Abstract
Streptococcus is a dominant genus in the human oral cavity, making up about 20 % of the more than 800 species of bacteria that have been identified, and about 80 % of the early biofilm colonizers. Oral streptococci include both health-compatible (e.g. Streptococcus gordonii and Streptococcus sanguinis) and pathogenic strains (e.g. the cariogenic Streptococcus mutans). Because the streptococci have similar metabolic requirements, they have developed defence strategies that lead to antagonism (also known as bacterial interference). S. mutans expresses bacteriocins that are cytotoxic toward S. gordonii and S. sanguinis, whereas S. gordonii and S. sanguinis differentially produce H2O2 (under aerobic growth conditions), which is relatively toxic toward S. mutans. Superimposed on the inter-bacterial combat are the effects of the host defensive mechanisms. We report here on the multifarious effects of bovine lactoperoxidase (bLPO) on the antagonism between S. gordonii and S. sanguinis versus S. mutans. Some of the effects are apparently counterproductive with respect to maintaining a health-compatible population of streptococci. For example, the bLPO system (comprised of bLPO+SCN−+H2O2) destroys H2O2, thereby abolishing the ability of S. gordonii and S. sanguinis to inhibit the growth of S. mutans. Furthermore, bLPO protein (with or without its substrate) inhibits bacterial growth in a biofilm assay, but sucrose negates the inhibitory effects of the bLPO protein, thereby facilitating adherence of S. mutans in lieu of S. gordonii and S. sanguinis. Our findings may be relevant to environmental pressures that select early supragingival colonizers.
INTRODUCTION
The term ‘microbial ecosystem’ has been defined as an organization of micro-organisms that live in a particular niche, while subject to the environmental pressures of the niche (Raes & Bork, 2008). These pressures may be biotic (e.g. self, other microbial species, plants and animals) and abiotic (e.g. temperature, pH, nutrients, etc.) in origin. Dental plaques are examples of dynamic and exceedingly complex microbial ecosystems that are thought to comprise at least 800 bacterial species (Kroes et al., 1999; Paster et al., 2006; Aas et al., 2005, 2008; Preza et al., 2008; Becker et al., 2002; Paster et al., 2001; Dethlefsen et al., 2007). Many of the environmental factors that influence the speciation of oral biofilms have been reviewed in other articles (Marsh, 2005; Overman, 2000; Rosan & Lamont, 2000; Sissons, 1997; Socransky & Haffajee, 2005; ten Cate, 2006), including the effects of pH (Burne & Marquis, 2000), nutrients (Sissons et al., 2007), fluoride (Bowden, 1990) and various antimicrobials (Dashper et al., 2007; Fine et al., 2001; Leung et al., 2005; Yang et al., 2006; Zaura-Arite et al., 2001). The effects of the host immune system on oral biofilms are less well understood. This paper focuses on the influence of defensive peroxidases on inter-streptococcal interactions.
There are two host-derived defensive peroxidases in the human oral cavity, salivary peroxidase (hSPO) and myeloperoxidase (hMPO) (Ashby, 2008; Ihalin et al., 2006). hSPO is a normal non-inducible component of the saliva of the parotid and submandibular glands (Riva et al., 1978), whereas hMPO is an offensive mechanism of neutrophilic polymorphonuclear leukocytes (PMNs). Employing hydrogen peroxide (H2O2) as an oxidant, the defensive peroxidases use inorganic ions to produce antimicrobials that are generally believed to be more effective than H2O2 itself. hMPO is capable of producing hypochlorous acid (HOCl), a powerful and comparatively indiscriminate biocide. While the production of HOCl by the hMPO system is possible in other environments (e.g. the gingival sulcus), it is disfavoured in the part of the oral cavity that is controlled by saliva (Ashby, 2008). Instead, hSPO and hMPO work in unison in the supragingival regime to exclusively oxidize thiocyanate (SCN−) to produce hypothiocyanite (OSCN−, a reactive inorganic species), which constantly bathes early oral plaques (Ashby, 2008). We have employed bovine milk lactoperoxidase (bLPO), a readily available enzyme, as a surrogate for hSPO. The two enzymes are structurally and catalytically similar (Mansson-Rahemtulla et al., 1988).
Streptococcus is a dominant genus in the human oral cavity, making up about 20 % of the more than 800 species of bacteria that have been identified (Marsh, 1999), and about 80 % of the early biofilm colonizers (Rosan & Lamont, 2000). Oral streptococci include both health-compatible (e.g. Streptococcus gordonii and Streptococcus sanguinis) and pathogenic species (e.g. the cariogenic Streptococcus mutans). Because the streptococci have similar metabolic requirements, they have developed defence strategies that lead to antagonism (also known as bacterial interference). S. mutans expresses bacteriocins that are cytotoxic toward S. gordonii and S. sanguinis (Hamada & Ooshima, 1975; Kreth et al., 2008a), whereas under aerobic growth conditions S. gordonii and S. sanguinis differentially produce H2O2, which is relatively toxic toward S. mutans (Kreth et al., 2008a).
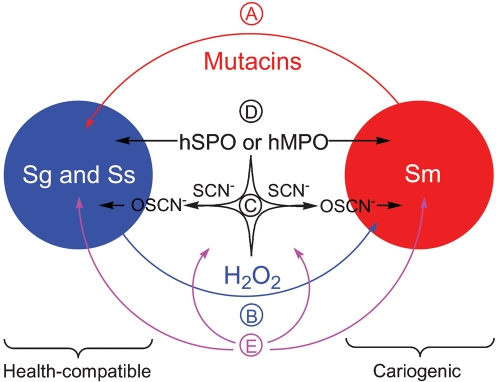
The present study focuses on the effects of components of the lactoperoxidase (LPO) system (comprising bLPO, SCN− and H2O2) on the pair-wise growth of S. mutans, S. gordonii and S. sanguinis and on the capacity of bLPO protein to influence the ability of individual bacterial species to form biofilm in an in vitro biofilm model. An effort has been made to simulate the aerobic growth conditions that probably exist during the initial colonization of tooth enamel (i.e. biofilm growth under low oxygen tension). The factors that we suggest are relevant to the subject of this study are summarized in Fig. 1. This is the first investigation of which we are aware that probes the influence of peroxidase systems on interspecies competition in a biofilm and on the biofilm-forming abilities of oral streptococci.
Fig. 1.
Competitive host peroxidase and streptococcal defence mechanisms in the supragingival region of the oral cavity. (A) Cariogenic S. mutans produces bacteriocins (mutacins) that are known to be cytotoxic to other oral streptococci. (B) Oral health-compatible S. gordonii and S. sanguinis differentially produce H2O2 under aerobic growth conditions, thereby promoting an ecological advantage. (C) The human supragingival peroxidase system (consisting of the enzyme hSPO or hMPO, the substrate SCN− and the oxidant H2O2) produces the antimicrobial hypothiocyanite (OSCN−). (D) The peroxidase proteins themselves are known to be antimicrobial. (E) Environmental pressures, which may be biotic or abiotic in origin, could affect the above defensive mechanisms.
METHODS
Bacterial strains and media.
