Abstract
Cystatins, the classical inhibitors of C1 cysteine proteinases, have been extensively studied and reviewed in the literature. Over the last 20 years, however, proteins containing cystatin domains but lacking protease inhibitory activities have been identified, and most likely more will be described in the near future. These proteins together with family 1, 2, and 3 cystatins constitute the cystatin superfamily. Mounting evidence points to the new roles that some members of the superfamily have acquired over the course of their evolution. This review is focused on the roles of cystatins in: 1) tumorigenesis, 2) stabilization of matrix metalloproteinases, 3) glomerular filtration rate, 4) immunomodulation, and 5) neurodegenerative diseases. It is the goal of this review to get as many investigators as possible to take a second look at the cystatin superfamily regarding their potential involvement in serious human ailments.
Keywords: Cystatin, fetuin-A, metalloproteinases, neurodegenerative, tumorigenesis
Structure-Function relationships of Cystatins
The cystatins are the reversible competitive inhibitors of C1 cysteine proteases. The major cysteine proteases which interact with cystatins include the plant derived papain and the mammalian cathepsins, B, H and L.1 Since their discovery, the cystatin family has grown and is now a superfamily that can be categorized into three major families. These are: 1) Stefins (stefin A and B; also known as cystatin A and B) that belong to family 1. They are unglycosylated inhibitors of ~11 kDa, lack signal sequence and disulfide bonds and are generally expressed intracellularly. 2) Family 2 (cystatins) have molecular masses in the range of 13–14 kDa, contain signal sequence and disulfide bonds at the carboxy terminus of the molecule. Some members of the family are glycosylated,2,3 and the family is represented by cystatin C, D, S, SA, and SN. 3) Family 3 (kininogens) have molecular weights in the range of 88–114 kDa, are glycosylated and have three family-2 cystatin domains, two of which (domains 2 and 3) have protease inhibitory activities (Table 1).
