Abstract
Pseudomonas aeruginosa exotoxin A (ETA) production depends on the virulence-factor regulator Vfr. Recent evidence indicates that the P. aeruginosa iron-starvation sigma factor PvdS also enhances ETA production through the ETA-regulatory gene regA. Mutants defective in vfr, regA and pvdS, plasmids that overexpress these genes individually and lacZ transcriptional/translational fusion plasmids were utilized to examine the relationship between vfr, regA and pvdS in regulating P. aeruginosa ETA production. ETA concentration and regA expression were reduced significantly in PAOΔvfr, but pvdS expression was not affected. Overexpression of Vfr produced a limited increase in ETA production in PAOΔpvdS, but not PAOΔregA. Additionally, overexpression of either RegA or PvdS did not enhance ETA production in PAOΔvfr. RT-PCR analysis showed that iron did not affect the accumulation of vfr mRNA in PAO1. These results suggest that: (i) Vfr enhances toxA expression in PAO1 both directly and indirectly through regA, but not through pvdS; (ii) vfr expression is not regulated by iron; and (iii) both Vfr and PvdS cooperate in the presence of RegA to achieve a maximum level of toxA expression.
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative, opportunistic pathogen that causes acute and chronic infections in immunocompromised hosts, including severely burned patients, individuals with cystic fibrosis and cancer patients undergoing chemotherapy (Davis et al., 1996; Pollack, 2000). The severity of these infections is due to the ability of P. aeruginosa to produce an arsenal of cell-associated and extracellular virulence factors (Frank, 1997; Govan & Deretic, 1996; Sato & Frank, 2004; Woods & Vasil, 1994). Among the extracellular virulence factors produced by P. aeruginosa are exotoxin A (ETA), proteins of the type III secretion system and the LasB protease (elastase) (Frank, 1997; Govan & Deretic, 1996; Pollack, 2000). ETA is an ADP-ribosyltransferase that catalyses the transfer of the NAD moiety to elongation factor 2 of eukaryotic cells, which inhibits protein synthesis and results in cell death (Iglewski & Kabat, 1975). Both clinical studies and animal models have demonstrated the importance of ETA in P. aeruginosa infection. For example, sputum samples obtained from cystic fibrosis patients contained toxA mRNA (Storey et al., 1998). Additionally, P. aeruginosa clinical isolates obtained from different sites produced ETA (Hamood et al., 1996). Furthermore, P. aeruginosa mutants defective in ETA production were less virulent in different animal models than their parent strains (Fogle et al., 2002; Matsumoto et al., 1999).
ETA production by P. aeruginosa is regulated by different environmental factors, including growth temperature and the level of iron in the growth medium (Hamood et al., 2004; Liu, 1973). Iron represses ETA production; P. aeruginosa produces maximum levels of ETA when it is grown in an iron-deficient medium (Hamood et al., 2004; Liu, 1973). ETA production by P. aeruginosa also involves several positive and negative regulators (Hamood et al., 2004). The most extensively analysed of these are the positive regulators RegA and PvdS (Hamood et al., 2004; Vasil & Ochsner, 1999). RegA enhances toxA transcription, although the mechanism by which this enhancement occurs is not completely understood (Hamood & Iglewski, 1990; Raivio et al., 1996). PvdS, the iron-starvation sigma factor, enhances the expression of toxA and is required for expression of the pyoverdine genes (Beare et al., 2003; Cunliffe et al., 1995; Vasil & Ochsner, 1999). Evidence suggests that the PvdS enhancement of toxA occurs through regA (Hamood et al., 2004; Ochsner et al., 1996). Negative regulation of ETA production occurs through the ferric-uptake regulator Fur (Hamood et al., 2004; Vasil & Ochsner, 1999). Iron-activated Fur represses pvdS transcription, thereby reducing regA and toxA transcription (Hamood et al., 2004; Vasil & Ochsner, 1999). Gaines et al. (2007) demonstrated that the PvdS–RNA polymerase (RNAP) holoenzyme complex binds specifically to the upstream region of regA and toxA.
The virulence-factor regulator Vfr, originally described as a factor that regulates ETA and protease production by P. aeruginosa, exerts a global regulatory effect on the production of multiple virulence factors (West et al., 1994b). Studies have demonstrated that Vfr regulates different genes of the las quorum-sensing system (including lasR and lasB), twitching motility, flagellar biosynthesis and the stationary-phase sigma factor RpoS (Albus et al., 1997; Ambrosi et al., 2002; Bertani et al., 2003; Dasgupta et al., 2002). Using microarray analysis, Wolfgang et al. (2003) identified more than 200 genes, including those that encode different components of the type III secretion system including exoS and exsA, that are regulated either positively or negatively by Vfr. Vfr is a homologue of the Escherichia coli catabolite-repressor protein CRP, which requires cAMP for its activation (West et al., 1994b). Kanack et al. (2006) showed that Vfr binds to a specific sequence within the upstream regions of toxA, regA, lasR, prpL, algD and fimS, although other genes, including pvdS, exoS and exsA, lack the Vfr-binding sequence (N. L. Carty & A. N. Hamood, unpublished results). In this study, we analysed the mechanism by which Vfr regulates ETA production in P. aeruginosa. Our results suggest that Vfr regulates ETA production directly by binding to the toxA upstream region and indirectly through regA. However, Vfr does not regulate pvdS expression; neither is vfr expression regulated by iron.
METHODS
Bacterial strains, plasmids and growth media and conditions.
Strains and plasmids utilized in this study are described in Table 1. For general growth experiments, strains were grown in Luria–Bertani (LB) broth (Miller, 1972). For analysis of ETA production, P. aeruginosa strains were grown in the iron-deficient medium TSBDC (Chelex-treated trypticase soy broth dialysate) to which glycerol (1 %, v/v) and monosodium glutamate (0.5 M) were added (Ohman et al., 1980). Iron-sufficient medium was prepared by the addition of FeCl3 to TSBDC to a final concentration of 25 μg ml−1 (TSBDC-Fe) (Ohman et al., 1980). Antibiotics were added to the growth medium as needed: carbenicillin (300 μg ml−1), chloramphenicol (50 μg ml−1), gentamicin (50 μg ml−1), kanamycin (500 μg ml−1), streptomycin (300 μg ml−1) and tetracycline (80 μg ml−1).