S. mutans UA140 (Qi et al., 2001), S. sanguinis SK36 (Xu et al., 2007) and S. gordonii DL1 (Pakula & Walczak, 1963) were grown aerobically (5 % CO2) at 37 °C either in brain heart infusion broth (BHI; Difco) without shaking or on BHI agar plates.
Competition assays on solid medium: effect of the peroxidase system.
To assess competitive growth, a protocol described previously was used with modifications (Kreth et al., 2008a). Briefly, 8 μl of an overnight culture of each species in BHI medium was inoculated as the pioneer colonizer onto a 90 mm BHI agar plate. The BHI medium and the agar plates were alternatively supplemented or not supplemented with 10 mM SCN−. bLPO (TATUA Co-operative Dairy Company) was introduced using two different methods: 100 μl PBS containing 250 μg bLPO was spread as a lawn using a Spiral Biotech Autoplate 4000, or 20 μg bLPO was added to the 8 μl of inoculum of both the pioneer and the competing species just before plating. A reference culture (spatially separated from the pioneer and subsequent competing inocula) was inoculated at the same time. After incubation for 16 h, 8 μl of the competing species was inoculated next to the pioneer colonizer such that the colonies almost touched each other. A reference of the competing colonizer was inoculated at the same time. The inoculation order was pioneer/competitor: S. mutans/S. gordonii, S. gordonii/S. mutans, S. mutans/S. sanguinis, S. sanguinis/S. mutans, S. gordonii/S. gordonii, and S. sanguinis/S. gordonii. The plates were prepared in triplicate. After incubation for an additional 16 h, the plates were photographed with an in-house-constructed, optical density-calibrated digital camera system. Growth inhibition was assessed by the presence of a proximal zone of inhibition at the intersection with the pioneer colony.
Competition assays on solid medium: effect of the catalase.
A protocol analogous to the one used to investigate the effects of the bLPO system was followed, except that no SCN− was added to the medium and 8 μg catalase (Sigma) was added to the 8 μl inocula of both the pioneer and the competing species just before plating. Alternatively, 8 μl PBS containing 8 μg catalase was added to the site of inoculation, and once absorbed, 8 μl of the inocula followed. Both procedures for introducing catalase gave the same visual result. The inoculation order was pioneer/competitor: S. mutans/S. sanguinis, S. sanguinis/S. mutans and S. sanguinis/S. sanguinis.
Biofilm quantification with 96-well microtitre dish assay.
Biofilm formation was measured using a modification of the Christensen microtitre plate test (Christensen et al., 1985). Microtitre wells (Falcon Microtest 96, Becton Dickinson) were inoculated from overnight BHI-grown cultures of S. mutans, S. gordonii or S. sanguinis diluted 1 : 60 in fresh BHI medium with or without the addition of bLPO and SCN−, as indicated (final volume 150 μl with 107 cells ml−1). The cells were grown for 16 h, and the medium was removed by inverting the dish with shaking. The remaining cells were stained with 150 μl per well of crystal violet (CV) (2.3 % w/v, Accustain Crystal Violet Solution, Sigma Diagnostics) for 15 min, and the microtitre dish was subsequently washed twice with water and air-dried. Biofilm formation was quantified by solubilization of the CV staining in 150 μl of 95 % ethanol per well. The absorbance of the resulting solution (100 μl) was measured at 570 nm with a microplate reader (model 680, Bio-Rad). Experiments were repeated three times with multiple replicates (at least eight) per microtitre dish.
Confocal laser scanning microscopy (CLSM).
Biofilms for CLSM imaging were grown essentially as described above for the 96-well plate assay. The cell suspensions were inoculated into the Lab-TekII Chamber Slide System (Nalge Nunc International), where the objective slide was replaced with a thin cover slide to accommodate the working distance of the microscope objective. Biofilms were grown aerobically for 16 h at 37 °C. Cells were stained with a LIVE/DEAD Bacterial Viability kit (Molecular Probes) according to the manufacturer's recommendations. CLSM was performed using a LSM-510META laser scanning confocal microscope (Carl Zeiss) equipped with detectors and filter sets for visualizing green and red fluorescence. Images were obtained with a ×63 water (1.2 numerical aperture) objective.
Rate of reaction of H2O2 with BHI.
The rate of reaction of H2O2 with BHI was determined by adding a 500 μM bolus of H2O2 to BHI broth followed by periodic sampling. The concentration of H2O2 was determined using a modification of a protocol described by Gilliland (1969). Briefly, 50 μl of 0.1 % w/v o-dianisidine (ICN) in methanol was added to 750 μl of 100 mM acetate buffer (pH 4.5) with 2.0 μg horseradish peroxidase (HRP; Sigma) in methanol, followed by 200 μl of the sample of BHI which contained H2O2. The reaction mixture was incubated at 20 °C for 10 min, and the chromophore was stabilized by adding 50 μl of 4 M HCl. The absorbance at 400 nm was determined using an identical solution (o-dianisidine+HRP+BHI) without H2O2 as a blank, and the concentration was calculated from a standard curve prepared using a 500 μM solution of H2O2 [from a 100 mM stock solution in water that was prepared from a commercial 30 % H2O2 solution (Sigma)]. The concentration of H2O2 in the 100 mM stock solution was determined spectrophotometrically (ε240=43.6 M−1 cm−1) using an HP 8452A diode array UV-visible spectrophotometer.
Rate of reaction of OSCN− with BHI.
The rate of reaction of OSCN− with BHI was determined by a colorimetric assay using 5-thio-2-nitrobenzoic acid (TNB) (Aune & Thomas, 1977). As H2O2 is known to interfere with the assay (Bulaj et al., 1998), it was first established that the BHI contained no measurable H2O2, using the above HRP/o-dianisidine method. TNB was prepared in situ by reduction of a 520 μM solution of 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB; TCI) in 0.1 M phosphate buffer (pH 7.4) with a 0.5 M stock solution of 2-mercaptoethanol in water. Excess 2-mercaptoethanol was avoided by monitoring the absorption of DTNB (λmax=324 nm, ε324=17 780 M−1 cm−1) (Riddles et al., 1979). The final concentration of TNB was determined spectrophotometrically (λmax=412 nm, ε412=14 150 M−1cm−1) (Eyer et al., 2003) as 860 μM (∼95 % reduction of the original DTNB). A 1 mM solution of OSCN− was prepared in 0.1 M phosphate buffer (pH 7.4) by reacting 1 mM H2O2 with 10 mM SCN− in the presence of 10 μg bLPO ml−1. The resulting solution of OSCN− was mixed 1 : 1 with BHI broth to give a 500 μM solution of OSCN− in 50 % BHI. The amount of OSCN− in 25 μl BHI solution was determined periodically by adding it to a 50 μM solution of TNB (prepared by adding 58 μl TNB stock solution to 917 μM 0.1 M phosphate buffer at pH 7.4. Taking into account the 2 : 1 stoichiometry of the TNB to OSCN− reaction, the concentration of OSCN− was determined spectrophotometrically by monitoring the absorption of TNB at 412 nm.
Concentration of thiols in BHI.
Thiols in BHI were quantified with Ellman's reagent (DTNB) using a published procedure (Bulaj et al., 1998; Ellman, 1959).