Table 1.
LE 1, THE CYSTATIN INHIBITORY MOTIFS IN THE DOMAINS OF THE VARIOUS CYSTATIN FAMILIES AND SUB-GROUPS
| Cystatin-family group | Inhibitory sequence motif |
|---|---|
| Family 1 (stefins) | D1 (G—Q-V-G—PW) |
| Family 2 (Cystatins) | D1 (G—Q-V-G—PW; S-S bonds present) |
| Family 3 (Kininogens) | DI (empty); D2 (G—Q-V-G); D3 (G—Q-V-G—PW); S-S |
| HRG (histidine-rich glycoprotein) | D1 (empty); D2 (empty); S-S bonds |
| Fetuins (ahsg) | D1 (empty); D2 (empty); S-S bonds |
| CRP (cystatin-related protein) | D1 (empty); S-S bonds |
| Spp24 | D1 (empty); S-S bonds |
| CRES (cystatin-related epididymal spermatogenic) | D1 (Q-X-X——PW); S-S bonds |
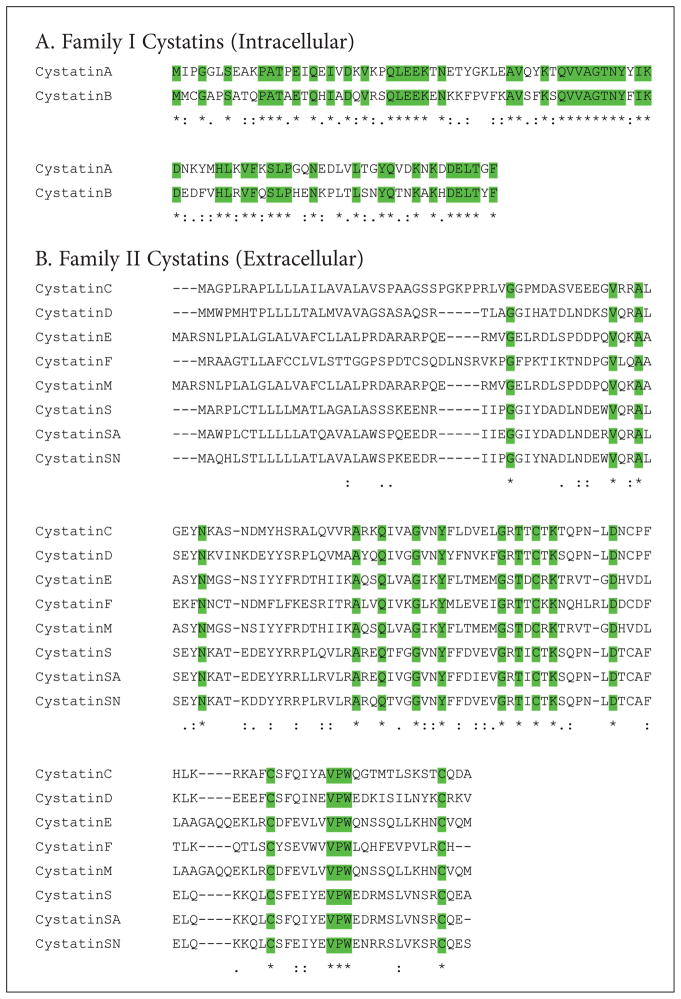
Amino acid sequence alignments of the family 1 cystatins (Stef A and B) show extensive sequence identity (>50%) and similarities. Mutagenesis and X-ray crystallographic studies have unraveled three conserved motifs in cystatins which form a wedge-shaped structure that blocks the active site of C1 cysteine proteases.1 These are an N-terminal glycine, a glutamine-valine-glycine (Q-X-V-X-G) loop and a second c-terminus hairpin loop consisting of proline-typtophan (PW) residues (Figure 1A). It should, however, be noted that in human stefins, the PW motif is replaced by PG in stefin A and PH in stefin B. These conserved residues are also reflected in the ClustalW alignment4 of the amino acid sequences of the family-2 cystatins (Figure 1B).
Figure 1.
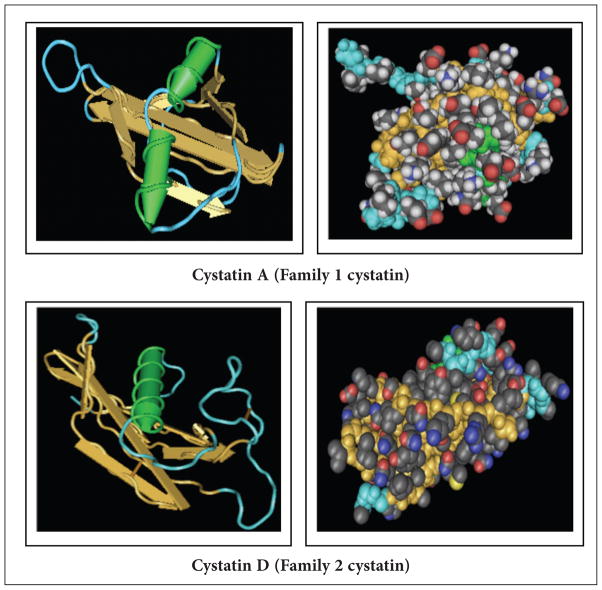
Although family-1 cystatins share conserved amino acid residues with members of family-2 proteins, their 3-D structures5,6 are distinct. Each cystatin structure has a core of five-stranded anti-parallel β-sheets wrapped around a core of a central helix as shown by the ribbon and space filling models of the structures (Figure 2).
Figure 2.
