEs supplying the small intestine are dependent on NT-4 for survival, and Raab et al. (2003) established that esophageal IGLEs depend on NT-3. Also consistent with BDNF supporting survival of gastric vagal afferents, BDNF is expressed in the outer muscle layers and lamina propria of the corpus and antrum of the developing stomach (Fox and Murphy, 2008), and trkB is expressed by developing no dose ganglion neurons (Ernfors et al., 1992; Huang et al., 1999; Huber et al., 2000).
To examine the hypothesis that BDNF is essential for survival of vagal afferent stomach innervation, several gastric vagal elements were quantitatively and qualitatively compared in wild-type mice (bdnf +/+) and mice lacking one (bdnf +/−) or both (bdnf−/−) copies of the bdnf gene at P0 and at P3-6. Additionally, since BDNF is involved in other aspects of sensory neuron development, including axon growth and guidance (Fass et al., 2004; Fritzsch et al., 2005; Krimm, 2007; Lykissas et al., 2007; Paves and Saarma, 1997) and differentiation (McFarlane, 2000; Miyamoto et al., 2006), we also investigated whether BDNF contributes to these aspects of gastric vagal sensory development.
METHODS
Animals
Mice heterozygous for a targeted mutation of bdnf were obtained from Jackson Laboratories(Bar Harbor, ME; stock no. 002266). The bdnf gene was inactivated using homologous recombination to replace a portion of exon 5, which contains the entire protein coding sequences, with the neo gene and associated phosphoglycerate kinase promoter and polyadenylation signals (Ernfors et al., 1994). This deletion left 49 N- and 30 C- terminus amino acids. BDNF mRNA in the cerebral cortex, which exhibits high levels of BDNF expression, was decreased in heterozygous mutants relative to wild types, and was absent in homozygous mutants. Mice had ad lib access to food (Laboratory Rodent Diet 5001, PMI Nutrition International, Saint Louis, MI, USA) and water, temperature was maintained at 22–24°C, and a 14:10 light/dark cycle was employed. Mice were genotyped by polymerase chain reaction using the following primer sequences: BDNF1: CAT GAA AGA AGT AAA CGT CCA C, BDNF2: CCA GCA GAA AGA GTA GAG GAG; BDNF-PGK: GGG AAC TTC CTG ACT AGG GG. All procedures were conducted in accordance with Principles of Laboratory Animal Care (NIH publication No. 86-23, revised 1985) and American Association for Accreditation of Laboratory Animal Care guidelines and were approved by the Purdue University Animal Care and Use Committee.
Mating
Heterozygous bdnf mutant mice were bred to obtain wild-type (bdnf +/+), and heterozygous (bdnf+/−) or homozygous (bdnf−/−) mutant mice. Cages were checked daily at 1700 h for births and litters born that day were designated as P0 and harvested. Additionally, several litters were not harvested until P3-6, and with the exception of one litter, only the rare litters that contained a homozygous bdnf−/ − mutant were studied.
Tissue Preparation
At P0, P3, P4, or P6, pups were deeply anesthetized with methohexital sodium (Brevital Sodium, Eli Lilly, Indianapolis, Indiana; 100 mg/kg) and perfused transcardially at 3 ml/min with warm saline for 5 min. For cuprolinic blue staining this was followed by ice-cold 4% paraformaldehyde (PF) in a 0.1M phosphate buffered saline (PBS, pH 7.4) at 3 ml/min for 30 min, and then tissues were stored 3 d at 4°C in 4% PF in PBS. For DiI nerve labeling the 4% PF in PBS contained 0.1% ethylenediamine tetraacetic acid (EDTA). EDTA was added to retard the diffusion of DiI out of membranes and therefore increase the specificity and longevity of the DiI label (Hofmann and Bleckmann, 1999; Murphy and Fox, 2007).
Experiment 1: Effects of BDNF deficiency on gastric vagal innervation at P0 and P3-6
DiI Application
After 3 d fixation, DiI was used to label vagal fibers and terminals in the stomach of bdnf +/+ (P0, n=12, P3, n=2; P4, n=2; P6, n=1; total P3-6, n=5), bdnf +/− (P0, n=12, P3, n=2; P4, n=3; P6, n=1, total P3-6, n=6), and bdnf−/ − (P0, n=11, P3, n=1; P4, n=2; P6, n=0; total P3-6, n=3) mice as previously described (Murphy and Fox, 2007). Briefly, the stomach, esophagus and associated vagal trunks and branches were exposed and one DiI crystal (D-3911, Invitrogen Corporation, Carlsbad, CA) was applied at the bifurcation of the hepatic and gastric vagal branches and then crushed into the nerve fibers (Fig. 1A). Precautions were taken to prevent redistribution of DiI crystal fragments as previously described (Murphy and Fox, 2007). Tissue was stored in 4% PF, 0.1% EDTA in PBS for 4 wk (P0), 5 wk (P3, P4), or 6 wk (P6) light protected at 37°C.
Fig. 1.
(A) A schematic diagram that illustrates the DiI (1, 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) application site. A single DiI crystal was applied to the anterior vagal trunk at the bifurcation of the hepatic and gastric branches as they course along the abdominal esophagus. This location was determined to be optimal for comprehensive labeling of vagal innervation of the stomach wall (Murphy and Fox, 2007). (B) A schematic diagram adapted from Swithers et al. (2002) that illustrates where the estimated boundaries of three major stomach compartments were located in early postnatal mice and how the sampling sites were distributed among them. The antrum included columns 1–3 (light shading), the corpus columns 4–6 (dark shading), and the forestomach columns 7–10 (medium shading). (C) A schematic diagram that illustrates the sampling and counting method modified from Wang and Powley (2000) and Fox et al. (2000). Based on length and width of each ventral stomach wall sampled, eighty sampling points were established at the intersections of a sampling grid that consisted of 8 equidistant rows and 10 equidistant columns. The microscope objective was systematically moved from one intersection to the next, and the underlying tissue imaged. Then a counting grid was laid over each successive optical section of the z-series collected at each sampling site and used to quantify vagal fibers, bundles and terminals. Sampling points with less than half of the imaged area containing stomach tissue were not included in the quantification and are represented by gray circles in the sampling grid portion of the schematic.
Vagotomy
To verify the vagal origin of DiI labeled elements in the stomach wall, the anterior vagal gastric branch was sectioned between the DiI application site and the stomach immediately after DiI application, bdnf +/+, bdnf +/−, and bdnf−/− (P0, n=2/gp; P3-6, n=1/gp at each age; P3-6 total, n=9).
Tissue Preparation
The stomach along with the pylorus, esophageal sphincter, and associated vagal branches were dissected out, and any milk remnants removed by rinsing with PBS. Stomachs were prepared as wholemounts, separated into dorsal and ventral walls by making a longitudinal cut along the lesser and greater curvatures, and mounted directly in PBS with 0.5% sodium azide, mucosal side facing down on glass microscope slides, coverslipped, sealed with nail polish, and stored (light protected) at 4°C until imaged.