Statistical methods.
Statistical analysis of two datasets was performed with QuickCalcs online calculators (http://www.graphpad.com/quickcalcs/index.cfm) using the t test software presenting mean and sd. Differences between group means (bLPO concentrations) were tested with ANOVA (http://statpages.org/anova1sm.html). Statistically significant differences were set at a P value of <0.05.
RESULTS
The bLPO system protects streptococci from the toxic effects of H2O2
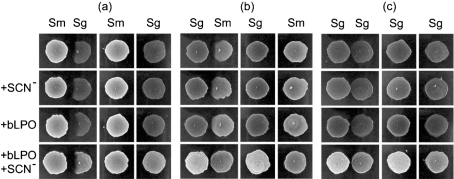
To assess the influence of each component of the bLPO system (comprised of bLPO, SCN− and H2O2), the effects of BHI (control), BHI+SCN−, BHI+bLPO, and BHI+bLPO+SCN− were investigated using a competition assay on agar. For the agar with SCN−, BHI was supplemented with 10 mM NaSCN before autoclaving. For the agar with bLPO, the protein was filter-sterilized and either spread as a lawn on the agar before inoculation or bLPO was added to the inocula. The two methods of introducing bLPO gave visually indistinguishable results. Typical results are illustrated in Fig. 2. Visually similar results were obtained when S. sanguinis was substituted for S. gordonii for experiments analogous to those of Fig. 2(a, b) (data not shown). Proximal zones of inhibition around competing colonies of S. gordonii and S. sanguinis were observed under all growth/medium conditions when S. mutans was the pioneer (e.g. Fig. 2a). In the absence of bLPO, proximal zones of inhibition around competing colonies of S. mutans were observed when S. gordonii (or S. sanguinis) was the pioneer (e.g. Fig. 2b). In some cases, bLPO alone resulted in a reduction in the zone of inhibition for competing colonies of S. mutans when S. gordonii or S. sanguinis was the pioneer (e.g. Fig. 2b for +bLPO). In all cases, bLPO in the presence of SCN− resulted in the elimination of the zone of inhibition for competing colonies of S. mutans when S. gordonii or S. sanguinis was the pioneer (e.g. Fig. 2b for bLPO+SCN−). Reduction of the zones of inhibition by the protein alone was never as notable as when the protein was added in conjunction with SCN−. Mature colonies of S. gordonii retarded the establishment of adjacent colonies of S. gordonii (Fig. 2c) and S. sanguinis (data not shown). For all colonies of S. gordonii and S. sanguinis (regardless of whether they were pioneers or competitors), the presence of bLPO+SCN− resulted in more dense colonies (e.g. S. gordonii in Fig. 2 for the control versus bLPO+SCN−).
Fig. 2.
Effect of the bLPO system on the growth of S. mutans and S. gordonii. (a) S. mutans was inoculated first and grown aerobically (5 % CO2) for 16 h at 37 °C on BHI agar with (+SCN−) or without 10 mM SCN− and with (+bLPO) or without 20 μg bLPO per 8 μl of inoculum. S. gordonii was then inoculated next to the pioneer colonizer (with or without bLPO in the inoculum), and the plates were incubated for 16 h and then photographed. The columns labelled Sm and Sg are reference cultures grown in spatial isolation. (b) The same protocol was followed, except that S. gordonii was the pioneer colonizer and S. mutans was the competing colonizer. (c) The same protocol was followed as for (a) and (b), except that S. gordonii was both the pioneer colonizer and the competing colonizer.
Catalase protects the streptococci from the toxic effects of H2O2
It has been previously suggested that catalase-negative oral bacteria that grow in the vicinity of catalase-positive bacteria are afforded protection from H2O2 (Jakubovics et al., 2008). Accordingly, we have observed that inhibition of competing colonies of S. mutans by pioneer colonies of S. gordonii and S. sanguinis was abolished by catalase (e.g. Fig. 3). Not surprisingly, the addition of catalase to the inocula had no effect on the bacteriocin-induced inhibition of competing colonies of S. gordonii and S. sanguinis by pioneer colonies of S. mutans (data not shown).
Fig. 3.
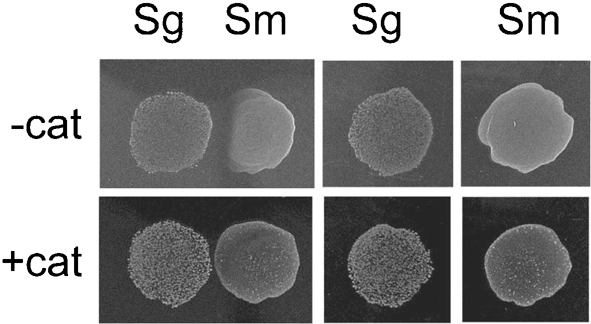
Effect of catalase on the growth of S. gordonii and S. mutans. The same protocol was followed as for Fig. 2, except that catalase was employed instead of bLPO.
Hypothiocyanite is not an obvious influencing factor
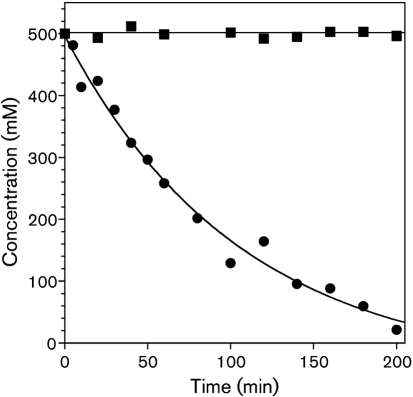
In contrast to H2O2, which is relatively inert towards thiol moieties, OSCN− is reactive, and in addition it spontaneously decomposes (via an unknown mechanism) in the absence of a reaction partner (Aune & Thomas, 1977; Christy & Egeberg, 2000; Tenovuo et al., 1986; Thomas, 1981). We determined that the half-life of OSCN− in BHI (Fig. 4) is similar to that in PBS under similar conditions (data not shown). The kinetics of decomposition of OSCN− in BHI are consistent with our observation that the BHI does not contain thiol groups (data not shown). At room temperature, 500 μM OSCN− decomposed in BHI in a first-order process with a half-life of about 1 h, with no detectable OSCN− remaining after about 4 h (Fig. 4). Under similar conditions at 37 °C, no OSCN− was detected after 1 h (data not shown). In contrast, no loss of H2O2 was observed in BHI over a period of 5 h at 20 °C (Fig. 4).
Fig. 4.
Rate of decomposition of an initial concentration of OSCN− of 500 μM at pH 7.4 and 19 °C in the presence of 50 % BHI (•). A first-order fit is illustrated (k=9.5±1.4×10−3 min−1; t1/2=73 min). H2O2 (initial concentration=500 μM) is stable under the same conditions (▪).
Our observations suggest that under the conditions of our competitive assays on solid media, the OSCN− that is produced decomposes before it has a marked effect on adjacent colonies. However, there is also no inhibitory effect of OSCN− on the pioneer colonies of S. gordonii and S. sanguinis (when grown in the presence of bLPO+SCN−). Indeed, denser colonies of these H2O2-producing bacteria were observed in the presence of bLPO+SCN−. This observation suggests that H2O2 is more cytotoxic to S. gordonii and S. sanguinis than the OSCN− that is presumably produced. This issue will be addressed further in the Discussion (see Fig. 8). We note that because OSCN− decomposes faster than H2O2, the competition assay on solid media does not address the issue of whether OSCN− or H2O2 is more cytotoxic to S. mutans.