Table 1.
Strains and plasmids used in this study
| Strain/plasmid | Description* | Source (reference) |
|---|---|---|
| Pseudomonas aeruginosa | ||
| PAO-SW | Prototrophic PAO1 strain | S. E. H. West (West et al., 1994b) |
| PAOΔvfr | vfr deletion of PAO-SW | S. E. H. West (Albus et al., 1997) |
| PAOΔpvdS | pvdS isogenic mutant of PAO-SW; pvdS disruption; Gmr | This study |
| PAOΔregA | regA isogenic mutant of PAO-SW; regA internal deletion; Gmr | This study |
| Plasmids | ||
| pSW205 | lacZ translational fusion vector that replicates stably in P. aeruginosa; Cbr | S. E. H. West (Storey et al., 1990) |
| pSW228 | toxA–lacZ translational fusion in pSW205; Cbr | S. E. H. West (West et al., 1994a) |
| pRL88 | regA(P1/P2)–lacZ translational fusion in pSW205; Cbr | D. Storey (Storey et al., 1990) |
| pAM21-2 | pUC18 recombinant plasmid in which toxA is expressed from the lac promoter carried on pKT230; Cbr, Kmr | This study |
| pIN9 | 1.5 kb AvaI–PstI fragment carrying intact regA from PA103 plus 40 bp of the regA upstream region expressed from the lac promoter in pUC18; Cbr | This study |
| pIN10 | pIN9 carrying the 1.8 kb stability fragment for replication of plasmid in P. aeruginosa; Cbr | This study |
| pUCP19 | E. coli–P. aeruginosa shuttle vector; Cbr | H. P. Schweizer (Schweizer, 1991) |
| pKF917 | pUCP19 carrying intact vfr; Cbr | S. E. H. West (West et al., 1994b) |
| pVLT31 | Broad-host-range expression vector; Tcr | M. Vasil (Ochsner et al., 1996) |
| pPVD31 | pVLT31 recombinant plasmid in which pvdS is expressed from the tac promoter; Tcr | M. Vasil (Ochsner et al., 1996) |
| pMP220 | Broad-host-range lacZ transcriptional fusion vector; Tcr | P. Visca (Spaink et al., 1987) |
| pMP220 : : PpvdS | pvdS–lacZ transcriptional fusion in pMP220; Tcr | P. Visca (Ambrosi et al., 2002) |
| pMP190 | Broad-host-range lacZ transcriptional fusion vector; Cmr, Smr | I. Lamont (Spaink et al., 1987) |
| pMP190 : : PpvdE | pvdE–lacZ transcriptional fusion in pMP190; Cmr, Smr | P. Visca (Leoni et al., 2000) |
| pJQpvdS : : Gm | pvdS interrupted with a Gm cassette carried in the sacB suicide vector pJQ200SK; Gmr | D. Storey (Hunt et al., 2002) |
| pSUP203-ΔregA : : Gm | Mobilizable suicide plasmid carrying a Gm cartridge between regA-flanking regions; Gmr | M. Vasil (Ochsner et al., 1996) |
*Cb, Carbenicillin; Cm, chloramphenicol; Gm, gentamicin; Km, kanamycin; Sm, streptomycin; Tc, tetracycline.
For the analysis of ETA production, as well as the expression of different genes, P. aeruginosa strains were first grown overnight in LB broth at 37 °C. An aliquot of the overnight culture was pelleted, washed and resuspended in TSBDC. The resuspended culture was used to inoculate fresh TSBDC or TSBDC-Fe to an OD540 of 0.03–0.05. The cultures were grown at 32 °C for 14–16 h with shaking at 250 r.p.m. Samples were obtained at specified time points for analysis. Each experiment was conducted in triplicate (three separate flasks), with three replicates per flask.
General DNA techniques.
Plasmid DNA extraction was performed by using the Wizard Plus MiniPreps DNA Purification system (Promega). Restriction digestion, ligation and transformation of E. coli were done as described by Sambrook & Russell (2001). Plasmids were introduced into P. aeruginosa by electroporation (Smith & Iglewski, 1989).
Construction of pIN10.
A 1.5 kb AvaI–PstI fragment was isolated from pDF18-202 (Frank et al., 1989) and cloned into the SalI/PstI sites of pUC18. The fragment carries the intact regA open reading frame from P. aeruginosa strain PA103, plus 40 bp of the region immediately upstream of the regA ATG codon. In the resulting recombinant plasmid (pIN9), regA is expressed constitutively from the lac promoter. The 1.8 kb stability fragment, which allows ColEI plasmids to replicate stably in P. aeruginosa (Olsen et al., 1982), was cloned into the PstI site of pIN9, generating pIN10.
Generation of pvdS and regA knockout mutants.
The mutants were constructed from PAO-SW, the strain from which the vfr mutant (PAOΔvfr) was generated, by using the gene-replacement technique as described previously (Hunt et al., 2002; Ochsner et al., 1996). Plasmid pJQpvdS : : Gm (Hunt et al., 2002) was utilized to construct PAOΔpvdS, whilst plasmid pSUP203-ΔregA : : Gm (Ochsner et al., 1996) was used to generate PAOΔregA. Construction of both mutants was confirmed by PCR.
RT-PCR.
Bacterial RNA was extracted by using a modified hot phenol method as described previously (Carty et al., 2003; Frank et al., 1989). Residual chromosomal DNA was removed with DNase I in the presence of the RNase inhibitor RNasin (Promega). Enzymes and buffer were then removed from the RNA solution by using an RNeasy kit (Qiagen). To synthesize cDNA, approximately 1 μg total RNA was reverse-transcribed at 42 °C for 90 min with StrataScript reverse transcriptase (Stratagene) and random hexamers (Promega) as primers. Specific primers were used to amplify regions of toxA (F-5′-GCGTGCTGCACTACTCCATG-3′; R-5′-GTTACCGGCGTTCAGTTCGT-3′), pvdS (F-5′-ACCGTAGATCCTGGTGAAGA-3′; R-5′-CGAGTATTTCTGTTCGAGCGC-3′) and vfr (F-5′-GCTGCGAAACGCTGTTCTTC-3′; R-5′-GCTGCCGAGGGTGTAGAGG-3′) from the cDNA. A region of the constitutively expressed rpsL gene (F-5′-GCAACTATCAACCAGCTG-3′; R-5′-GCTGTGCTCTTGCAGGTTGTG-3′) was amplified as a positive control (Sobel et al., 2003). PCR extension was conducted at temperatures appropriate for each primer for 30 cycles. To exclude DNA contamination, each RNA sample was subjected to PCR without reverse transcriptase. The products were examined on ethidium bromide-stained agarose gels.