Microscopy
Specimens were first briefly examined using Leica DM5000 B microscope with filter cube Y3 to determine whether the DiI labeling of vagal fibers and terminals met several criteria required for inclusion in quantitative analyses: (1) A significant proportion of vagal fibers and terminals in all stomach compartments were labeled with DiI, and this labeling extended distally as far as the greater curvature and pylorus. (2) DiI labeling remained stable during transport and analysis, i.e. DiI did not leak out of vagal fibers. (3) DiI did not redistribute from the injection site to produce non-specific labeling. Three specimens (1 P0 bdnf +/+, 1 P0 bdnf +/−, and 1 P4 bdnf +/−) failed to meet all of these criteria and were not further analyzed. DiI fluorescence (460 nm excitation, 565 nm emission) was then visualized with standard rhodamine filters and optical sections obtained using an Olympus BX-DSU spinning disk confocal microscope equipped with a 60X water immersion objective (total magnification = 600X). Processing of three-dimensional image data was performed using Slidebook (v.4.1, Intelligent Imaging Innovations, USA). To control for fluorescence fading, all specimens were imaged within 5 h of initial inspection after removal from incubation and each was sampled in the same sequence.
Identification of Vagal Fibers, Bundles and Terminals
Criteria that have been published for identifying mature forms of vagal fibers, bundles, efferent terminals, IGLEs and IMAs were used to identify neonatal vagal fibers, bundles, efferent terminals and putative IGLE and IMA precursors as previously described (Murphy and Fox, 2007). Briefly, identification of IGLE and IMA precursors was possible because they exhibited some or all of the unique features of the mature mechanoreceptor forms, although the receptors were smaller in size and their nerve terminal components were less numerous or less dense. Also, the changes in their form over the course of development were characterized by selecting examples at P8 that could be matched to mature profiles and working backwards sequentially one day in age at a time to the ages utilized in the present study and as early as E16.
Criteria for identification of single fibers
(1) Consists of a single DiI-labeled axon coursing through the myenteric plexus, (2) not contained within a vagal fiber bundle.
Criterion for identification of an axon bundle
Consists of two or more DiI-labeled axons coursing through the myenteric plexus in parallel and in close apposition.
Criteria for identification of putative IGLE precursors
Consists of (1) a laminar (2) aggregate of fine terminal puncta (3) within the neuropil of a myenteric ganglion and (4) covering all or part of the ganglion (Berthoud et al., 1995; Neuhuber, 1987; Rodrigo et al., 1982; Rodrigo et al., 1975). For the present purposes, criterion (4) was determined by locating terminal processes in either the z-plane immediately above or below the planes containing myenteric neurons, the myenteric plexus, and vagal efferent terminals.
Criteria for identification of putative IMA precursors
Consists of (1) an array of parallel axonal telodendria (2) in close proximity, (3) interconnected by bridging axonal elements and (4) located in either the longitudinal or circular muscle layer (Berthoud and Powley, 1992; Fox et al., 2000; Wang and Powley, 2000). For the present purposes, criterion (4) was determined by locating terminal processes in the series of z-planes that included longitudinal or circular muscle layers either above or below the planes containing the myenteric plexus, respectively.
Criteria for identification of putative efferent terminal precursors
Consists of (1) a network of characteristic rings of fiber arborizations and terminal complexes (2) circling the myenteric neurons (3) within the myenteric ganglia (Holst et al., 1997; Kirchgessner and Gershon, 1989; Phillips et al., 2003).
Sampling and Quantification
To assess innervation throughout the stomach wall, a sampling grid was used to locate equidistant sampling sites. The sampling grid established a proportional scale that normalized each wholemounted stomach so that the variability due to differences in stomach size or distention was minimized (Fig. 1C). At each sampling location, a counting grid was used to quantify each labeled vagal element as described below. Counts were made blind to the genotype of the specimen and independent counts of a small subset of the specimens were made by a second investigator to validate the identification criteria. There were no significant differences in estimations of any quantified vagal elements observed between counters (not shown).
At each sampling point a confocal microscope was used to scan a series of optical sections through the entire thickness of the stomach wall, which ranged from 20–150μm in thickness, at 1 μm intervals. Each optical section was 147μm × 114μm in size (.01676 mm2 in area). Eighty sites were sampled for each stomach wall, covering about 6% of the stomach surface area in P0 stomachs and 4% in P3-6 stomachs, which appeared to sample a consistent portion of the vagal innervation in each stomach, and thus provided an acceptable baseline for assessing changes in mutants.
The counting grid consisted of six equidistant rows and eight equidistant columns that spanned the entire width and height of an optical section and were drawn on transparency film paper that was attached to the computer screen where the optical sections were viewed. The location of each fiber on the counting grid was used to register positional information about individual fibers onto grid paper by assigning successive rows on the grid paper to successive optical sections (based on Cheng et al., 1997). In this way, each fiber was tracked along its 3-dimensional (3D) path so as to avoid counting a fiber more than once. Axons were then counted starting with the first section and then sequentially through each section. Using this technique, Cheng et al., (1997) were able to distinguish fibers separated by as little as 1 μm. The density of individual fibers and IMA telodendria were quantified by applying this method, and counting the total number of gridline crossings that occurred. Numbers of IGLEs (aggregates of terminal puncta, or leaves) were estimated by counting each individual IGLE leaf identified within each sequential image (optical section) at each sampling site. Counts of vagal efferent terminals and axon bundles were made from 2D projections of the 3D series of optical sections collected at each sampling site. Each myenteric neuron encircled by DiI-labeled fibers was counted as one vagal efferent terminal in this 2D projection. Also, each vagal bundle present in a 2D projection was counted.
For statistical comparisons, data collected from sampling points throughout the stomach wall were also grouped by location within the forestomach, corpus, or antrum (Fig. 1B). These regions were established by dropping two vertical lines, one from each side of the outer surface of the lower esophageal sphincter resulting in the antrum consisting of columns 1–3, the corpus columns 4–6, and the forestomach columns 7–10.
Statistical analysis and illustration
Results of quantification of fibers, endings and bundles are expressed as means and standard errors. These data were represented in graphs created using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Statistical analyses for all groups were carried out by one-way analysis of variance (ANOVA), followed by FSD-tests for normality and Tukey’s post-hoc tests using Statistica 6.0 (StatSoft, Inc., Tulsa, OK). Photoshop software (version 6.0 Adobe Systems, Mountain View, CA) was used to a) apply scale bars and text, b) adjust brightness and contrast, and c) organize the final layouts.
Experiment 2: Effect of BDNF deficiency on gastric myenteric neurons at P0
A cuprolinic blue stain for enteric neurons in adult rodent GI wholemounts (Holst and Powley, 1995) was adapted to label these neurons in P0 mouse stomachs (bdnf +/+, bdnf +/−, and bdnf−/−; n=5/gp). Cuprolinic blue was chosen due to its greater completeness and specificity for staining myenteric neurons as compared with other methods (Heinicke et al., 1987; Karaosmanoglu et al., 1996). Cuprolinic blue (quinolinic phthalocyanine, Polysciences, Warrington, PA) stain was prepared by dissolving it in 0.05 M sodium acetate buffer (0.5% w/v, pH 4.9) and filtering it (0.22 μm; Millipore, Billerica, MA).