Fig. 8.
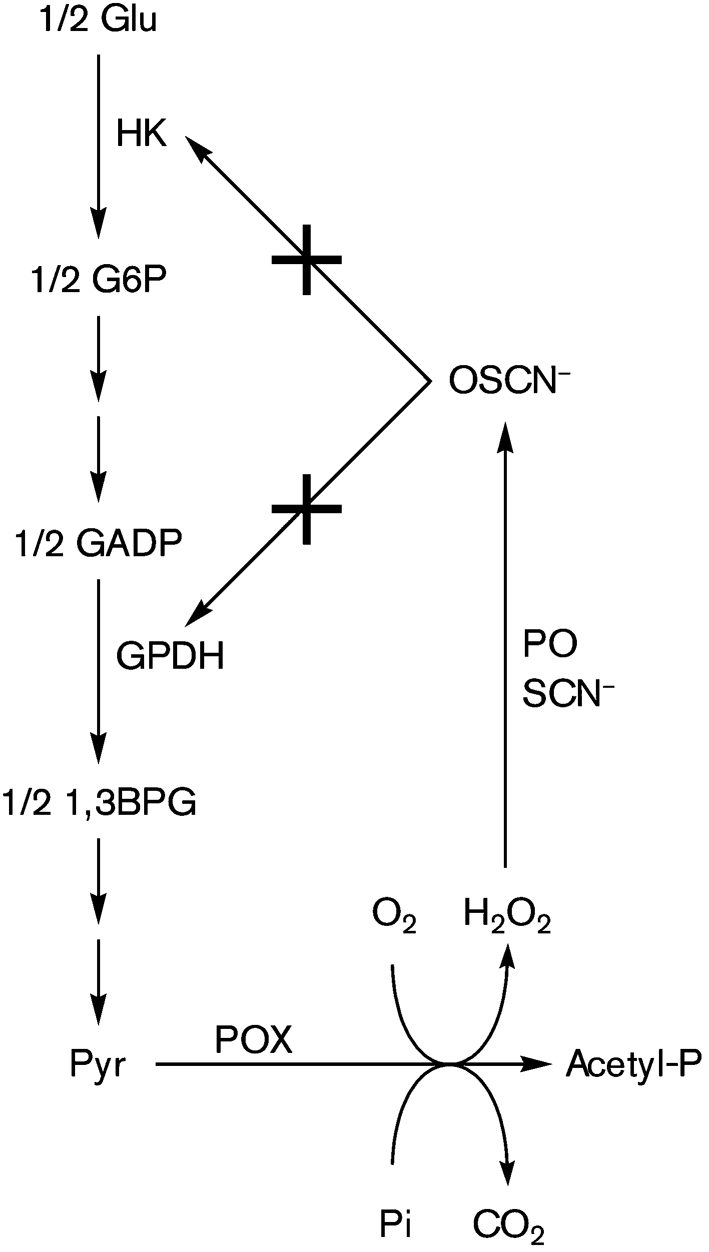
Feedback limitation of H2O2 production by pyruvate oxidase (POX)-catalysed oxidation of pyruvate (Pyr) through inhibition of the upstream glycolytic enzymes hexokinase (HK) and glyceraldehyde-3-phosphate dehydrogenase (GPDH) by host peroxidase (PO=hSPO and hMPO)-catalysed production of OSCN−. G6P, glucose 6-phosphate; GADP, glyceraldehyde 3-phosphate; 1,3BPG, 1,3-bisphosphoglycerate.
LPO inhibits biofilm formation by streptococci, but sucrose overcomes the effect for S. mutans only
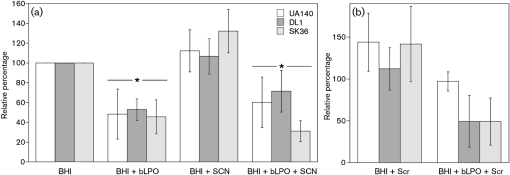
Oral streptococci must attach to the tooth surface to avoid eradication by saliva flow. After attachment, the cells develop into stratified biofilm structures. This process is accompanied by the presence of saliva and salivary components, including the defensive peroxidase systems. To learn whether the LPO system influences the biofilm formation of S. sanguinis, S. gordonii and S. mutans, the relative biomass of attached cells after overnight biofilm development was assessed with a microtitre plate biofilm assay. Compared with the BHI control, cells grown in the presence of 1 mg bLPO ml−1 showed a reduced ability to form biofilm on the polystyrene surface. The biomass decreased to about 50 % (Fig. 5a). A similar decrease was observed when SCN−-containing medium was supplemented with bLPO. Both reductions were significant for all strains as determined by Student's t test (P<0.05). In contrast, BHI supplemented with SCN− did not decrease in biomass, indicating that bLPO alone is sufficient to exert an effect on the biofilm-forming abilities of oral streptococci. The terminal cell density of all species (determined spectrophotometrically) was not influenced by bLPO, SCN− or bLPO+SCN− (versus the BHI control) under the tested conditions, suggesting that the reduced biomass was not a consequence of decreased growth (data not shown).
Fig. 5.
Quantification of attached biomass using a microtitre plate biofilm assay. Biofilm formation of S. mutans (UA140, white), S. gordonii (DL1, dark grey) and S. sanguinis (SK36, light grey) on microtitre plates was quantified with CV staining. Values were normalized to the BHI control, which was set to 100 %. (a) Biofilm biomass of cultures in BHI medium, BHI plus bLPO (1 mg ml−1), BHI plus SCN− (10 mM) and BHI plus bLPO (1 mg ml−1) and SCN− (10 mM). (b) Biofilm biomass of cultures supplemented with 0.5 % sucrose and 0.5 % sucrose plus bLPO (1 mg ml−1). Data presented are the means±sd from three independent experiments. Asterisks indicate statistical significance.
Earlier reports have shown that S. mutans is better able to withstand shear stress (Yang et al., 2006) and antimicrobials (Kreth et al., 2008b) when grown in the presence of sucrose. This has been attributed to the production of extracellular glucan polymers. Addition of 0.5 % sucrose increased the attached biomass of S. mutans and S. sanguinis slightly, but seemed to have no effect on S. gordonii. Addition of 0.5 % sucrose to cultures with bLPO, however, increased the attached biomass of S. mutans to a value that was similar to that for the BHI control. Accordingly, the difference between bLPO and bLPO with sucrose was statistically significant for the attached biomass of S. mutans (P<0.05). Sucrose addition did not influence the biomass of S. sanguinis and S. gordonii when grown in the presence of bLPO (Fig. 5b). This suggests that sucrose-dependent glucan formation can overcome the inhibitory effect of bLPO during S. mutans biofilm formation.