Sandwich ELISA and β-galactosidase assays.
The sandwich ELISA was done as described previously (Gaines et al., 2005). Strains carrying various plasmids were grown in TSBDC or TSBDC-Fe for 14 h at 32 °C. ETA levels within the supernatant were determined by sandwich ELISA. Values were standardized by dividing the amount of ETA in pg μl−1 by the OD600 of the culture from which the fraction was obtained. Assays for β-galactosidase were performed as reported previously (Carty et al., 2006; Miller, 1972; Stachel et al., 1985).
RESULTS AND DISCUSSION
It has been suggested that both Vfr and PvdS regulate toxA and regA at the transcriptional level, although PvdS appears to regulate toxA expression through regA (Ochsner et al., 1996; West et al., 1994a). The regA upstream region contains an iron-starvation box (Hunt et al., 2002; Ochsner et al., 2002). Kanack et al. (2006) provided evidence that Vfr regulation may occur through its binding to the toxA and regA upstream region. However, Vfr does not bind to the pvdS upstream region (Kanack et al., 2006). Thus, to understand the relationship between vfr and toxA, regA and pvdS, we addressed the following questions. (i) Does Vfr regulate toxA expression directly? (ii) Does Vfr regulate toxA expression through regA, pvdS or both? (iii) Is vfr regulated by iron, similarly to toxA, regA and pvdS? To examine the roles of regA and pvdS in the effect of Vfr on ETA production, we constructed mutants that carry specific deletions in either regA (PAOΔregA) or pvdS (PAOΔpvdS) as described previously (Hunt et al., 2002; Ochsner et al., 1996). The mutants were generated from PAO-SW, the PAO1 strain from which PAOΔvfr was generated (Table 1) (Albus et al., 1997).
Vfr regulates toxA expression at the transcriptional level only
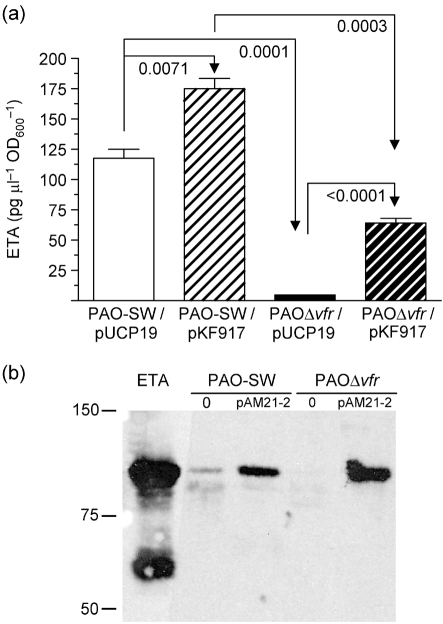
Using sandwich ELISA, we confirmed the effect of vfr deletion on ETA production. Compared with its parent strain PAO-SW carrying vector pUCP19, PAOΔvfr/pUCP19 produced 25-fold less ETA (P<0.0001) (Fig. 1a). The introduction of vfr into plasmid pKF917 increased the level of ETA produced by PAOΔvfr significantly (P<0.0001), but did not restore it to wild-type level (Fig. 1a). To determine whether the effect of Vfr on ETA occurs at the transcriptional level, we examined toxA expression from the toxA–lacZ fusion plasmid pSW228 in PAO-SW and PAOΔvfr. Throughout the growth cycle, toxA expression in PAOΔvfr/pSW228 was significantly lower than that in PAO-SW/pSW228 (data not shown). As a global regulator, Vfr may regulate other cellular functions that affect ETA levels indirectly, including a general effect on protein synthesis or protein secretion. Wolfgang et al. (2003) suggested that a vfr mutation affected the type II secretion system through which ETA is secreted by P. aeruginosa. Therefore, we examined the level of ETA produced by PAOΔvfr/pAM21-2, in which toxA is expressed constitutively from the lac promoter. ETA production in PAO1 is deregulated with respect to iron in the presence of pAM21-2 (A. N. Hamood, unpublished results). If vfr mutation affects the type II secretion pathway, we would expect to see very little or no ETA in the supernatant fraction of PAOΔvfr/pAM21-2 grown under iron-sufficient conditions. However, PAO-SW/pAM21-2 and PAOΔvfr/pAM21-2 produced comparable levels of ETA (Fig. 1b). Thus, Vfr affects toxA transcription, but does not affect ETA secretion in PAO1.
Fig. 1.
(a) Vfr is required for optimum production of ETA by PAO1. PAO-SW and PAOΔvfr carrying vector plasmid (pUCP19) or the vfr plasmid pKF917 were grown in TSBDC for 14 h at 32 °C. ETA levels within the supernatant were determined by sandwich ELISA. Values were standardized by dividing the amount of ETA in pg μl−1 by the OD600 of the culture from which the fraction was obtained. Values represent the mean±sem of three independent experiments. Unpaired, two-tailed t-tests were used to determine statistical significance (P<0.05) between pairs. (b) Vfr does not affect ETA secretion. PAO-SW and PAOΔvfr carrying pAM21-2 in which toxA is expressed from the lac promoter were grown in TSBDC-Fe for 14 h at 32 °C. Supernatants were harvested and concentrated; 10 μg protein was separated by SDS-PAGE and transferred to a membrane. ETA levels within the supernatant fractions were determined by immunoblotting with polyclonal anti-ETA. Molecular mass standards in kDa are indicated on the left; 0, no plasmid present.