Tissue Preparation
Stomachs were prepared as wholemounts (as described above for preparation of tissue with DiI labeled elements) and the outer smooth muscle layers were separated from the mucosal and submucosal layers using #7 curved fine forceps and placed in a drop of cuprolinic blue on a glass microscope slide. The tissue was then covered with a piece of parafilm and stained for 1 h in a humidified slide-warming tray at 40 °C. After staining, wholemounts were drained and rinsed in distilled water, differentiated in 0.05M sodium acetate buffer (pH 5.6) and rinsed in 0.02M KPBS. Tissue was mounted on slides, air dried, dehydrated in a series of graded alcohols (70%, 95%, 2 × 100%; 2 min each), cleared in xylene (2 × 2 min), and then coverslipped with Cytoseal.
Imaging, Sampling, and Quantification
Myenteric neurons stained with cuprolinic blue were visualized with brightfield illumination using an Olympus BX-DSU microscope. For counting myenteric neurons, the same magnification and sampling grid described above for counting DiI-labeled vagal fibers and terminals were used so these two sets of counts were obtained in as similar a manner as possible. At each sampling location all stained cells within the field of view were counted. Individual myenteric neurons are distinct – with blue stained Nissl substance clearly outlining the cytoplasm (which is unstained) and a stained nucleolus. Myenteric glial cells are not known to be stained by this method, so all of the stained cells were considered neuronal. Sampling points with excessive folds or tears in the tissue were excluded. For each stomach wall sampled, myenteric neuron numbers counted at each sampling site were totaled, and those from sampling sites within the antrum, corpus and forestomach compartments totaled separately. These data were represented and statistically analyzed as described for Experiment 1. Images were acquired using a Leica DM5000 B microscope, a video camera (Spot RT Slider; Diagnostic Instruments, Inc., Sterling Heights, MI) and SpotSoftware (v4.5, Diagnostic Instruments) and adjusted using Photoshop software as in Experiment 1.
RESULTS
Experiment 1: Effects of BDNF deficiency on gastric vagal innervation at P0
Vagal Innervation in the stomach of wild-type mice at P0
DiI-labeled vagal fibers and terminals in the P0 stomach revealed a dense pattern of innervation reaching from the gastric branches traversing the lower esophagus to the greater curvature of the stomach. Dense innervation of the myenteric plexus included individual vagal fibers, bundles, putative IGLE precursors (Fig. 2A,B) and efferent terminals (Fig. 3). Putative IMA precursors innervated the circular and longitudinal smooth muscle layers with telodendria orientated parallel to one another and to muscle fibers as previously described for adult IMAs (Fig. 2C–E). Vagal innervation was also observed within the mucosa and submucosa, consisting mostly of fibers and free endings (Fig. 2F). The myenteric plexus in the antrum was more dense and tightly organized than in the forestomach (cf. Fig. 3A,B). Also, axon bundles were larger in the corpus (Fig. 3C) than in the antrum or forestomach probably because most bundles arising directly from the esophageal gastric branch initially traversed the corpus.
Fig. 2.
Putative Intraganglionic laminar ending (IGLE; A,B) and intramuscular array (IMA; C,D,E) precursors and mucosal/submucosal processes and terminals (F) labeled with DiI in the stomach wall of wild-type P0 mice. (A) A confocal image illustrating putative IGLE precursors (in outlined area) in the forestomach. At this age IGLEs are small and often consist of only a few terminal processes that vary from small puncta to relatively large growth cone-like structures. (B) Higher magnification image of the area outlined in A that consists of optical sections restricted to a tissue plane immediately below a myenteric ganglion that contains the putative IGLE leaves (arrows). (C) A confocal image taken from the forestomach that includes optical sections through the longitudinal muscle, myenteric plexus and circular muscle layers that contain IMAs and myenteric vagal axons and fiber bundles. (D) A confocal image of the same region as in C, but optical sections were restricted to the circular muscle layer containing putative IMA precursors (arrows). (E) A confocal image of the same region as in C and D, but optical sections were restricted to the longitudinal muscle layer containing putative IMAs (arrows). (F) Vagal fibers (arrows) and free endings (arrowheads) with imaging restricted to the mucosal and submucosal layers of the forestomach. Scale bars = 25 μm (A, C–F) or 10μm (B).
Fig. 3.
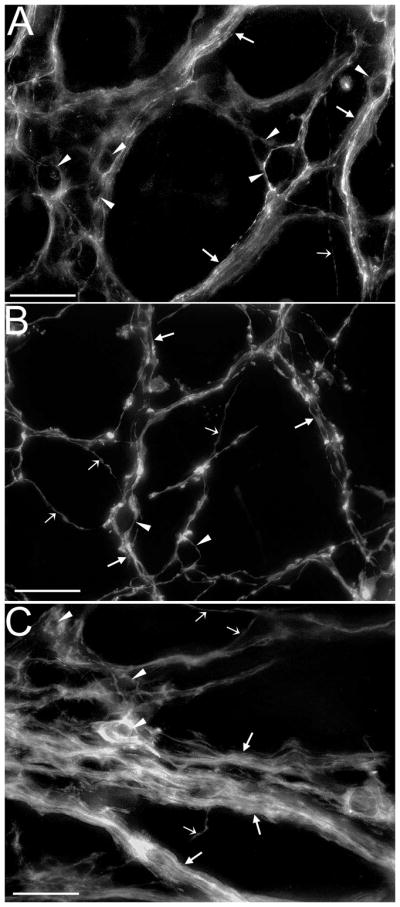
The organization of vagal fibers and myenteric axon bundles is different in each of the three major stomach compartments at P0. (A–C) Confocal images of DiI labeled vagal bundles (arrows), fibers (smaller open arrows), and efferent terminals in myenteric ganglia (arrowheads) within the antrum (A), forestomach (B), and corpus (C). In the antrum fiber bundles were large in diameter and along with efferent terminals were tightly packed, whereas in the forestomach axon bundles were smaller in diameter and along with fibers were more loosely organized. Organization of fibers, axon bundles and efferents and in the corpus was similar to the forestomach, but additionally large diameter bundles extended from the cardia. Scale bars = 25μm.
Quantification of DiI-labeled elements in wild-type mice, including individual vagal fiber density, fiber bundle number, putative IGLE numbers, IMA density and efferent terminal number yielded no significant differences among the three stomach compartments (Suppl. Fig. 1A–E; longitudinal and circular IMAs shown separately in Fig. 4D, open bars).
Fig. 4.
Quantitative comparisons of DiI-labeled vagal elements among wild types and heterozygous or homozygous bdnf mutants at P0. Elements were quantified over the entire ventral stomach wall and included axon bundle number (A), individual fiber density (B), putative IGLE precursor number (C), putative IMA precursor density (D), and efferent terminal number (E). IMA density in D plots circular and longitudinal IMAs separately and combined (total); the histogram bar shading represent the genotypes in a similar manner as in the other panels. The only significant difference was a 50% reduction of putative IGLEs in bdnf −/− mice compared with wild types. * Significantly different from wild type, p<0.05.