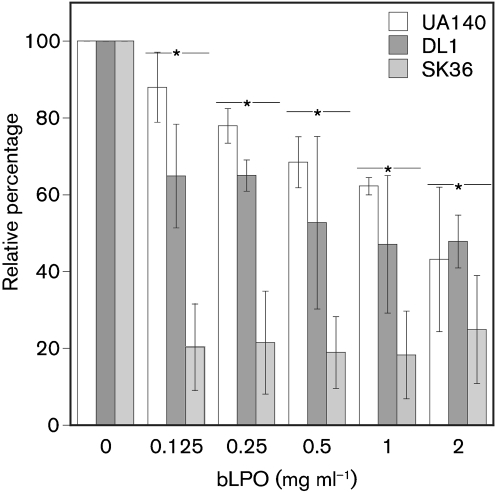
Biofilm inhibition by LPO is dose-dependent
Addition of varying concentrations of bLPO from 0.125 to 2 mg ml−1 resulted in a significant dose-dependent inhibition of biofilm growth for S. mutans (P=0.0065) and S. gordonii (P=0.04) (Fig. 6), as determined by a one-way ANOVA statistical test. Interestingly, for S. sanguinis the inhibition under the tested conditions was similar for all tested concentrations of bLPO. This suggests a differential response of oral streptococci to the presence of bLPO during biofilm formation.
Fig. 6.
Dose-dependent effect of bLPO protein (no SCN−) upon biofilm growth of S. mutans (UA140, white), S. gordonii (DL1, dark grey) and S. sanguinis (SK36, light grey) using the 96-well plate assay. Cultures were grown in BHI medium containing different concentrations of LPO (ranging from 0.125 to 2 mg ml−1). Values were normalized to the BHI control, which was set to 100 %. Data presented are the means±sd from three independent experiments. Asterisks indicate statistical significance.
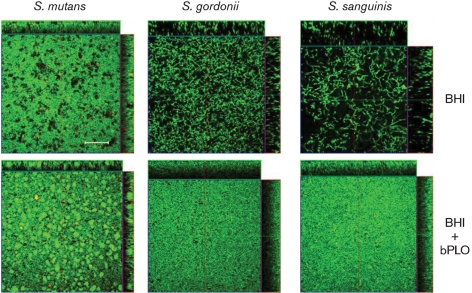
LPO alters biofilm architecture
Addition of bLPO to the growth medium during biofilm formation has an obvious effect on the biofilm mass of all three tested oral streptococci. To further characterize the effect of bLPO on biofilms of S. mutans, S. sanguinis and S. gordonii, the biofilm architecture was investigated with CLSM. Static overnight biofilms were grown and stained with LIVE/DEAD viability stain for visualization. S. mutans grown in BHI shows a characteristic biofilm architecture (a confluent layer of cells punctuated by void areas) that has been described previously (Kreth et al., 2008b). Cells grown with bLPO seemed to cluster in dense micro-colonies with an absence of void areas. Overall, the biofilm of S. mutans appeared denser when grown in the presence of bLPO (Fig. 7). The biofilm architectures of S. sanguinis and S. gordonii appeared similar to one another in BHI medium. Both species formed long chains that seemed to be loosely attached and tangled together, leaving larger void areas between chains. Growth in the presence of bLPO again changed the biofilm appearance dramatically. Both S. sanguinis and S. gordonii appeared densely packed, the void areas disappeared completely, and the height of the biofilm was reduced. In summary, the biofilms of S. mutans, S. sanguinis and S. gordonii grown in the presence of bLPO seemed to be more compact than their counterparts grown in BHI alone. However, LIVE/DEAD viability stain did not reveal any differences between BHI and BHI+bLPO-grown cells (Fig. 7).
Fig. 7.
Biofilm architecture of cultures grown with and without LPO. CLSM images of S. mutans, S. sanguinis and S. gordonii biofilms grown in the Lab-TekII Chamber Slide system. Cells were stained with the LIVE/DEAD viability fluorescent stain. Bar, 100 μm.
DISCUSSION
The results we have presented illustrate the influences of the bLPO system on the interspecies competition among three oral bacteria. Fig. 1 summarizes the competitive host and bacterial defence mechanisms that are potentially relevant to the subject of our study. (A) Cariogenic S. mutans produces bacteriocins, including lantibiotic mutacin I and non-lantibiotic mutacin IV, that are known to be cytotoxic to other oral streptococci (Hamada & Ooshima, 1975; Kreth et al., 2008a). (B) Commensal (oral health-compatible) S. gordonii and S. sanguinis differentially produce H2O2 under aerobic growth conditions, thereby promoting an ecological advantage (Kreth et al., 2008a). (C) The human supragingival peroxidase system (consisting of the enzyme hSPO or hMPO, the substrate SCN−, and the oxidant H2O2) produce the antimicrobial hypothiocyanite (OSCN−). (D) The peroxidase proteins themselves are known to be antimicrobial (Tenovuo & Knuuttila, 1977b). (E) Environmental pressures that may be biotic (e.g. other microbial species) or abiotic (e.g. nutrients) in origin could affect the above defensive mechanisms. The last category is intended to draw attention to the fact that the in vitro models we have employed in our investigation are incomplete. Thus, the intent of the present study has been to uncover parameters that may be relevant in the oral cavity, but, as a caveat, these data do not unequivocally demonstrate the dominance of (A–D) over (E) in vivo. Indeed, some of the factors that enhance or diminish the influence of (A–D) are discussed next.
Inhibition of oral streptococci by hypothiocyanite
It has been previously demonstrated that S. mutans is inhibited by the LPO+SCN−+H2O2 system when H2O2 is added exogenously (Donoghue et al., 1985; Hoogendoorn, 1976; Kersten et al., 1981; Mansson-Rahemtulla et al., 1987; Rupf et al., 2001; Tenovuo & Knuuttila, 1977a; Tenovuo & Knuuttila, 1977b; Thomas et al., 1983; Thomas et al., 1994; van der Hoeven & Camp, 1993). Furthermore, Thomas et al. (1983) have shown that the amount of H2O2 that is produced by some strains of S. mutans during aerobic growth is sufficient to activate the bLPO system. In contrast to the large number of studies of the effects of the LPO and related systems on S. mutans, there have been relatively few studies of the effect on S. sanguinis (formally sanguis) (Carlsson, 1980; Carlsson et al., 1983; Courtois et al., 1995; van der Hoeven & Camp, 1993), and we are not aware of any efforts to quantify the effects of the peroxidase systems on S. gordonii. Nonetheless, a number of other streptococcal species have been shown to be susceptible to inhibition by OSCN− in sufficient concentration, including Streptococcus oralis (van der Hoeven & Camp, 1993), Streptococcus mitior (Donoghue et al., 1987), Streptococcus rattus (Donoghue et al., 1987), Streptococcus uberis (Marshall et al., 1986), Streptococcus dysgalactiae (Mickelson & Brown, 1985), Streptococcus agalactiae (Mickelson, 1979) and Streptococcus pyogenes (Mickelson, 1966). Importantly, many factors determine susceptibility to inhibition by OSCN−, including pH, the flux of the peroxidase system (availability of H2O2), cell thiol content, stored carbohydrate content, and the ability of the medium to quench OSCN−. Peroxidase systems produce OSCN− at the expense of H2O2, but both chemical species can be cytotoxic; apparently, H2O2 can be more so under some circumstances (see below).