Vfr regulates ETA production indirectly through regA and directly through toxA
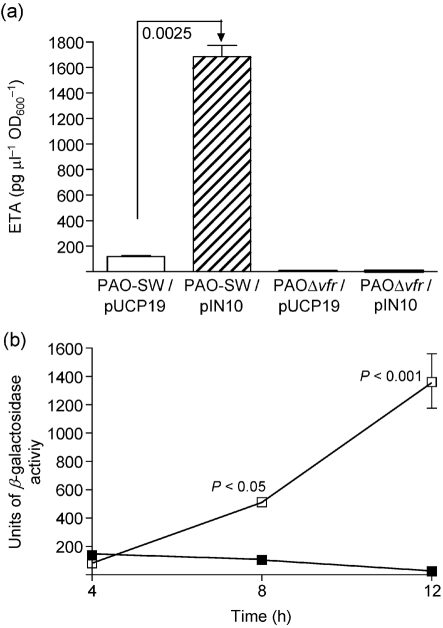
The DNA/protein gel-shift analyses reported by Kanack et al. (2006) showed that the Vfr-protected sequence within the regA upstream region is located 51–77 bp 5′ of the regA P1 transcriptional start site. To determine whether Vfr regulates toxA expression through regA, we utilized pIN10, in which regA is expressed from the constitutive lac promoter; therefore, regA expression is not influenced by any P. aeruginosa regulatory factor. The presence of pIN10 in the regA deletion mutant PAOΔregA complemented its defect in ETA production and deregulated it with respect to iron (data not shown). As shown in Fig. 2(a), compared with PAO-SW/pUCP19, ETA production by PAO-SW/pIN10 was increased significantly (P<0.0001). However, pIN10 did not increase ETA production by PAOΔvfr (Fig. 2a). The failure of overexpressed RegA to complement the defect of PAOΔvfr in ETA production suggests that Vfr does not regulate toxA expression solely through regA. In addition, the results show that RegA requires a functional vfr to induce toxA expression in P. aeruginosa. Next, we determined whether vfr deletion affects regA expression in PAO1. Monitoring regA expression from the regA–lacZ fusion plasmid pRL88 showed that regA expression in PAOΔvfr was the same as that in PAO-SW at 4 h. However, at 8 and 12 h, regA expression in PAOΔvfr was significantly (P<0.05 and <0.001, respectively) lower than that in PAO-SW (Fig. 2b). Therefore, Vfr may regulate toxA expression both directly and through regA. The Vfr-protected sequence within the toxA upstream region is located −53 to −78 bp 5′ of the toxA S1a start site (Kanack et al., 2006), supporting the idea that Vfr regulates ETA production in PAO1 directly, bypassing regA. To examine this possibility, we determined the effect of the vfr plasmid pKF917 on ETA production by PAOΔregA. In both iron-deficient and iron-sufficient medium, pKF917 produced no significant increase in ETA production by PAOΔregA (Supplementary Fig. S1, available with the online version of this paper).
Fig. 2.
(a) Constitutive expression of regA from the lac promoter in pIN10 does not bypass the defect of PAOΔvfr in ETA production. Strains were grown and ETA levels determined as described in the legend to Fig. 1. Values represent the mean±sem of three independent experiments. (b) vfr mutation interferes with regA expression at late stages of growth. PAO-SW/pRL88 (regA–lacZ transcriptional fusion plasmid; □) and PAOΔvfr/pRL88 (▪) were grown in TSBDC for 12 h; samples were obtained at 4, 8 and 12 h post-inoculation. Cells were pelleted and the level of β-galactosidase activity was determined (Methods). Data were analysed by one-way ANOVA with a Tukey–Kramer multiple comparisons post-test. Values represent the mean±sem of three independent experiments.
Results of these first three experiments revealed that Vfr regulates both toxA and regA expression at the transcriptional level, as the vfr deletion reduced expression of both genes significantly (Fig. 2b; data not shown). However, the failure of the vfr plasmid to enhance ETA production in PAOΔregA indicates that RegA is essential for the enhancement in ETA production by Vfr.
Vfr does not regulate pvdS; neither does it regulate toxA through pvdS
Evidence indicates that ETA production in P. aeruginosa is regulated positively by the iron-starvation sigma factor PvdS. In the absence of functional PvdS, ETA production and toxA expression in P. aeruginosa are reduced considerably (Ochsner et al., 1996). Whilst the toxA and regA upstream regions contain the Vfr-binding consensus, this sequence is not present in the pvdS upstream region (Kanack et al., 2006), suggesting that Vfr does not regulate pvdS directly. Thus, we investigated whether Vfr regulates ETA production through pvdS.
First, we examined the effect of vfr mutation on pvdS transcription in PAO1. PAO-SW and PAOΔvfr carrying the pvdS–lacZ transcriptional fusion plasmid pMP220 : : PpvdS were grown in iron-deficient medium and the level of pvdS expression was assessed at 4, 8, 12 and 16 h. As shown in Fig. 3(a), at 4 h (early stage of growth), PAO-SW/pMP220 : : PpvdS and PAOΔvfr/pMP220 : : PpvdS showed low levels of pvdS expression. However, by 8 h, pvdS expression in PAO-SW increased sharply and continued unchanged until the 16 h time point (late stage of growth) (Fig. 3a). In contrast, the level of pvdS expression in PAOΔvfr/pMP220 : : PpvdS increased until the 12 h time point and then declined to a level similar to that seen at 8 h (Fig. 3a). These results suggest that pvdS expression in PAO1 does not require functional Vfr. To confirm these results and to exclude the possibility that Vfr regulates pvdS expression post-transcriptionally, we measured the amount of accumulated pvdS mRNA in PAO-SW and PAOΔvfr by using RT-PCR. As shown in Fig. 3(b), there was no apparent difference in the amount of the accumulated pvdS mRNA between PAO-SW and PAOΔvfr under iron-deficient conditions.
Fig. 3.