Vagal Innervation in the stomach of BDNF-deficient mice as compared with wild types at P0
Visual inspection of confocal images from sampled sites revealed two striking qualitative differences in vagal innervation of the stomach in bdnf −/− mice compared to wild types that were not evident in statistical comparisons. First, vagal innervation in portions of the antrum appeared more dense and disorganized than in either bdnf +/+ (cf. Fig. 5A,B; cf. also Fig. 6C,D in Fox and Murphy, 2008) or bdnf +/− mice. These changes appeared to result from an increase in putative growth cones found in the myenteric plexus and a decrease in space between fiber bundles and ganglia. This decrease may have resulted from a change in the arrangement of axon bundles, and an increase in their diameter. This altered organization was apparent in approximately 21% of sampled sites distributed throughout the antrum. The second difference occurred in the forestomach of bdnf −/− mice, where the processes of putative IMAs appeared shorter in length and larger in diameter than those present in either bdnf +/+ (cf. Fig. 5C,D) or bdnf +/− mice. This occurred in an average of 20% of sampled sites containing IMAs in the forestomach for all bdnf −/− mice, ranging in severity from 10–30%.
Fig. 5.
At P0, bdnf KO was associated with altered vagal innervation of the antrum (A,B) and changes in the morphology of developing putative IMAs in the forestomach (C,D). (A,B) In bdnf KO mice vagal innervation of the antrum appeared to become disorganized as compared with wild types and heterozygous mutants. In particular, spacing between myenteric ganglia was reduced and there was an increase of growth cone-like structures. Confocal images illustrating the normal organization of vagal fibers, bundles and ganglia in the antrum of a wild-type mouse (A), and the apparent disorganization of these elements in a bdnf KO mouse (B). (C) Confocal image that includes optical sections restricted to the circular muscle layer, demonstrating normal IMA structure (arrows) observed in the forestomach of a wild-type mouse. Normal IMA telodendria are long rectilinear processes. (D) Similarly restricted confocal image as in C, illustrating the shorter, larger diameter IMA telodendria (arrows) present in some regions of the forestomach of a bdnf KO mouse. Scale bars = 25μm.
Quantification of numbers of vagal elements sampled across the entire stomach wall in bdnf +/+, bdnf +/−, and bdnf −/− mice revealed a nearly 50% decrease in putative IGLE numbers in homozygous mutants as compared with wild types (p<0.05; Fig. 4C). In contrast, numbers of vagal bundles and efferent terminals, and densities of individual fibers and putative IMA precursors did not differ across genotypes (Fig. 4A,B,D,E).
Comparisons of quantified elements of vagal innervation grouped by stomach compartment were not significantly different between bdnf +/+, bdnf +/−, and bdnf −/− mice (Suppl. Fig. 2A–E).
Vagotomies performed in bdnf +/+, bdnf +/−, and bdnf −/− mice to verify vagal origin of DiI labeling prevented DiI diffusion in vagal axons from reaching the stomach in all cases, resulting in no DiI-labeled fibers observed (not shown).
Experiment 1: Effects of BDNF deficiency on gastric vagal innervation at P3 - 6
To assess whether the effects of BDNF deficiency observed at P0 persisted, recovered or worsened at later stages of development, DiI-labeled vagal innervation of the stomach of mice aged P3-6 was examined. Mice of several ages were combined because most homozygous bdnf −/− mutants die shortly after birth, making it difficult to obtain them at older ages.
Vagal Innervation in the stomach wall of wild-type mice at P3-6
DiI labeled vagal fibers and terminals in the P3-6 stomach revealed a dense pattern of innervation similar to that seen at P0. However, it was more mature in appearance, exhibiting more numerous vagal fibers and bundles. Also, putative IMAs, IGLEs and efferent terminals had more mature structures than was observed at P0 (Figs. 2,3 vs. 6,7). For example, myenteric vagal axon bundles appeared to be separated by greater distances, which may have been due in part to the growth of the stomach from P0 to P3-6, and IGLEs were larger in size, which made their laminar organization more evident (Fig. 6A,B). The putative IMAs that innervated the smooth muscle layers at P3-6 exhibited more numerous telodendria that were greater in length than at P0 (circular and longitudinal; Fig. 6C–E). Vagal innervation was also observed within the mucosal and submucosal layers (Fig. 6F). Among the three defined stomach compartments, qualitative differences observed at P0 were also present. The myenteric plexus in the antrum was much more dense and tightly organized than it was in the forestomach (cf. Fig. 7A,B). Also, larger bundles were observed in the corpus, as they descended from the esophagus, than were found in the antrum or forestomach (Fig. 7C).
Fig. 6.
Putative IGLE (A,B) and IMA (C,D,E) precursors and mucosal/submucosal processes and terminals (F) labeled with DiI in the stomach wall of wild-type P3-6 mice. (A) A confocal image illustrating putative IGLE precursors (in outlined area) in the forestomach. At this age IGLEs were slightly larger than at P0 and often began to take on a more laminar appearance, but otherwise had not changed significantly in structure. (B) Higher magnification image of the area outlined in A that consists of optical sections restricted to the tissue plane that contained the putative IGLE leaves, which was immediately below a myenteric ganglion. (C) A confocal image taken from the forestomach that includes optical sections through the longitudinal muscle, myenteric plexus and circular muscle layers and thus contains putative IMAs and vagal axons and fiber bundles. At this age IMA telodendria appeared to have increased in number and length as compared with P0. (D) A confocal image of the same region as in C, but optical sections were restricted to the circular muscle layer containing putative IMA precursors (arrows). (E) A confocal image of the same region as in C and D, but optical sections were restricted to the longitudinal muscle layer containing putative IMAs (arrows). (F) Vagal fibers (arrows) with imaging restricted to the mucosal and submucosal layers of the forestomach. Scale bars = 25μm (A, C–F) or 10μm (B).
Fig. 7.

The organization of vagal fibers and myenteric axon bundles was different in each of the three major stomach compartments at P3-6. (A–C) Confocal images of DiI labeled vagal bundles (arrow), fibers (open arrow), and efferent terminals in myenteric ganglia (arrowheads) within the antrum (A), forestomach (B), and corpus (C). Although the vagal innervation pattern in each stomach compartment had matured slightly by P3-6 as compared with P0, the differences described between compartments at P0 were largely maintained at P3-6. Scale bars = 25μm.
Quantification of DiI-labeled elements of vagal stomach innervation yielded significant differences in IMA density and axon bundle number among the three stomach compartments in wild-type mice. There was a 97% increase in putative IMA density in the forestomach as compared with the antrum and 123% as compared with the corpus (p<0.05; Fig 8D). Also, there were 53% more fiber bundles in the forestomach than the antrum (p<0.05; Fig. 8A). In contrast, there were no differences observed for individual axon densities, or putative IGLE and efferent terminal numbers (Fig. 8B,C,E).
Fig. 8.
Quantitative comparisons of DiI-labeled vagal elements among the antrum, corpus and forestomach of wild-type mice at P3-6. The quantified elements included axon bundle number (A), individual fiber density (B), putative IGLE precursor number (C), putative IMA precursor density (D), and efferent terminal number (E). The forestomach contained more fiber bundles than the antrum (*, p<0.05). Moreover, the approximate doubling of IMA density in the forestomach as compared with the corpus and antrum was significant for both of these compartments (#, p<0.05).