The peroxidase system protects streptococci from H2O2 toxicity
Although H2O2 production is part of the lifestyle of streptococci, they lack conventional defence mechanisms against the toxicity of H2O2 (e.g. catalase and glutathione). Resistance of streptococci to H2O2 toxicity has been attributed to the absence of haemoproteins and related metalloenzymes (Dolin, 1961). The relative importance of H2O2 as a toxic agent in the oral cavity is difficult to assess because varying amounts act on microcosm plaques in diverse environments. Individual susceptibilities of oral streptococci to H2O2 toxicity vary. Some mutans streptococci accumulate up to 2 mM H2O2 in their media during growth on glucose (Thomas & Pera, 1983; Thomas, 1985), whereas other oral streptococci are inhibited by micromolar concentrations of H2O2 (Adamson & Carlsson, 1982; Carlsson et al., 1983; Donoghue et al., 1987). Furthermore, there are likely to be synergisms in multi-species plaques. For example, while pure cultures of oral streptococci produce H2O2, H2O2 is not found in dental plaque or salivary sediment, despite streptococci being major components of their mixed bacterial populations. This could be due to the rapid consumption of H2O2 by the hSPO and hMPO systems. Indeed, it has been suggested that the human peroxidases of the oral cavity protect host tissues (Carlsson, 1987; Tipton et al., 1995) and H2O2-sensitive bacteria (Adamson & Carlsson, 1982) by consuming endogenous H2O2 (with the production of the comparatively less toxic OSCN−). An alternative mechanism for the consumption of free H2O2 is by catalase-positive species of bacteria (e.g. Neisseria, Haemophilus, Actinomyces and Staphylococcus species; see Fig. 3) (Ryan & Kleinberg, 1995).
S. sanguinis and S. gordonii produce H2O2 under conditions of aerobic mixed-acid fermentation during the oxidation of pyruvate (Pyr) as catalysed by pyruvate oxidase (Pox) (Kreth et al., 2008a). Under the conditions of our agar plate assay, bLPO+SCN− protects S. mutans from the cytotoxic effects of H2O2 produced by S. sanguinis and S. gordonii (Fig. 2b). While this might be considered counterproductive for a host defence mechanism that presumably protects the oral cavity from cariogenic pathogens, we note that bLPO+SCN− also facilitates the growth of the health-compatible S. sanguinis and S. gordonii (see Fig. 2 for the control versus bLPO+SCN−). Thus, bLPO+SCN− is capable of protecting H2O2-producing bacteria from themselves. Of particular relevance are the results shown in Fig. 2(c), which suggest that regrowth adjacent to more mature, H2O2-producing colonies may be facilitated by the peroxidase system. The presence of bLPO+SCN− results in denser colonies of S. gordonii and S. sanguinis, which implies that the OSCN− that is presumably generated by the peroxidase system is less cytotoxic than the H2O2 that is consumed in the process. However, compounding factors may be relevant: it is known that the bLPO+SCN−+H2O2 system is capable of inhibiting enzymes in the glycolytic metabolic pathway, including hexokinase (HK) (Adamson & Pruitt, 1981; Pruitt & Adamson, 1977) and glyceraldehyde-3-phosphate dehydrogenase (GPDH) (Shin et al., 2001). It is therefore conceivable that a feedback mechanism exists by which the peroxidase system simultaneously destroys H2O2 and inhibits key metabolic steps in the glycolytic pathway that may disfavour the production of H2O2 by Pox (Fig. 8). We note that the cell density that is achieved for S. gordonii (Fig. 3) and S. sanguinis (data not shown) in the presence of catalase is less than that achieved in the presence of bLPO+SCN− (e.g. Fig. 2 for S. gordonii), which is consistent with the hypothesis shown in Fig. 8.
Which factors dominate in the oral cavity?
The presumed role of the innate components of the host defences in the oral cavity is to place selective pressures on pathogens while passively facilitating the growth of health-compatible bacterial species. Using in vitro models, we have illustrated herein two major effects of a (model) host peroxidase system on oral streptococcal speciation: (1) the peroxidase system protects all of the streptococci from H2O2; and (2) the peroxidase protein inhibits biofilm formation. We note that our observations are consistent with previous studies (which have primarily employed planktonic cultures). While the overall effect of innate defence mechanisms such as the peroxidases benefits the host, there may be unintended consequences of the non-specific host defensive stratagems at the bacterial species level. For example, the peroxidase system suppresses the H2O2-induced antagonism of cariogenic S. mutans by health-compatible S. gordonii and S. sanguinis. Also, in the case of S. mutans (but not the health-compatible streptococci), sucrose overcomes the inhibition of biofilm growth by the bLPO protein, perhaps thereby giving the pathogen an ecological advantage. However, what are missing from this simple description are the differential effects of the innate defence mechanisms. While in vitro experiments such as those described here are capable of identifying relevant parameters that may affect speciation of multispecies biofilms, they do not necessarily indicate the dominant factors in vitro. Finally, we note that the present study has focused on conditions that are pertinent to the early colonization of teeth (i.e. aerobic growth conditions), and it is possible that the peroxidase systems afford host defence under different conditions, such as during the development of mature plaque biofilms. A challenge that remains for researchers is the transition from in vitro models such as the ones herein to in vivo experiments that assess the environmental factors that influence the microbial shifts associated with oral infectious diseases.
Acknowledgments
This study was supported by NIH grants R21 DE016889-01A2 to M. T. A. and 1K99DE018400-01 to J. K. We thank Dr Chris Sissons (University of Otago, New Zealand) for the gift of bLPO.
