Abstract
The molecular basis of abrogation of Fv-1 restriction in mouse cells by murine leukemia virus was investigated. Two different lines of experimentation indicated that high-molecular-weight viral RNA is required for abrogation. First, the decay of abrogating ability of virus stocks heated at 43 degrees C was quantitatively correlated with a loss of intact virion 35S RNA. Second, Act D virions, which lack such RNA although they contain normal structural proteins, failed to abrogate. These findings imply that abrogation does not result from the mere entry of virion structural proteins into a cell. Additional data indicate that the role of viral RNA in abrogation is not that of a template for DNA synthesis. Virus particles lacking reverse transcriptase activity as a result of either mutation or heat inactivation exhibit abrogating activity even though they do not synthesize detectable viral DNA. In addition, abrogation was shown to take place in the presence of cytosine arabinoside, an inhibitor of DNA synthesis. Thus, abrogation does not depend on viral or cellular DNA synthesis, and the role of viral RNA in this process must involve some other function. The nature of this viral function and its occurrence in Fv-1 permissive cells are discussed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- BADER J. P. TRANSFORMATION OF ROUS SARCOMA VIRUS: A REQUIREMENT OF DNA SYNTHESIS. Science. 1965 Aug 13;149(3685):757–758. doi: 10.1126/science.149.3685.757. [DOI] [PubMed] [Google Scholar]
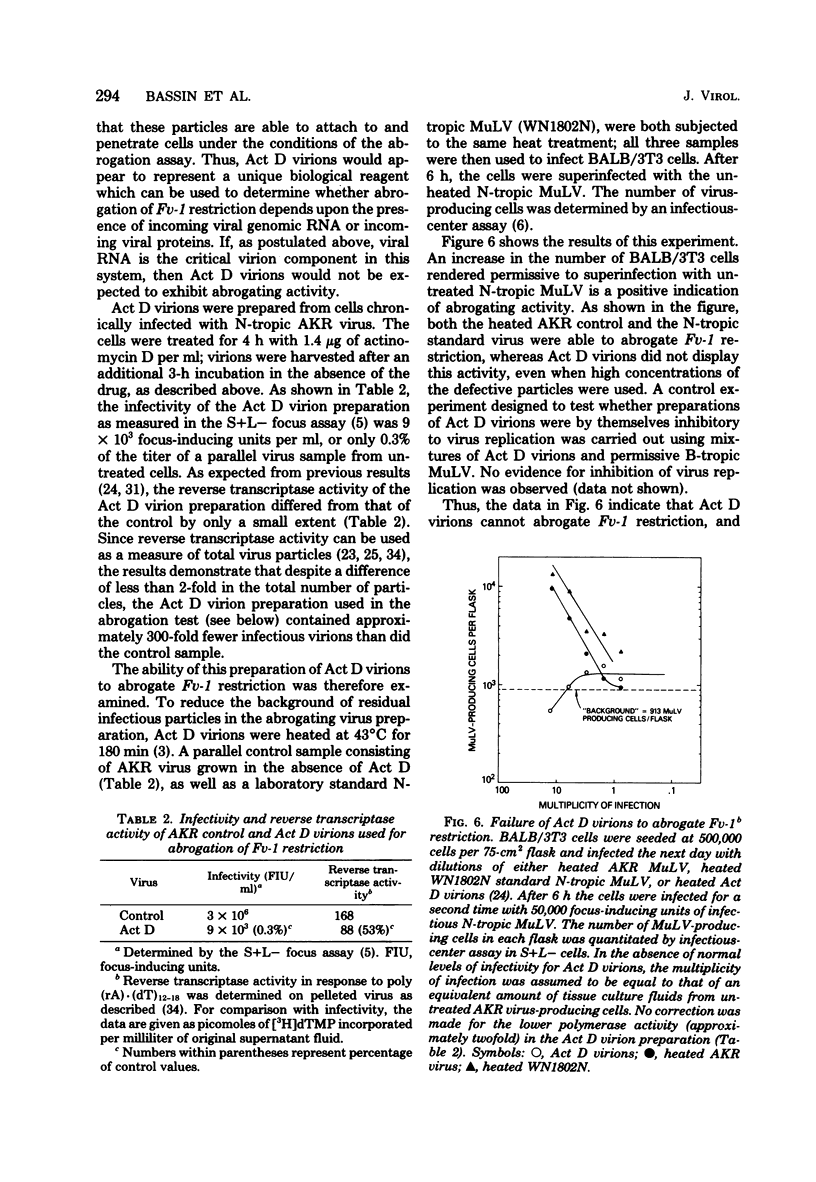
- Bassin R. H., Duran-Troise G., Gerwin B. I., Rein A. Abrogation of Fv-1b restriction with murine leukemia viruses inactivated by heat or by gamma irradiation. J Virol. 1978 May;26(2):306–315. doi: 10.1128/jvi.26.2.306-315.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Benjers B. M., Bassin R. H., Rein A., Gerwin B. I., Duran-Troise G. Mechanism of restriction of murine leukemia viruses varies between different strains of Fv-1n mice. Int J Cancer. 1979 Nov 15;24(5):600–607. doi: 10.1002/ijc.2910240513. [DOI] [PubMed] [Google Scholar]
- Bryant M. L., Klement V. Clonal heterogeneity of wild mouse leukemia viruses: host ranges and antigenicity. Virology. 1976 Sep;73(2):532–536. doi: 10.1016/0042-6822(76)90415-3. [DOI] [PubMed] [Google Scholar]
- Declève A., Niwa O., Gelmann E., Kaplan H. S. Replication kinetics of N- and B-tropic murine leukemia viruses on permissive and nonpermissive cells in vitro. Virology. 1975 Jun;65(2):320–332. doi: 10.1016/0042-6822(75)90038-0. [DOI] [PubMed] [Google Scholar]
- Duran-Troise G., Bassin R. H., Rein A., Gerwin B. I. Loss of Fv-1 restriction in Balb/3T3 cells following infection with a single N tropic murine leukemia virus particle. Cell. 1977 Mar;10(3):479–488. doi: 10.1016/0092-8674(77)90035-6. [DOI] [PubMed] [Google Scholar]
- Gallis B. M., Eisenman R. N., Diggelmann H. Synthesis of the precursor to avian RNA tumor virus internal structural proteins early after infection. Virology. 1976 Oct 15;74(2):302–313. doi: 10.1016/0042-6822(76)90337-8. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Meier H. A short-term quantitative XC assay for murine leukemia virus. Virology. 1976 Jul 15;72(2):509–513. doi: 10.1016/0042-6822(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Levin J. G. Interactions of murine leukemia virus core components: characterization of reverse transcriptase packaged in the absence of 70S genomic RNA. J Virol. 1977 Nov;24(2):478–488. doi: 10.1128/jvi.24.2.478-488.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Milstien J. B. An oligonucleotide affinity column for RNA-dependent DNA polymerase from RNA tumor viruses. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2599–2603. doi: 10.1073/pnas.69.