Vagal Innervation in the stomach of BDNF-deficient mice as compared with wild types at P3 - 6
The abnormal organization of vagal innervation observed qualitatively in the antrum of bdnf −/− mice at P0 was still present at P3–6, but to a lesser degree (cf. Fig. 9A,B). In contrast, the aberrant morphology of putative IMA precursors in the forestomach of mice at P0 was still present at P3-6. While some IMAs in P3-6 bdnf −/ − specimens were similar to wild types, having longer, more mature processes than at P0, others had stunted telodendria compared to either bdnf +/+ or bdnf +/− mice (cf. Figs. 6E and 9C).
Fig. 9.
At P3-6, bdnf KO was associated with altered vagal innervation of the antrum (A,B) and changes in the morphology of developing putative IMAs in the forestomach (C). In bdnf KO mice vagal innervation of the antrum still appeared to be disorganized and denser compared with wild types and heterozygous mutants, but to a lesser degree that observed at P0. (A,B) Confocal images illustrating these differences between the antrum of a wild-type mouse (A), and a bdnf KO mouse (B). Also, in P3-6 bdnf KO mice, some IMA telodendria were stunted, but normal diameter. (C) Confocal image that includes optical sections restricted to the longitudinal muscle layer, illustrating the shorter IMA telodendria (arrows) observed in the forestomach of a bdnf KO mouse. To compare these IMAs with those of a wild type see Fig. 6E, which is a similarly restricted confocal image from a wild-type at this age. Scale bars = 25μm.
Quantification of DiI-labeled vagal innervation in P3-6 bdnf +/+, bdnf +/− and bdnf −/ − mice over all the sampling sites of the stomach wall did not identify significant differences between these genotypes for any of the vagal elements studied (Suppl. Fig. 3A–E). Moreover, quantitative comparisons of vagal innervation among bdnf +/+, bdnf +/− and bdnf −/ − mice within each stomach compartment did not reveal significant differences for any of the DiI-labeled vagal elements examined (Suppl. Fig. 4).
Similar to vagotomies performed at P0 in bdnf +/+, bdnf +/−, and bdnf −/ − mice to verify vagal origin of DiI labeling, those done for each genotype at P3-6 also prevented DiI diffusion in vagal axons from reaching the stomach in all instances (not shown).
Experiment 2: Effect of BDNF deficiency on gastric myenteric neuron numbers at P0
The total number of cuprolinic blue-stained myenteric neurons (Fig. 10A) counted in the stomach muscle wall of bdnf +/− or bdnf −/ − mice were not significantly different from the total in wild-type mice (Fig. 10B). However, the total number for the bdnf −/ − group was significantly greater than for the bdnf +/− group (p< 0.05). Analysis of neuron counts by compartment revealed that a similar group difference occurred, but it was restricted to the antrum (p<0.05; Fig. 10C).
Fig. 10.
Analysis of gastric myenteric neurons. (A) Brightfield photomicrograph of a myenteric ganglion in the stomach muscle wall of a wild-type P0 mouse stained with cuprolinic blue (three neuron cell bodies are indicated by arrows). The differentiation of the stain from background was not as prominent as in the adult GI muscle wall, but was sufficient to identify neurons according to the criteria. Scale bar = 25μm. (B,C) Quantification of myenteric neurons stained with cuprolinic blue at P0 among wild types and heterozygous or homozygous bdnf mutants, grouped by whole stomach (B) or by stomach compartment (C; antrum, corpus and forestomach). The histogram bar fills represent the genotypes in a similar manner as in B. When grouped by whole stomach neither heterozygous nor homozygous mutants were different from wild types (B). However, the decreased counts in heterozygotes compared with wild types were significantly different from the increased counts in homozygotes. This difference was mainly due to the significant difference between these groups in the antrum (C). # Significant difference between heterozygous and homozygous bdnf mutants at p<0.05 level.
DISCUSSION
DiI labeled vagal sensory innervation of the early postnatal stomach wall was compared in bdnf knockout (KO) mice and their heterozygous and wild-type littermates to investigate whether BDNF may be involved in their survival, differentiation, or axon growth or guidance. In wild-type mice at P0, vagal fibers, bundles, efferent terminals and putative IGLE and IMA precursors were present throughout the stomach wall. However, the adult distributions of IMAs and IGLEs were not evident and their morphology was immature. By P3-6 the morphology of IMAs and IGLEs was slightly more mature and although IMAs had begun to concentrate in the forestomach as in adults, the IGLE distribution still had not matured. Examination of this innervation in bdnf KO mice at P0 surprisingly revealed the presence of a significant amount of vagal stomach wall innervation. Nevertheless, bdnf KO resulted in the loss of about 50% of IGLEs compared to wild types without affecting the number or distribution of their target cells, myenteric neurons. Additionally, bdnf KO was associated with truncated telodendria of some forestomach IMAs and abnormal organization of vagal innervation in portions of the antrum. At P3-6, in bdnf KO mice IGLE number had recovered to wild-type levels, the disorganization of antrum innervation was less severe than at P0, and some forestomach IMAs still exhibited abnormal morphology. The reduced IGLE number at P0 could have resulted from loss of BDNF support of their survival, or of their axon’s growth or guidance required for them to the reach the stomach wall. Alternatively, recovery of IGLE numbers by P3-6 could imply that the main effect of BDNF deficiency was to delay IGLE development. Loss of axon guidance function of BDNF could also have contributed to the disorganized antrum innervation and loss of BDNF support of neuronal differentiation could have affected IMA formation in the forestomach.
There was no effect of bdnf KO on myenteric neuron and vagal efferent terminal numbers as compared with wild types, suggesting the effects of BDNF deficiency on developing vagal gastric afferents were specific, and were not secondary to loss of IGLE target cells. However, these findings do not exclude the possibility that the primary effects of BDNF deficiency were on structures closely associated with vagal afferents such as sympathetic gastric innervation, or gastric myenteric axons or terminals. The increased numbers of myenteric neurons in the stomachs of bdnf −/− as compared with bdnf +/− mice were perplexing. However, they were counted blind and a recount yielded the same result.
Organization of vagal stomach innervation in wild-type mice at early postnatal ages
Previously, it has been suggested that development of vagal innervation is relatively complete at birth (e.g., Rinaman, 2006). In the stomach wall of adult rats and mice, IMAs are concentrated in the forestomach, and IGLEs are found throughout with peak density occurring in the corpus (Fox et al., 2000; Wang and Powley, 2000). However, shortly after birth in the present study, IMAs and IGLEs were equally distributed across compartments, suggesting their distributions were immature. In contrast, the distribution of myenteric neurons across stomach regions at P0 did appear mature as it was similar to that reported for adult male Fischer 344 rats (Phillips and Powley, 2001). At P3-6, IMA density was significantly greater in the forestomach than in the antrum or corpus, suggesting the adult distribution had begun to form, whereas IGLEs were equally dispersed across stomach compartments. These results suggest that growth, maturation, and differentiation of vagal mechanoreceptors continue after birth, consistent with previous qualitative observations made at several embryonic and postnatal ages (Murphy and Fox, 2007).
BDNF may regulate survival, or axon growth or guidance of gastric IGLEs
The reduced density of gastric IGLEs in BDNF-deficient mice at P0 could have resulted because BDNF is required for their survival during development. BDNF has been established as a survival factor for vagal sensory neurons (Conover et al., 1995; Jones et al., 1994; Liu et al., 1995). Moreover, it would be consistent with the apparent dependence of IGLEs in other GI organs on the neurotrophins NT-3 and NT-4 for survival (Fox et al., 2001b; Raab et al., 2003). Loss of IGLEs occurred in all stomach compartments, but BDNF is only expressed in the developing antrum and corpus (Fox and Murphy, 2008). Thus, if BDNF supports forestomach IGLE survival, the BDNF source could have been the nodose ganglion, or blood vessels that supply the stomach (Ernfors et al., 1992; Fox and Murphy, 2008).