Abbreviations
bLPO, bovine milk lactoperoxidase
CLSM, confocal laser scanning microscopy
CV, crystal violet
DTNB, 5,5′-dithio-bis(2-nitrobenzoic acid)
hMPO, human myeloperoxidase
HRP, horseradish peroxidase
hSPO, human salivary peroxidase
LPO, lactoperoxidase
References
- Aas, J. A., Paster, B. J., Stokes, L. N., Olsen, I. & Dewhirst, F. E. (2005). Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43, 5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aas, J. A., Griffen, A. L., Dardis, S. R., Lee, A. M., Olsen, I., Dewhirst, F. E., Leys, E. J. & Paster, B. J. (2008). Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol 46, 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson, M. & Carlsson, J. (1982). Lactoperoxidase and thiocyanate protect bacteria from hydrogen peroxide. Infect Immun 35, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson, M. & Pruitt, K. M. (1981). Lactoperoxidase-catalyzed inactivation of hexokinase. Biochim Biophys Acta 658, 238–247. [DOI] [PubMed] [Google Scholar]
- Ashby, M. T. (2008). Inorganic chemistry of defensive peroxidases in the human oral cavity. J Dent Res 87, 900–914. [DOI] [PubMed] [Google Scholar]
- Aune, T. M. & Thomas, E. L. (1977). Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem 80, 209–214. [DOI] [PubMed] [Google Scholar]
- Becker, M. R., Paster, B. J., Leys, E. J., Moeschberger, M. L., Kenyon, S. G., Galvin, J. L., Boches, S. K., Dewhirst, F. E. & Griffen, A. L. (2002). Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40, 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden, G. H. (1990). Effects of fluoride on the microbial ecology of dental plaque. J Dent Res 69, 653–659. [DOI] [PubMed] [Google Scholar]
- Bulaj, G., Kortemme, T. & Goldenberg, D. P. (1998). Ionization–reactivity relationships for cysteine thiols in polypeptides. Biochemistry 37, 8965–8972. [DOI] [PubMed] [Google Scholar]
- Burne, R. A. & Marquis, R. E. (2000). Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193, 1–6. [DOI] [PubMed] [Google Scholar]
- Carlsson, J. (1980). Bactericidal effect of hydrogen peroxide is prevented by the lactoperoxidase–thiocyanate system under anaerobic conditions. Infect Immun 29, 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson, J. (1987). Salivary peroxidase: an important part of our defense against oxygen toxicity. J Oral Pathol 16, 412–416. [DOI] [PubMed] [Google Scholar]
- Carlsson, J., Iwami, Y. & Yamada, T. (1983). Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun 40, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, G. D., Simpson, W. A., Younger, J. J., Baddour, L. M., Barrett, F. F., Melton, D. M. & Beachey, E. H. (1985). Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22, 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy, A. A. & Egeberg, P. K. (2000). Oxidation of thiocyanate by hydrogen peroxide – a reaction kinetic study by capillary electrophoresis. Talanta 51, 1049–1058. [DOI] [PubMed] [Google Scholar]
- Courtois, P., Vanden Abbeele, A., Amrani, N. & Pourtois, M. (1995). Streptococcus sanguis survival rates in the presence of lactoperoxidase-produced OSCN− and OI−. Med Sci Res 23, 195–197. [Google Scholar]
- Dashper, S. G., Liu, S. W. & Reynolds, E. C. (2007). Antimicrobial peptides and their potential as oral therapeutic agents. Int J Pept Res Ther 13, 505–516. [Google Scholar]
- Dethlefsen, L., McFall-Ngai, M. & Relman, D. A. (2007). An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 449, 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin, M. I. (1961). Cytochrome-independent electron transport enzymes of bacteria. In The Bacteria, Metabolism, pp. 425–460. Edited by I. C. Gunsalus & R. Y. Stanier. New York: Academic Press.
- Donoghue, H. D., Perrons, C. J. & Hudson, D. E. (1985). The role of H2O2 and the lactoperoxidase-SCN−-H2O2 system on the interaction between two bacteria originating from human dental plaque, Streptococcus rattus (mutans) BHT and Streptococcus mitior LPA-1, grown on human teeth in an artificial mouth. Arch Oral Biol 30, 519–523. [DOI] [PubMed] [Google Scholar]
- Donoghue, H. D., Hudson, D. E. & Perrons, C. J. (1987). Effect of the lactoperoxidase system on streptococcal acid production and growth. J Dent Res 66, 616–618. [DOI] [PubMed] [Google Scholar]
- Ellman, G. L. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82, 70–77. [DOI] [PubMed] [Google Scholar]
- Eyer, P., Worek, F., Kiderlen, D., Sinko, G., Stuglin, A., Simeon-Rudolf, V. & Reiner, E. (2003). Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal Biochem 312, 224–227. [DOI] [PubMed] [Google Scholar]
- Fine, D. H., Furgang, D. & Barnett, M. L. (2001). Comparative antimicrobial activities of antiseptic mouthrinses against isogenic planktonic and biofilm forms of Actinobacillus actinomycetemcomitans. J Clin Periodontol 28, 697–700. [DOI] [PubMed] [Google Scholar]
- Gilliland, S. E. (1969). Enzymatic determination of residual hydrogen peroxide in milk. J Dairy Sci 52, 321–324. [Google Scholar]
- Hamada, S. & Ooshima, T. (1975). Production and properties of bacteriocins (mutacins) from Streptococcus mutans. Arch Oral Biol 20, 641–648. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn, H. (1976). Microbial aspects of dental caries. The inhibitory action of the lactoperoxidase system on Streptococcus mutans and other microorganisms. Microb Aspects Dent Caries Proc Workshop 2, 353–357. [Google Scholar]
- Ihalin, R., Loimaranta, V. & Tenovuo, J. (2006). Origin, structure, and biological activities of peroxidases in human saliva. Arch Biochem Biophys 445, 261–268. [DOI] [PubMed] [Google Scholar]
- Jakubovics, N. S., Gill, S. R., Vickerman, M. M. & Kolenbrander, P. E. (2008). Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol Ecol 66, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten, H. W., Moorer, W. R. & Wever, R. (1981). Thiocyanate as a cofactor in myeloperoxidase activity against Streptococcus mutans. J Dent Res 60, 831–837. [DOI] [PubMed] [Google Scholar]
- Kreth, J., Zhang, Y. & Herzberg, M. C. (2008a). Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol 190, 4632–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth, J., Zhu, L., Merritt, J., Shi, W. & Qi, F. (2008b). Role of sucrose in the fitness of Streptococcus mutans. Oral Microbiol Immunol 23, 213–219. [DOI] [PubMed] [Google Scholar]
- Kroes, I., Lepp, P. W. & Relman, D. A. (1999). Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A 96, 14547–14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, K. P., Crowe, T. D., Abercrombie, J. J., Molina, C. M., Bradshaw, C. J., Jensen, C. L., Luo, Q. & Thompson, G. A. (2005). Control of oral biofilm formation by an antimicrobial decapeptide. J Dent Res 84, 1172–1177. [DOI] [PubMed] [Google Scholar]
- Mansson-Rahemtulla, B., Baldone, D. C., Pruitt, K. M. & Rahemtulla, F. (1987). Effects of variations in pH and hypothiocyanite concentrations on S. mutans glucose metabolism. J Dent Res 66, 486–491. [DOI] [PubMed] [Google Scholar]
- Mansson-Rahemtulla, B., Rahemtulla, F., Baldone, D. C., Pruitt, K. M. & Hjerpe, A. (1988). Purification and characterization of human salivary peroxidase. Biochemistry 27, 233–239. [DOI] [PubMed] [Google Scholar]
- Marsh, P. D. (1999). Microbiologic aspects of dental plaque and dental caries. Dent Clin North Am 43, 599–614. [PubMed] [Google Scholar]