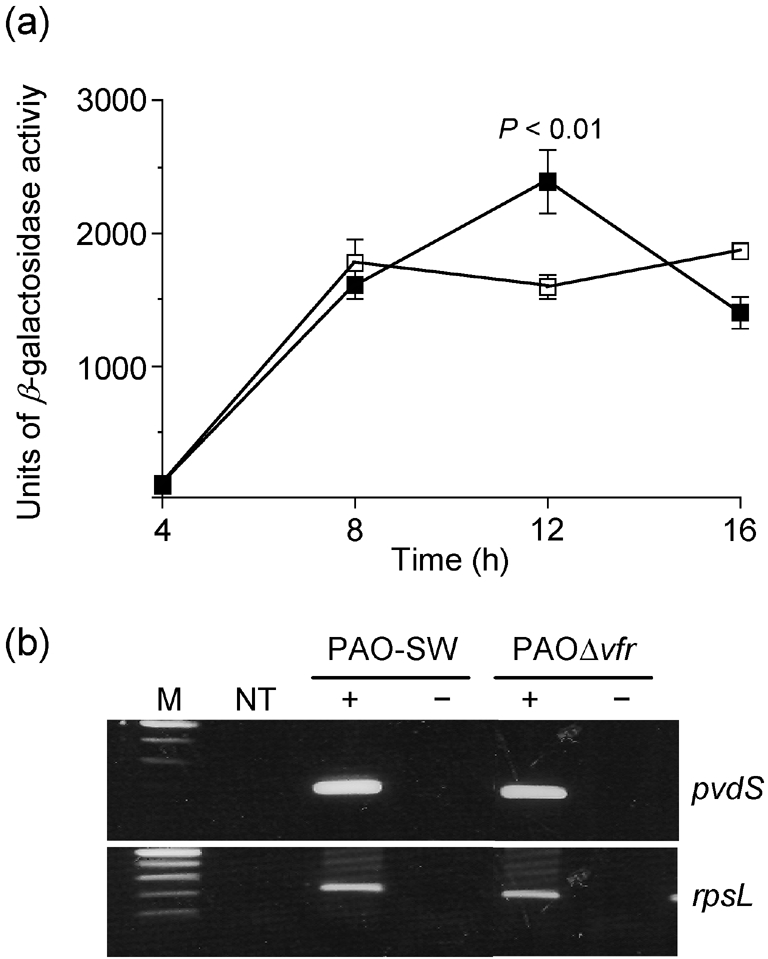
vfr mutation does not affect pvdS expression. (a) PAO-SW (□) and PAOΔvfr carrying the pvdS–lacZ transcriptional fusion plasmid pMP220 : : PpvdS (▪) were grown in TSBDC for 16 h at 32 °C; samples were obtained at 4, 8, 12 and 16 h and the level of β-galactosidase was determined as described in the legend to Fig. 2. Values represent the mean±sem of three independent experiments. (b) Total RNA was extracted from PAO-SW and PAOΔvfr grown in TSBDC for 14 h at 32 °C and the accumulation of pvdS mRNA was determined by RT-PCR. Transcript accumulation of rpsL was examined as a positive control. M, DNA size marker; NT, no-template control; −, no reverse transcriptase; +, with reverse transcriptase.
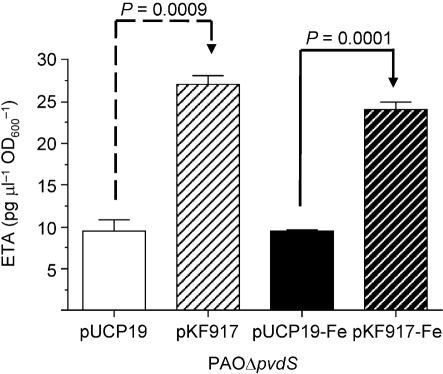
To explore further the relationship between PvdS and Vfr in regulating ETA production, we determined whether increased levels of Vfr would compensate for the loss of functional PvdS in PAO1 (PAOΔpvdS). As shown in Fig. 4, in both iron-deficient and iron-sufficient media, PAOΔpvdS/pKF917 produced significantly higher levels of ETA than PAOΔpvdS/pUCP19 (P=0.0009 and 0.0001, respectively). The level of ETA produced by PAOΔpvdS/pKF917 in iron-deficient medium paralleled the amount produced in iron-sufficient medium (Fig. 4). Comparison of these results with those shown in Fig. 1 suggests that, in the absence of functional PvdS, the effect of Vfr on ETA production is limited. In addition, whilst iron represses ETA production in PAO-SW/pKF917, it does not in PAOΔpvdS/pKF917, indicating that Vfr is not one of the factors through which iron regulates ETA production in P. aeruginosa.
Fig. 4.
Overexpression of Vfr enhances ETA production under low-iron and high-iron conditions in PAOΔpvdS. Strains were grown in TSBDC and TSBDC-Fe and ETA levels were determined as described in the legend to Fig. 1. Values represent the mean±sem of three independent experiments.
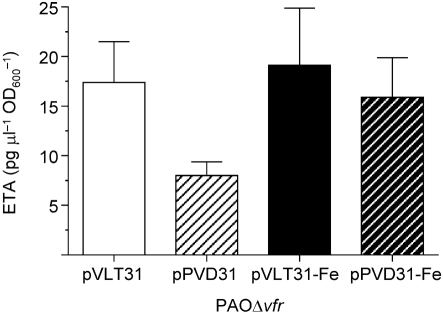
We also excluded the possibility that Vfr regulates ETA production through pvdS by examining the effect of pPVD31, in which pvdS is expressed constitutively from the tac promoter, on ETA production in PAOΔvfr. Similarly to regA expression in pIN10, pvdS expression in pPVD31 is not influenced by any P. aeruginosa regulator. Ochsner et al. (1996) showed that pPVD31 deregulated ETA production in PAO1 with respect to iron. Plasmid pPVD31 did not affect ETA production by PAOΔvfr in iron-deficient or iron-sufficient medium, suggesting that Vfr does not regulate ETA production in PAO1 through pvdS (Fig. 5).
Fig. 5.
Overexpression of PvdS does not increase ETA production in PAOΔvfr under low-iron or high-iron conditions. Strains were grown and ETA levels determined as described in the legend to Fig. 1. Values represent the mean±sem of three independent experiments. There were no significant differences in the levels of ETA produced by PAOΔvfr carrying either plasmid.
These experiments clarify the relationship of Vfr to pvdS and toxA. Firstly, Vfr does not regulate pvdS; levels of pvdS expression, as well as the accumulation of pvdS mRNA, were comparable in PAO1 and PAOΔvfr (Fig. 3a, b). Secondly, whilst an increase in the level of Vfr produced a limited increase in ETA production in PAOΔpvdS (Fig. 4), pvdS overexpression did not affect ETA production by PAOΔvfr (Fig. 5). Finally, overexpression of Vfr in PAOΔpvdS enhanced ETA production, irrespective of the presence of iron (Fig. 4).