An alternative possibility is that BDNF is required en route between the nodose ganglion and the gut to guide vagal sensory axons, or promote their growth toward the stomach wall. Consistent with this possibility, at embryonic ages when vagal axons grow along blood vessels toward the stomach, BDNF is expressed in blood vessels that supply the stomach wall (Fox, 2006; Fox and Murphy, 2008). NT-3 expressed by blood vessels appeared to provide a similar function for the sympathetic innervation of some organs (Kuruvilla et al., 2004). Consistent with this interpretation, several studies have suggested BDNF has both axonal growth and guidance properties. In vitro studies have demonstrated an ability of BDNF to produce neurite outgrowth from cultured neurons, including sensory ganglia (Li et al., 2005; Rochlin et al., 2000; Song et al., 2008; Ulmann et al., 2007), whereas in vivo manipulations of BDNF levels have shown that BDNF may be necessary for axons to find and enter a target tissue (Ernfors et al., 1994; Fritzsch et al., 2005; Hellard et al., 2004; Krimm, 2007; Ma et al., 2009). Distinguishing the contributions of BDNF survival and axon guidance functions to reduced gastric IGLE number in bdnf KO mice will require examination of vagal afferents at earlier stages of development.
Although the loss of IGLEs at P0 in BDNF KO mice may reflect loss of BDNF survival or guidance function, another possibility raised by the recovery of IGLE loss between P0 and P3-6 is that the main effect of BDNF deficiency was to delay the normal developmental timetable of some gastric IGLEs. One mechanism that could account for such a delay would be that another factor expressed during development compensated for BDNF deficiency, preventing a more severe loss of putative IGLEs at P0, and supporting recovery from that loss by P3-6. A good candidate for such a factor is another neurotrophin, NT-3. Nt-3 KO results in a 41% loss of vagal sensory neurons and bdnf KO a 59% loss, but bdnf −/ −; nt-3 −/ − double KO’s only produce a 66% reduction (ElShamy and Ernfors, 1997). As this is only a 7% increase in addition to the loss produced by bdnf KO alone, it suggests there is large overlap of the vagal sensory neuron populations supported by BDNF and NT-3 (ElShamy and Ernfors, 1997). The potential for NT-3 to compensate for BDNF deficiency is further supported by the overlapping expression patterns of NT-3 and BDNF in the stomach wall, associated blood vessels, and nodose ganglion (Ernfors et al., 1992; Fox, 2006; Fox and Murphy, 2008), and by the capacity for NT-3 to activate trkB (Barbacid, 1994). Compensation for BDNF deficiency by NT-3 or other factors could be investigated by determining whether bdnf −/ −/nt-3 −/ − double KO (or double KO of BDNF and one of the other factors expressed in the stomach wall) resulted in greater IGLE loss as compared with bdnf KO alone (Erickson et al., 1996). If NT-3 or another molecule did compensate for the effects of BDNF deficiency on IGLE neuron survival or axon guidance, one way normal IGLE numbers could be achieved would be for the reduced number of IGLE axons present in the stomach wall to increase their branching or sprout additional axons with IGLE terminals. Consistent with this mechanism, Elshamy et al. (1996) found that even though a large proportion of sympathetic neurons failed to survive in NT-3 KO mice, some of the target organs exhibited normal innervation density, probably as a result of compensation by NGF or another factor supporting expansion of the innervation derived from the surviving neurons.
Further, If NT-3 or another factor did compensate for BDNF deficiency, the rare P3-6 bdnf KO mice might have survived beyond P0 because they had more effective compensation for BDNF deficiency than the more typical bdnf KO mice that die shortly after birth. Such a difference could have resulted from differences in background genes. If greater compensation had occurred in the P3-6 KO mice it might also account for the lack of effect of their BDNF deficiency on IGLE number and the reduced effect on antrum innervation as compared with P0 KO mice. Consistent with this possibility, a small percentage of P0 bdnf KO mice exhibited loss of IGLEs in the range observed at P3-6.
One final possibility that could account for the loss of only about half of the putative IGLE precursors in bdnf KO mice at P0 is the existence of IGLE populations with different phenotypes, some dependent on BDNF for survival, others not. Several analyses are consistent with this possibility. Gastric IGLEs exhibited a different morphology from small intestinal IGLEs (Jarvinen et al., 1998), NT-4 KO resulted in loss of small intestinal IGLEs, but not gastric IGLEs (Fox et al., 2001b), some gastric and esophageal IGLEs survive capsaicin treatment, whereas the remaining IGLEs in these organs and most in the intestine are lost (Berthoud et al., 1997b). Further, consistent with the existence of different stomach IGLE populations, mechanosensitive gastric afferents that expressed TRPV1, and thus were capsaicin-sensitive had a lower threshold for mechanical activation and responded to stomach stretch, whereas TRPV1-negative afferents had higher thresholds and did not respond to stretch (Bielefeldt et al., 2006).
BDNF regulation of vagal axon guidance could contribute to altered antrum innervation
Disorganized patterns of vagal innervation in some regions of the antrum involved abnormal arrangement of myenteric axon bundles and increased density of growth cones. Interestingly, the locus of this abnormal patterning coincided with the stomach region that expresses BDNF in the smooth muscle layers and the lamina propria of the mucosa (Fox and Murphy, 2008). Thus, if BDNF secreted from these tissues acted as a guidance factor or chemoattractant as proposed above for IGLE loss, its loss from the muscle wall could alter axon bundle formation. Further, BDNF loss from the lamina propria could have led to failure of axons to exit the muscle layer and enter the mucosa, resulting in sprouting of terminals and growth cones. Similar disruptions of normal gastric sensory vagal organization in the stomach have been observed in c-Kit and steel mutant mice that lack intramuscular interstitial cells of Cajal, the target cells of IMAs in the forestomach (Fox et al., 2002; Fox et al., 2001a). The disorganized vagal antrum innervation was probably not detected by quantitative measures because it did not occur at enough sampling sites, or because it involved more of a shift in location of fibers and axon bundles rather than a change in number. Also, the apparent increase in growth cones was not quantified because an antibody that specifically detected growth cones and was compatible with our DiI labeling method could not be identified.
BDNF may regulate differentiation of a subpopulation of IMAs
A subset of the putative IMA precursors in the forestomach of bdnf homozygous mutants at P0 and P3-6 displayed stunted telodendria, suggesting their growth was halted, or delayed at an early stage of formation. This was probably not detected as a decrease in IMA density because it did not occur at a sufficient number of sampling sites, or because compensatory growth of unaffected IMAs occurred.
This abnormal IMA morphology suggests BDNF may be required for specific aspects of differentiation of a subpopulation of IMAs, including growth of terminal processes. During early postnatal development BDNF is not expressed in the forestomach (Fox and Murphy, 2008), suggesting altered IMA structure may have resulted from loss of BDNF normally expressed in the nodose ganglion, or in the blood vessel walls along which IMA axons grow to reach the stomach (Ernfors et al., 1992; Fox and Murphy, 2008).