- Marsh, P. D. (2005). Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol 32, 7–15. [DOI] [PubMed] [Google Scholar]
- Marshall, V. M. E., Cole, W. M. & Bramley, A. J. (1986). Influence of the lactoperoxidase system on susceptibility of the udder to Streptococcus uberis infection. J Dairy Res 53, 507–514. [DOI] [PubMed] [Google Scholar]
- Mickelson, M. N. (1966). Effect of lactoperoxidase and thiocyanate on the growth of Streptococcus pyogenes and Streptococcus agalactiae in a chemically defined culture medium. J Gen Microbiol 43, 31–43. [DOI] [PubMed] [Google Scholar]
- Mickelson, M. N. (1979). Antibacterial action of lactoperoxidase-thiocyanate-hydrogen peroxide on Streptococcus agalactiae. Appl Environ Microbiol 38, 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson, M. N. & Brown, R. W. (1985). Physiological characteristics of Streptococcus dysgalactiae and Streptococcus uberis and the effect of the lactoperoxidase complex on their growth in a chemically-defined medium and milk. J Dairy Sci 68, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Overman, P. R. (2000). Biofilm: a new view of plaque. J Contemp Dent Pract 1, 18–29. [PubMed] [Google Scholar]
- Pakula, R. & Walczak, W. (1963). Nature of competence of transformable streptococci. J Gen Microbiol 31, 125–133. [DOI] [PubMed] [Google Scholar]
- Paster, B. J., Boches, S. K., Galvin, J. L., Ericson, R. E., Lau, C. N., Levanos, V. A., Sahasrabudhe, A. & Dewhirst, F. E. (2001). Bacterial diversity in human subgingival plaque. J Bacteriol 183, 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster, B. J., Olsen, I., Aas, J. A. & Dewhirst, F. E. (2006). The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 42, 80–87. [DOI] [PubMed] [Google Scholar]
- Preza, D., Olsen, I., Aas, J. A., Willumsen, T., Grinde, B. & Paster, B. J. (2008). Bacterial profiles of root caries in elderly patients. J Clin Microbiol 46, 2015–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt, K. M. & Adamson, M. (1977). Enzyme activity of salivary lactoperoxidase adsorbed to human enamel. Infect Immun 17, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, F., Chen, P. & Caufield, P. W. (2001). The group I strain of Streptococcus mutans, UA140, produces both the lantibiotic mutacin I and a nonlantibiotic bacteriocin, mutacin IV. Appl Environ Microbiol 67, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes, J. & Bork, P. (2008). Molecular eco-systems biology: towards an understanding of community function. Nat Rev Microbiol 6, 693–699. [DOI] [PubMed] [Google Scholar]
- Riddles, P. W., Blakeley, R. L. & Zerner, B. (1979). Ellman's reagent: 5,5′-dithiobis(2-nitrobenzoic acid) – a reexamination. Anal Biochem 94, 75–81. [DOI] [PubMed] [Google Scholar]
- Riva, A., Puxeddu, P., Del Fiacco, M. & Testa-Riva, F. (1978). Ultrastructural localization of endogenous peroxidase in human parotid and submandibular glands. J Anat 127, 181–191. [PMC free article] [PubMed] [Google Scholar]
- Rosan, B. & Lamont, R. J. (2000). Dental plaque formation. Microbes Infect 2, 1599–1607. [DOI] [PubMed] [Google Scholar]
- Rupf, S., Merte, K., Eschrich, K., Stosser, L. & Kneist, S. (2001). Peroxidase reaction as a parameter for discrimination of Streptococcus mutans and Streptococcus sobrinus. Caries Res 35, 258–264. [DOI] [PubMed] [Google Scholar]
- Ryan, C. S. & Kleinberg, I. (1995). Bacteria in human mouths involved in the production and utilization of hydrogen peroxide. Arch Oral Biol 40, 753–763. [DOI] [PubMed] [Google Scholar]
- Shin, K., Hayasawa, H. & Lonnerdal, B. (2001). Inhibition of Escherichia coli respiratory enzymes by the lactoperoxidase-hydrogen peroxide-thiocyanate antimicrobial system. J Appl Microbiol 90, 489–493. [DOI] [PubMed] [Google Scholar]
- Sissons, C. H. (1997). Artificial dental plaque biofilm model systems. Adv Dent Res 11, 110–126. [DOI] [PubMed] [Google Scholar]
- Sissons, C. H., Anderson, S. A., Wong, L., Coleman, M. J. & White, D. C. (2007). Microbiota of plaque microcosm biofilms: effect of three times daily sucrose pulses in different simulated oral environments. Caries Res 41, 413–422. [DOI] [PubMed] [Google Scholar]
- Socransky, S. S. & Haffajee, A. D. (2005). Periodontal microbial ecology. Periodontol 2000 38, 135–187. [DOI] [PubMed] [Google Scholar]
- ten Cate, J. M. (2006). Biofilms, a new approach to the microbiology of dental plaque. Odontology 94, 1–9. [DOI] [PubMed] [Google Scholar]
- Tenovuo, J. & Knuuttila, M. L. E. (1977a). The antibacterial action of the various components of the lactoperoxidase system on a cariogenic strain of Streptococcus mutans. J Dent Res 56, 1603–1607. [DOI] [PubMed] [Google Scholar]
- Tenovuo, J. & Knuuttila, M. L. E. (1977b). Antibacterial effect of salivary peroxidases on a cariogenic strain of Streptococcus mutans. J Dent Res 56, 1608–1613. [DOI] [PubMed] [Google Scholar]
- Tenovuo, J., Pruitt, K. M., Mansson-Rahemtulla, B., Harrington, P. & Baldone, D. C. (1986). Products of thiocyanate peroxidation: properties and reaction mechanisms. Biochim Biophys Acta 870, 377–384. [DOI] [PubMed] [Google Scholar]
- Thomas, E. L. (1981). Lactoperoxidase-catalyzed oxidation of thiocyanate: equilibriums between oxidized forms of thiocyanate. Biochemistry 20, 3273–3280. [DOI] [PubMed] [Google Scholar]
- Thomas, E. L. (1985). Bacterial hydrogen peroxide production. In The Lactoperoxidase System: Chemistry and Biological Significance, pp. 179–202. Edited by K. M. Pruitt & J. O. Tenovuo. New York: Marcel Dekker Inc.
- Thomas, E. L. & Pera, K. A. (1983). Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol 154, 1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, E. L., Pera, K. A., Smith, K. W. & Chwang, A. K. (1983). Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect Immun 39, 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, E. L., Milligan, T. W., Joyner, R. E. & Jefferson, M. M. (1994). Antibacterial activity of hydrogen peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infect Immun 62, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton, D. A., Braxton, S. D. & Dabbous, M. K. (1995). Role of saliva and salivary components as modulators of bleaching agent toxicity to human gingival fibroblasts in vitro. J Periodontol 66, 766–774. [DOI] [PubMed] [Google Scholar]
- van der Hoeven, J. S. & Camp, P. J. M. (1993). Mixed continuous cultures of Streptococcus mutans with Streptococcus sanguis or with Streptococcus oralis as a model to study the ecological effects of the lactoperoxidase system. Caries Res 27, 26–30. [DOI] [PubMed] [Google Scholar]
- Xu, P., Alves, J. M., Kitten, T., Brown, A., Chen, Z., Ozaki, L. S., Manque, P., Ge, X., Serrano, M. G. & other authors (2007). Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol 189, 3166–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Sreenivasan, P. K., Subramanyam, R. & Cummins, D. (2006). Multiparameter assessments to determine the effects of sugars and antimicrobials on a polymicrobial oral biofilm. Appl Environ Microbiol 72, 6734–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura-Arite, E., van Marle, J. & ten Cate, J. M. (2001). Confocal microscopy study of undisturbed and chlorhexidine-treated dental biofilm. J Dent Res 80, 1436–1440. [DOI] [PubMed] [Google Scholar]