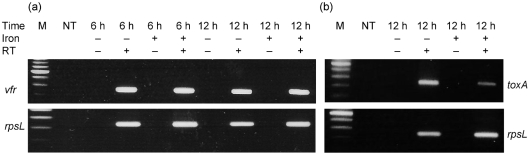
These results also suggest that, unlike toxA, regA and pvdS, vfr may not be regulated by iron. To address this possibility, we examined the accumulation of vfr mRNA in PAO-SW that was grown in iron-deficient or iron-sufficient medium. As shown in Fig. 6(a), there was no apparent difference in the accumulation of vfr mRNA in the presence or absence of iron. As a control, we monitored the accumulation of toxA mRNA in PAO-SW under those same conditions (Fig. 6b). Thus, Vfr regulation of regA and toxA may be responsible for the low level of ETA production that occurs under high-iron conditions.
Fig. 6.
(a) Accumulation of vfr mRNA in PAO-SW is not influenced by the level of iron in the growth medium. (b) Accumulation of toxA mRNA in PAO-SW is influenced by the level of iron in the growth medium (a control representing an iron-regulated gene). Total RNA was extracted from PAO-SW grown in TSBDC or TSBDC-Fe at 32 °C for 6 or 12 h (for vfr) or 12 h (for toxA) and the accumulation of mRNA was determined by RT-PCR. Transcript accumulation of rpsL was examined as a positive control. M, DNA size marker; NT, no-template control; RT, reverse transcriptase; −, absence of iron and/or RT; +, presence of iron and/or RT.
Vfr does not affect pyoverdine production in PAO1
As Vfr overrides pvdS to regulate ETA production, we determined whether Vfr affects the regulation of another PvdS-regulated virulence factor, pyoverdine. Compared with its parent strain, expression of the pyoverdine genes and the level of pyoverdine produced are reduced significantly in a PAO1 pvdS deletion mutant (Cunliffe et al., 1995; Leoni et al., 2000). We utilized the pvdE–lacZ fusion plasmid pMP190 : : PpvdE to examine the effect of Vfr on the expression of the pyoverdine genes. Transcriptional analysis revealed that the level of pvdE expression in PAOΔvfr was similar to that by PAO-SW (data not shown). Additionally, the presence of pKF917 in PAOΔpvdS failed to complement the defect in pyoverdine production (data not shown). Furthermore, whilst pvdS expressed constitutively from pPVD31 failed to affect ETA production in PAOΔvfr, it deregulated pyoverdine production with respect to iron (data not shown). These results suggest that Vfr affects ETA production directly, but has no effect on pyoverdine production.
Conclusion
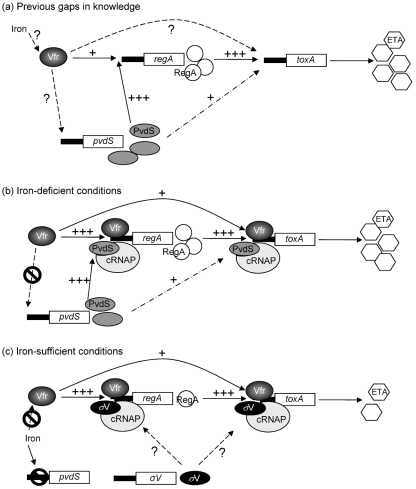
Results of this study indicate that Vfr, RegA and PvdS are required for optimum ETA production in P. aeruginosa (Fig. 7). Based on these results, we propose a model for toxA regulation by Vfr under iron-deficient (Fig. 7b) and iron-sufficient (Fig. 7c) conditions. PvdS binds to a specific sequence within the upstream region of several genes, including toxA, regA and the pyoverdine genes (Leoni et al., 2000; Wilson et al., 2001). Thus, binding of the PvdS-recruited core RNAP (PvdS–RNAP complex) to this sequence enhances the transcription of these genes. In low-iron medium and under aerobic conditions, both the PvdS–RNAP complex and Vfr bind to the regA upstream region, leading to an increase in regA transcription and the level of RegA protein, which leads to a moderate level of ETA production (Fig. 7b). Under the same conditions, the PvdS–RNAP complex would bind efficiently to the toxA promoter and enhance toxA transcription. This binding is strengthened further by the binding of Vfr and its interaction with the PvdS–RNAP complex, producing the maximum level of ETA. However, in the absence of RegA, binding of PvdS and Vfr to the toxA upstream region is not sufficient to enhance ETA production. Therefore, maximum expression of toxA occurs when functional Vfr, PvdS and RegA are present (Fig. 7b).
Fig. 7.
(a) Diagram illustrating the current knowledge and existing gaps in our understanding of the complicated interactions between different toxA-regulatory genes. (b, c) Modification of (a) based on the results obtained in the present study. (b) Interactions between the toxA-regulatory genes under iron-deficient conditions. (c) Interactions of these genes under iron-sufficient conditions. ? indicates a previously undetermined mechanism of regulation; a dotted arrow indicates a potential regulatory pathway based only on DNA-binding studies with the PvdS–RNAP complex and the toxA upstream region. cRNAP, Core RNA polymerase; σV, any sigma factor other than PvdS.
Under iron-sufficient conditions, iron–Fur represses pvdS expression and reduces the cellular level of PvdS severely. Consequently, PvdS will be replaced within the RNAP complex by another sigma factor that recognizes the promoters of toxA and regA less efficiently, resulting in a reduced level of toxA expression (Fig. 7c). However, binding of Vfr to the toxA and regA upstream region would not be altered by iron, i.e. Vfr binds at the same efficiency under iron-deficient and iron-sufficient conditions. Accordingly, an increase in the intracellular concentration of Vfr would enhance the binding of the RNAP complex to the toxA and regA promoter regions (Fig. 7c). This effect still depends on RegA. Under iron-sufficient conditions, a basal level of RegA exists in P. aeruginosa. The ability of sigma factors other than PvdS to transcribe the toxA promoter is supported by the findings of Walker et al. (1995), who showed that the E. coli RNAP holoenzyme recognizes the toxA promoter and transcribes toxA in an in vitro transcription system.