Previous work in other systems is consistent with the possibility of BDNF involvement in IMA differentiation or growth. In culture, BDNF has been shown to stimulate increases in the length and number of growth cone filopodia (Fass et al., 2004). Moreover, Miyamoto et al. (2006) found that BDNF influences cellular morphology by activating endogenous Rho GTPases, which results in alteration of the actin cytoskeleton. More recently, another group identified geranylgeranyl-transferase I (GGT), which is activated by BDNF and mediates prenylation, a lipid modification, as contributing to BDNF effects on the Rho GTPases (Zhou et al., 2008). Further, evidence from the somatosensory system, including mechanoreceptors – a receptor type that may share properties with vagal mechanoreceptors (Fox, 2006) – has demonstrated that BDNF can have powerful control of the structure of peripheral sensory nerve terminals. An in vitro study of embryonic DRG neurons (Lentz et al., 1999) found that BDNF significantly increased the terminal branching of subsets of these neurons. In vivo studies are consistent with this result since bdnf KO mice most often show decreases in size and density of the terminal fields of cutaneous receptors, including mechanoreceptors, whereas mice that overexpress BDNF in the skin show increases (LeMaster et al., 1999; Rice et al., 1998).
Supplementary Material
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: NS046716
Preliminary reports of the present findings were presented in abstract form at the annual meeting of the Society for Neuroscience (Murphy and Fox, 2007 and 2008), the IVth International Congress of the International Society for Autonomic Neuroscience (Fox and Murphy, 2007), and the Biannual Ingestive Behavior Research Center Symposium, Purdue University (Fox and Murphy, 2007), and in a review based on this presentation (Fox and Murphy, 2008). This manuscript was based in part on a dissertation submitted by M. Murphy in partial fulfillment of the requirements of the Ph.D. degree.
LITERATURE CITED
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25(11):1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191(3):203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol. 1997a;195(2):183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Willing AE, Mueller K, Neuhuber WL. Capsaicin-resistant vagal afferent fibers in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Res. 1997b;746(1–2):195–206. doi: 10.1016/s0006-8993(96)01222-x. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319(2):261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Zhong F, Koerber HR, Davis BM. Phenotypic characterization of gastric sensory neurons in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G987–997. doi: 10.1152/ajpgi.00080.2006. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ., 3rd A laser confocal microscopic study of vagal afferent innervation of rat aortic arch: chemoreceptors as well as baroreceptors. J Auton Nerv Syst. 1997;67(1–2):1–14. doi: 10.1016/s0165-1838(97)00085-4. [DOI] [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, Friedman B, Wiegand S, Vejsada R, Kato AC, DeChiara TM, Yancopoulos GD. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375(6528):235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- ElShamy WM, Ernfors P. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 complement and cooperate with each other sequentially during visceral neuron development. J Neurosci. 1997;17(22):8667–8675. doi: 10.1523/JNEUROSCI.17-22-08667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElShamy WM, Linnarsson S, Lee KF, Jaenisch R, Ernfors P. Prenatal and postnatal requirements of NT-3 for sympathetic neuroblast survival and innervation of specific targets. Development. 1996;122(2):491–500. doi: 10.1242/dev.122.2.491. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci. 1996;16(17):5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368(6467):147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells Expressing Messenger-Rna For Neurotrophins and Their Receptors During Embryonic Rat Development. Eur J Neurosci. 1992;4(11):1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Fass J, Gehler S, Sarmiere P, Letourneau P, Bamburg JR. Regulating filopodial dynamics through actin-depolymerizing factor/cofilin. Anat Sci Int. 2004;79(4):173–183. doi: 10.1111/j.1447-073x.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Fox EA. A genetic approach for investigating vagal sensory roles in regulation of gastrointestinal function and food intake. Auton Neurosci. 2006;126–127:9–29. doi: 10.1016/j.autneu.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Fox EA, Murphy MC. Factors regulating vagal sensory development: potential role in obesities of developmental origin. Physiol Behav. 2008;94(1):90–104. doi: 10.1016/j.physbeh.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, Powley TL. Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J Neurosci. 2001b;21:8602–8615. doi: 10.1523/JNEUROSCI.21-21-08602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Byerly MS, Baronowsky EA, Chi MM, Powley TL. Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c-Kit receptor ligand. Anat Embryol. 2002;205:325–342. doi: 10.1007/s00429-002-0261-x. [DOI] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: Morphology and topography. J Comp Neurol. 2000;428(3):558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. C-Kit Mutant Mice Have a Selective Loss of Vagal Intramuscular Mechanoreceptors that Innervate Gastric Smooth Muscle. Anat Embryol. 2001a;204:11–26. doi: 10.1007/s004290100184. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hear Res. 2005;206(1–2):52–63. doi: 10.1016/j.heares.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R, Leek BF. Gastro-duodenal receptor responses to chemical and mechanical stimuli, investigated by a ‘single fibre’ technique. J Physiol (Lond) 1972;222(2):139P–140P. [PubMed] [Google Scholar]
- Heinicke EA, Kiernan JA, Wijsman J. Specific, selective, and complete staining of neurons of the myenteric plexus, using cuprolinic blue. J Neurosci Methods. 1987;21(1):45–54. doi: 10.1016/0165-0270(87)90101-4. [DOI] [PubMed] [Google Scholar]
- Hellard D, Brosenitsch T, Fritzsch B, Katz DM. Cranial sensory neuron development in the absence of brain-derived neurotrophic factor in BDNF/Bax double null mice. Dev Biol. 2004;275(1):34–43. doi: 10.1016/j.ydbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Hofmann MH, Bleckmann H. Effect of temperature and calcium on transneuronal diffusion of DiI in fixed brain preparations. J Neurosci Methods. 1999;88(1):27–31. doi: 10.1016/s0165-0270(99)00007-2. [DOI] [PubMed] [Google Scholar]
- Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;381(1):81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Holst MC, Powley TL. Cuprolinic blue (quinolinic phthalocyanine) counterstaining of enteric neurons for peroxidase immunocytochemistry. J Neurosci Methods. 1995;62(1–2):121–127. doi: 10.1016/0165-0270(95)00064-x. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Wilkinson GA, Farinas I, Backus C, Zang K, Wong SL, Reichardt LF. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development. 1999;126(10):2191–2203. doi: 10.1242/dev.126.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K, Kuehnel F, Wyatt S, Davies AM. TrkB expression and early sensory neuron survival are independent of endogenous BDNF. J Neurosci Res. 2000;59(3):372–378. doi: 10.1002/(SICI)1097-4547(20000201)59:3<372::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Iggo A. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q J Exp Physiol Cogn Med Sci. 1957;42(4):398–409. doi: 10.1113/expphysiol.1957.sp001284. [DOI] [PubMed] [Google Scholar]
- Jarvinen MK, Powrozek TA, Wollmann WJ, Powley TL. Specializations of vagal intraganglionic laminar endings in the rat stomach and duodenum: a quantitative analysis. Soc Neurosci Abstr. 1998;24:1123. [Google Scholar]