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Rein A., Levin J. G., Bassin R. H., Benjers B. M., Kashmiri S. V., Hopkins D., O'Neill B. J. Mutant of B-tropic murine leukemia virus synthesizing an altered polymerase molecule. J Virol. 1979 Sep;31(3):741–751. doi: 10.1128/jvi.31.3.741-751.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Smith S. G., Peebles P. T. Two active forms of RD-114 virus DNA polymerase in infected cells. Cell. 1975 Sep;6(1):45–52. doi: 10.1016/0092-8674(75)90072-0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Selective decrease in the rate of cleavage of an intracellular precursor to Rauscher leukemia virus p30 by treatment of infected cells with actinomycin D. J Virol. 1976 Sep;19(3):1054–1072. doi: 10.1128/jvi.19.3.1054-1072.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr Top Microbiol Immunol. 1979;86:67–122. doi: 10.1007/978-3-642-67341-2_3. [DOI] [PubMed] [Google Scholar]
- Kelloff G. J., Hatanaka M., Gilden R. V. Assay of C-type virus infectivity by measurement of RNA-dependent DNA polymerase activity. Virology. 1972 Apr;48(1):266–269. doi: 10.1016/0042-6822(72)90135-3. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Grimley P. M., Ramseur J. M., Berezesky I. K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974 Jul;14(1):152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Rosenak M. J. Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1154–1158. doi: 10.1073/pnas.73.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Selective packaging of host tRNA's by murine leukemia virus particles does not require genomic RNA. J Virol. 1979 Jan;29(1):328–335. doi: 10.1128/jvi.29.1.328-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T., Prestidge L., Hogness D. S. A novel arrangement of tandemly repeated genes at a major heat shock site in D. melanogaster. Cell. 1978 Aug;14(4):901–919. doi: 10.1016/0092-8674(78)90345-8. [DOI] [PubMed] [Google Scholar]
- Lovinger G. G., Klein R., Ling H. P., Gilden R. V., Hatanaka M. Kinetics of murine type C virus-specific DNA synthesis newly infected cells. J Virol. 1975 Oct;16(4):824–831. doi: 10.1128/jvi.16.4.824-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Niwa O., Decléve A., Kaplan H. S. Conversion of restrictive mouse cells to permissiveness during sequential and mixed double infection by murine leukemia viruses. Virology. 1976 Oct 1;74(1):140–153. doi: 10.1016/0042-6822(76)90136-7. [DOI] [PubMed] [Google Scholar]
- Paskind M. P., Weinberg R. A., Baltimore D. Dependence of Moloney murine leukemia virus production on cell growth. Virology. 1975 Sep;67(1):242–248. doi: 10.1016/0042-6822(75)90421-3. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A. L., Gerwin B. I., Bassin R. H., Schwarm L., Schidlovsky G. A replication-defective variant of Moloney murine leukemia virus. I. Biological characterization. J Virol. 1978 Jan;25(1):146–156. doi: 10.1128/jvi.25.1.146-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtz R., Dolev S., Aboud M., Salzberg S. Viral genome RNA serves as messenger early in the infectious cycle of murine leukemia virus. J Virol. 1979 Sep;31(3):668–676. doi: 10.1128/jvi.31.3.668-676.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sveda M. M., Soeiro R. Host restriction of Friend leukemia virus: synthesis and integration of the provirus. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2356–2360. doi: 10.1073/pnas.73.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. K., Kiggans J. O., Yang D. M., Ou C. Y., Tennant R. W., Brown A., Bassin R. H. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2994–2998. doi: 10.1073/pnas.77.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H. Ultraviolet inactivation of murine leukemia virus and its assay in permissive and non-permissive cells. Int J Cancer. 1973 May;11(3):739–746. doi: 10.1002/ijc.2910110325. [DOI] [PubMed] [Google Scholar]