The effect of vfr and other genes on ETA production by P. aeruginosa has been examined in several studies. Our current understanding of the interaction between these genes and/or their products in regulating ETA production is outlined in the Introduction. Despite the wealth of knowledge in this area, several gaps still exist (Fig. 7a). For example, it is not known whether Vfr regulates ETA production directly or indirectly (through regA, pvdS or both), whether Vfr requires RegA, PvdS or both for its effect on ETA production, or whether iron interferes with the enhancement of ETA production by Vfr (Fig. 7a). As shown in Fig. 7(b, c), information provided by the present study fills these gaps. Our results provide the following new critical components. (i) Vfr does not enhance ETA production through PvdS (Fig. 7b). In the absence of PvdS, Vfr still enhances ETA production (Fig. 4). However, this effect is less dramatic than that in the presence of functional PvdS (Figs 1 and 4). Thus, maximum production of ETA requires the function of PvdS and Vfr. (ii) Vfr does not enhance ETA production through RegA (Fig. 7b). However, a minimal level of RegA is essential for the effect of Vfr on ETA production. Under conditions in which very low levels of RegA are produced, such as in iron-sufficient medium (Frank et al., 1989) or when pvdS has been mutated, Vfr enhances ETA production (Fig. 4). On the other hand, in the absence of functional RegA (PAOΔregA), Vfr does not enhance ETA production (Supplementary Fig. S1). (iii) Vfr enhances the expression of toxA and regA, possibly by binding to the upstream region of each gene, but does not enhance pvdS expression (Figs 2b, 7b) (West et al., 1994a). (iv) Vfr enhances ETA production under iron-sufficient conditions (Fig. 4), indicating that vfr is not part of the regulatory network through which iron regulates ETA production (Fig. 7c). This is due to the fact that, unlike toxA, regA and pvdS expression, vfr expression is not repressed by iron (Fig. 6) (Ochsner et al., 2002; Palma et al., 2003). In summary, our results indicate that maximum production of ETA by P. aeruginosa requires the cooperation of PvdS and Vfr, as well as the presence of functional RegA.
Acknowledgments
The authors would like to thank Douglas Storey, Michael Vasil, Iain Lamont, Herbert Schweizer, Paolo Visca and Susan West for the plasmids and strains. This work was supported by grant AI33386 from the National Institutes of Health to A. N. H. The authors thank Joanna Swickard for her assistance in editing the manuscript.
Abbreviations
ETA, exotoxin A
RNAP, RNA polymerase
Footnotes
A supplementary figure showing that overexpression of Vfr does not enhance ETA production in PAOΔregA is available with the online version of this paper.
References
- Albus, A. M., Pesci, E. C., Runyen-Janecky, L. J., West, S. E. & Iglewski, B. H. (1997). Vfr controls quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179, 3928–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi, C., Leoni, L. & Visca, P. (2002). Different responses of pyoverdine genes to autoinduction in Pseudomonas aeruginosa and the group Pseudomonas fluorescens–Pseudomonas putida. Appl Environ Microbiol 68, 4122–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare, P. A., For, R. J., Martin, L. W. & Lamont, I. L. (2003). Siderophore-mediated cell signalling in Pseudomonas aeruginosa: divergent pathways regulate virulence factor production and siderophore receptor synthesis. Mol Microbiol 47, 195–207. [DOI] [PubMed] [Google Scholar]
- Bertani, I., Sevo, M., Kojic, M. & Venturi, V. (2003). Role of GacA, LasI, RhlI, Ppk, PsrA, Vfr and ClpXP in the regulation of the stationary-phase sigma factor rpoS/RpoS in Pseudomonas. Arch Microbiol 180, 264–271. [DOI] [PubMed] [Google Scholar]
- Carty, N. L., Rumbaugh, K. P. & Hamood, A. N. (2003). Regulation of toxA by PtxR in Pseudomonas aeruginosa PA103. Can J Microbiol 49, 450–464. [DOI] [PubMed] [Google Scholar]
- Carty, N. L., Layland, N., Colmer-Hamood, J. A., Calfee, M. W., Pesci, E. C. & Hamood, A. N. (2006). PtxR modulates the expression of QS-controlled virulence factors in the Pseudomonas aeruginosa strain PAO1. Mol Microbiol 61, 782–794. [DOI] [PubMed] [Google Scholar]
- Cunliffe, H. E., Merriman, T. R. & Lamont, I. L. (1995). Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J Bacteriol 177, 2744–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta, N., Ferrell, E. P., Kanack, K. J., West, S. E. & Ramphal, R. (2002). fleQ, the gene encoding the major flagellar regulator of Pseudomonas aeruginosa, is sigma70 dependent and is downregulated by Vfr, a homolog of Escherichia coli cyclic AMP receptor protein. J Bacteriol 184, 5240–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, P. B., Drumm, M. & Konstan, M. W. (1996). Cystic fibrosis. Am J Respir Crit Care Med 154, 1229–1256. [DOI] [PubMed] [Google Scholar]
- Fogle, M. R., Griswold, J. A., Oliver, J. W. & Hamood, A. N. (2002). Anti-ETA IgG neutralizes the effects of Pseudomonas aeruginosa exotoxin A. J Surg Res 106, 86–98. [DOI] [PubMed] [Google Scholar]
- Frank, D. W. (1997). The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol 26, 621–629. [DOI] [PubMed] [Google Scholar]
- Frank, D. W., Storey, D. G., Hindahl, M. S. & Iglewski, B. H. (1989). Differential regulation by iron of regA and toxA transcript accumulation in Pseudomonas aeruginosa. J Bacteriol 171, 5304–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines, J. M., Carty, N. L., Colmer-Hamood, J. A. & Hamood, A. N. (2005). Effect of static growth and different levels of environmental oxygen on toxA and ptxR expression in the Pseudomonas aeruginosa strain PAO1. Microbiology 151, 2263–2275. [DOI] [PubMed] [Google Scholar]
- Gaines, J. M., Carty, N. L., Tiburzi, F., Davinic, M., Visca, P., Colmer-Hamood, J. A. & Hamood, A. N. (2007). Regulation of the Pseudomonas aeruginosa toxA, regA and ptxR genes by the iron-starvation sigma factor PvdS under reduced levels of oxygen. Microbiology 153, 4219–4233. [DOI] [PubMed] [Google Scholar]
- Govan, J. R. & Deretic, V. (1996). Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60, 539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood, A. N. & Iglewski, B. H. (1990). Expression of the Pseudomonas aeruginosa toxA positive regulatory gene (regA) in Escherichia coli. J Bacteriol 172, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood, A. N., Griswold, J. A. & Duhan, C. M. (1996). Production of extracellular virulence factors by Pseudomonas aeruginosa isolates obtained from tracheal, urinary tract, and wound infections. J Surg Res 61, 425–432. [DOI] [PubMed] [Google Scholar]
- Hamood, A. N., Colmer-Hamood, J. A. & Carty, N. L. (2004). Regulation of Pseudomonas aeruginosa exotoxin A synthesis. In Pseudomonas: Virulence and Gene Regulation, pp. 389–423. Edited by J.-L. Ramos. New York: Kluwer Academic/Plenum.