- Jiang Y, Liu MT, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258(2):364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76(6):989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaosmanoglu T, Aygun B, Wade PR, Gershon MD. Regional differences in the number of neurons in the myenteric plexus of the guinea pig small intestine and colon: an evaluation of markers used to count neurons. Anat Rec. 1996;244(4):470–480. doi: 10.1002/(SICI)1097-0185(199604)244:4<470::AID-AR5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87(2):175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Gershon MD. Identification of vagal efferent fibers and putative target neurons in the enteric nervous system of the rat. J Comp Neurol. 1989;285(1):38–53. doi: 10.1002/cne.902850105. [DOI] [PubMed] [Google Scholar]
- Krimm RF. Factors that regulate embryonic gustatory development. BMC Neurosci. 2007;8(Suppl 3)(8):S4. doi: 10.1186/1471-2202-8-S3-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118(2):243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- LeMaster AM, Krimm RF, Davis BM, Noel T, Forbes ME, Johnson JE, Albers KM. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci. 1999;19(14):5919–5931. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz SI, Knudson CM, Korsmeyer SJ, Snider WD. Neurotrophins support the development of diverse sensory axon morphologies. J Neurosci. 1999;19(3):1038–1048. doi: 10.1523/JNEUROSCI.19-03-01038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434(7035):894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375(6528):238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Liu X, Jaenisch R. Severe peripheral sensory neuron loss and modest motor neuron reduction in mice with combined deficiency of brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5. Dev Dyn. 2000;218(1):94–101. doi: 10.1002/(SICI)1097-0177(200005)218:1<94::AID-DVDY8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Luikart BW, Nef S, Shipman T, Parada LF. In vivo role of truncated trkb receptors during sensory ganglion neurogenesis. Neuroscience. 2003;117(4):847–858. doi: 10.1016/s0306-4522(02)00719-4. [DOI] [PubMed] [Google Scholar]
- Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4(2):143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- Ma L, Lopez GF, Krimm RF. Epithelial-derived brain-derived neurotrophic factor is required for gustatory neuron targeting during a critical developmental period. J Neurosci. 2009;29(11):3354–3364. doi: 10.1523/JNEUROSCI.3970-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane S. Dendritic morphogenesis: building an arbor. Mol Neurobiol. 2000;22(1–3):1–9. doi: 10.1385/MN:22:1-3:001. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A. 2006;103(27):10444–10449. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Fox EA. Anterograde tracing method using DiI to label vagal innervation of the embryonic and early postnatal mouse gastrointestinal tract. J Neurosci Methods. 2007;163(2):213–225. doi: 10.1016/j.jneumeth.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20(3):243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Clerc N. Afferent innervation of the esophagus in cat and rat. In: Zenker W, Neuhuber WL, editors. The primary afferent neuron. A survey of recent morpho-functional aspects. New York and London: Plenum Press; 1990. pp. 93–107. [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87(4):2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Paves H, Saarma M. Neurotrophins as in vitro growth cone guidance molecules for embryonic sensory neurons. Cell Tissue Res. 1997;290(2):285–297. doi: 10.1007/s004410050933. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Afferent innervation of gastrointestinal tract smooth muscle by the hepatic branch of the vagus. J Comp Neurol. 1997;384(2):248–270. [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Long-term regeneration of abdominal vagus: efferents fail while afferents succeed. J Comp Neurol. 2003;455(2):222–237. doi: 10.1002/cne.10470. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. As the gut ages: Timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434(3):358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- Raab M, Worl J, Brehmer A, Neuhuber WL. Reduction of NT-3 or TrkC results in fewer putative vagal mechanoreceptors in the mouse esophagus. Auton Neurosci. 2003;108(1–2):22–31. doi: 10.1016/j.autneu.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ratcliffe EM, D’Autreaux F, Gershon MD. Laminin terminates the Netrin/DCC mediated attraction of vagal sensory axons. Dev Neurobiol. 2008;68(7):960–971. doi: 10.1002/dneu.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe EM, Setru SU, Chen JJ, Li ZS, D’Autreaux F, Gershon MD. Netrin/DCC-mediated attraction of vagal sensory axons to the fetal mouse gut. J Comp Neurol. 2006;498(5):567–580. doi: 10.1002/cne.21027. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, Barbacid M, DeChiara TM, Yancopoulos GD, Dunne CE, Fundin BT. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Dev Biol. 1998;198(1):57–81. [PubMed] [Google Scholar]
- Rinaman L. Ontogeny of hypothalamic-hindbrain feeding control circuits. Dev Psychobiol. 2006;48(5):389–396. doi: 10.1002/dev.20146. [DOI] [PubMed] [Google Scholar]
- Robinson M, Adu J, Davies AM. Timing and regulation of trkB and BDNF mRNA expression in placode-derived sensory neurons and their targets. Eur J Neurosci. 1996;8(11):2399–2406. doi: 10.1111/j.1460-9568.1996.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, O’Connor R, Giger RJ, Verhaagen J, Farbman AI. Comparison of neurotrophin and repellent sensitivities of early embryonic geniculate and trigeminal axons. J Comp Neurol. 2000;422(4):579–593. [PubMed] [Google Scholar]
- Rodrigo J, de Felipe J, Robles-Chillida EM, Perez Anton JA, Mayo I, Gomez A. Sensory vagal nature and anatomical access paths to esophagus laminar nerve endings in myenteric ganglia. Determination by surgical degeneration methods. Acta Anat. 1982;112(1):47–57. doi: 10.1159/000145496. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Hernandez J, Vidal MA, Pedrosa JA. Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta Anat. 1975;92(1):79–100. doi: 10.1159/000144431. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tebar A, Dechant G, Gotz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. Embo J. 1992;11(3):917–922. doi: 10.1002/j.1460-2075.1992.tb05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Assmann P, Lefebvre O, Bellissent-Waydelich A, Olsen J, Orian-Rousseau V, De Arcangelis A. The laminins: role in intestinal morphogenesis and differentiation. Ann N Y Acad Sci. 1998;859:46–64. doi: 10.1111/j.1749-6632.1998.tb11110.x. [DOI] [PubMed] [Google Scholar]
- Song XY, Li F, Zhang FH, Zhong JH, Zhou XF. Peripherally-derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury. PLoS ONE. 2008;3(3):e1707. doi: 10.1371/journal.pone.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Rodeau JL, Danoux L, Contet-Audonneau JL, Pauly G, Schlichter R. Trophic effects of keratinocytes on the axonal development of sensory neurons in a coculture model. Eur J Neurosci. 2007;26(1):113–125. doi: 10.1111/j.1460-9568.2007.05649.x. [DOI] [PubMed] [Google Scholar]
- Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421(3):302–324. [PubMed] [Google Scholar]
- Yang X, Liu R, Brookes SJH. Intraganglionic laminar endings act as mechanoreceptors of vagal afferent nerve in guinea pig esophagus. Acta Physiol Sinica. 2006;58(2):171–176. [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJH. Intraganglionic laminar endings are mechanotransduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534.1:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XP, Wu KY, Liang B, Fu XQ, Luo ZG. TrkB-mediated activation of geranylgeranyltransferase I promotes dendritic morphogenesis. Proc Natl Acad Sci U S A. 2008;105(44):17181–17186. doi: 10.1073/pnas.0800846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