- Hunt, T. A., Peng, W. T., Loubens, I. & Storey, D. G. (2002). The Pseudomonas aeruginosa alternative sigma factor PvdS controls exotoxin A expression and is expressed in lung infections associated with cystic fibrosis. Microbiology 148, 3183–3193. [DOI] [PubMed] [Google Scholar]
- Iglewski, B. H. & Kabat, D. (1975). NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci U S A 72, 2284–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanack, K. J., Runyen-Janecky, L. J., Ferrell, E. P., Suh, S. J. & West, S. E. (2006). Characterization of DNA-binding specificity and analysis of binding sites of the Pseudomonas aeruginosa global regulator, Vfr, a homologue of the Escherichia coli cAMP receptor protein. Microbiology 152, 3485–3496. [DOI] [PubMed] [Google Scholar]
- Leoni, L., Orsi, N., de Lorenzo, V. & Visca, P. (2000). Functional analysis of PvdS, an iron starvation sigma factor of Pseudomonas aeruginosa. J Bacteriol 182, 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. V. (1973). Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis 128, 506–513. [DOI] [PubMed] [Google Scholar]
- Matsumoto, T., Furuya, N., Tateda, K., Miyazaki, S., Ohno, A., Ishii, Y., Hirakata, Y. & Yamaguchi, K. (1999). Effect of passive immunotherapy on murine gut-derived sepsis caused by Pseudomonas aeruginosa. J Med Microbiol 48, 765–770. [DOI] [PubMed] [Google Scholar]
- Miller, J. H. (1972). Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Ochsner, U. A., Johnson, Z., Lamont, I. L., Cunliffe, H. E. & Vasil, M. L. (1996). Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol Microbiol 21, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Ochsner, U. A., Wilderman, P. J., Vasil, A. I. & Vasil, M. L. (2002). GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol Microbiol 45, 1277–1287. [DOI] [PubMed] [Google Scholar]
- Ohman, D. E., Sadoff, J. C. & Iglewski, B. H. (1980). Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun 28, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, R. H., DeBusscher, G. & McCombie, W. R. (1982). Development of broad-host-range vectors and gene banks: self-cloning of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol 150, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma, M., Worgall, S. & Quadri, L. E. (2003). Transcriptome analysis of the Pseudomonas aeruginosa response to iron. Arch Microbiol 180, 374–379. [DOI] [PubMed] [Google Scholar]
- Pollack, M. (2000). Pseudomonas aeruginosa. In Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases, pp. 2310–2335. Edited by G. L. Mandell, J. E. Bennett & R. Dolin. Philadelphia, PA: Churchill Livingstone.
- Raivio, T. L., Hoffer, D., Prince, R. W., Vasil, M. L. & Storey, D. G. (1996). Linker insertion scanning of regA, an activator of exotoxin A production in Pseudomonas aeruginosa. Mol Microbiol 22, 239–254. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. & Russell, D. W. (2001). Molecular Cloning: a Laboratory Manual, 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Sato, H. & Frank, D. W. (2004). ExoU is a potent intracellular phospholipase. Mol Microbiol 53, 1279–1290. [DOI] [PubMed] [Google Scholar]
- Schweizer, H. P. (1991). Escherichia–Pseudomonas shuttle vectors derived from pUC18/19. Gene 97, 109–121. [DOI] [PubMed] [Google Scholar]
- Smith, A. W. & Iglewski, B. H. (1989). Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res 17, 10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel, M. L., McKay, G. A. & Poole, K. (2003). Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother 47, 3202–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink, H. P., Okker, R. J. H., Wijffelman, C. A., Pees, E. & Lugtenberg, B. J. J. (1987). Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol Biol 9, 27–39. [DOI] [PubMed] [Google Scholar]
- Stachel, S. E., An, G., Flores, C. & Nester, E. W. (1985). A Tn3 lacZ transposon for the random generation of β-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J 4, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey, D. G., Frank, D. W., Farinha, M. A., Kropinski, A. M. & Iglewski, B. H. (1990). Multiple promoters control the regulation of the Pseudomonas aeruginosa regA gene. Mol Microbiol 4, 499–503. [DOI] [PubMed] [Google Scholar]
- Storey, D. G., Ujack, E. E., Rabin, H. R. & Mitchell, I. (1998). Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect Immun 66, 2521–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil, M. L. & Ochsner, U. A. (1999). The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol Microbiol 34, 399–413. [DOI] [PubMed] [Google Scholar]
- Walker, S. L., Hiremath, L. S. & Galloway, D. R. (1995). ToxR (RegA) activates Escherichia coli RNA polymerase to initiate transcription of Pseudomonas aeruginosa toxA. Gene 154, 15–21. [DOI] [PubMed] [Google Scholar]
- West, S. E., Kaye, S. A., Hamood, A. N. & Iglewski, B. H. (1994a). Characterization of Pseudomonas aeruginosa mutants that are deficient in exotoxin A synthesis and are altered in expression of regA, a positive regulator of exotoxin A. Infect Immun 62, 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, S. E., Sample, A. K. & Runyen-Janecky, L. J. (1994b). The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J Bacteriol 176, 7532–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M. J., McMorran, B. J. & Lamont, I. L. (2001). Analysis of promoters recognized by PvdS, an extracytoplasmic-function sigma factor protein from Pseudomonas aeruginosa. J Bacteriol 183, 2151–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang, M. C., Lee, V. T., Gilmore, M. E. & Lory, S. (2003). Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell 4, 253–263. [DOI] [PubMed] [Google Scholar]
- Woods, D. E. & Vasil, M. L. (1994). Pathogenesis of Pseudomonas aeruginosa infections. In Pseudomonas aeruginosa: Infections and Treatment, pp. 21–50. Edited by A. L. Baltch & R. P. Smith. New York: Marcel Dekker.