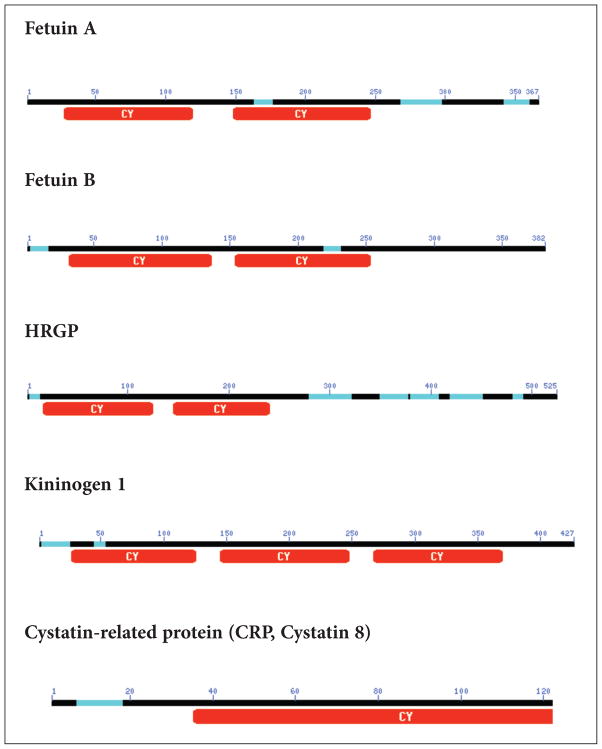
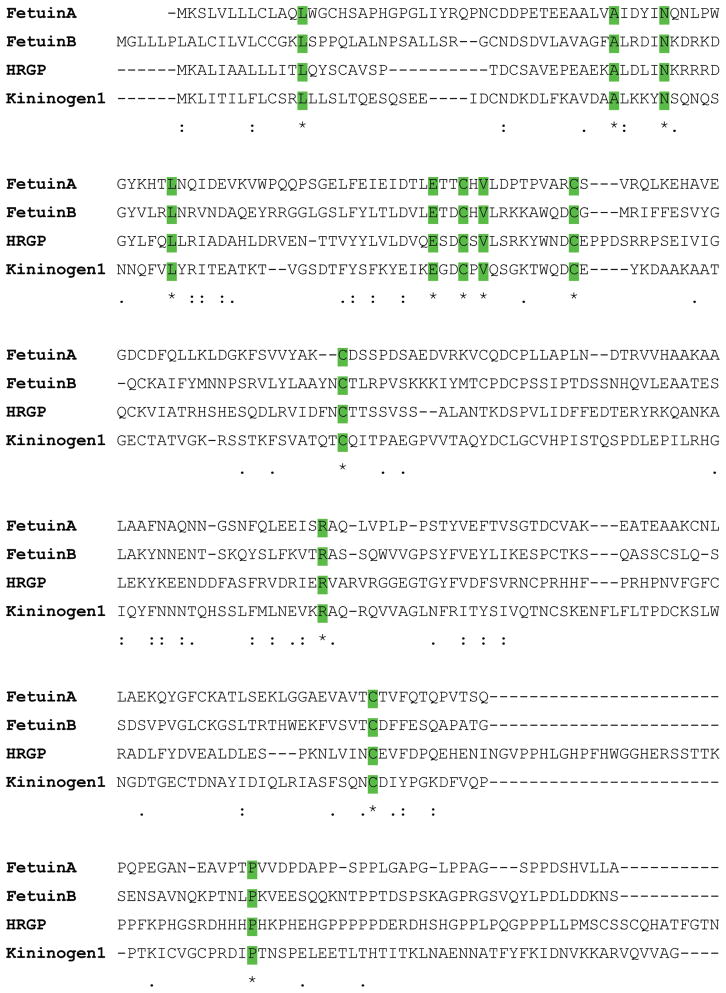
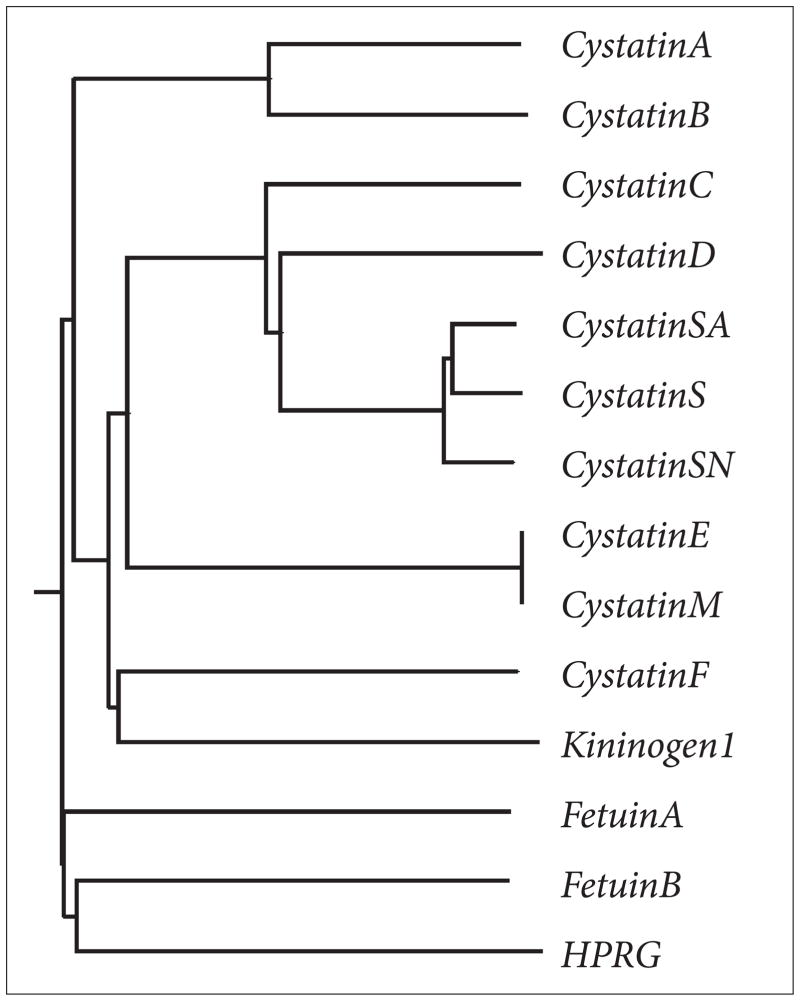
Apart from the three established members of the superfamily, there are several other proteins that have been identified that contain cystatin like sequences but lack cysteine protease inhibitory properties and thus represent new families. The evolution of cystatins predicts that archetypal cystatin gave rise to the family-1 cystatins. The introduction of disulfide bonds then gave rise to family-2 members, and then gene duplication events gave rise to family-3 cystatins via a two cystatin domain intermediate.2 The two cystatin domain intermediate with inhibitory activities is, however, missing in the puzzle.2,7 This ‘missing link’ would have given rise to a fourth family with new functions (Figure 3). For example, Histidine-rich glycoprotein (HRG) contains two cystatin-like domains and the conserved disulfide bond but are not inhibitory in protease assays.7 The physiological function of HRG is not known. On the other hand, a related protein known as fetuin-A,7,8 has been shown to be involved in a number of physiological functions including some that it shares with the other cystatin-family members and discussed in this review. Like HRP, bovine fetuin-A and its human homologue alpha 2HS glycoprotein (ahsg) contain two cystatin like domains but lack the cysteine protease inhibitory activities. Sequence similarities among family 3 cystatins, HRG and fetuin-A is depicted in Figure 4 and the dendogram showing how the cystatin family members are related is depicted in Figure 5.
Figure 3.
Figure 4.
Figure 5.
Other proteins that contain cystatin-like domains but lack the inhibitory activities are the cystatin-related protein (CRP), an androgen-regulated protein expressed in prostate9,10 and Spp24, a bone phosphoprotein.11 These duos contain disulfide bonds, but only a single cystatin domain which lack inhibitory activity and may have evolved from a family-2 precursor. Other cystatins that may eventually be classified as a new sub-group are cystatin E/M and F. Although they are capable of the protease inhibitory function, they have low sequence homology with the other family-2 members.3,12,13 Even more intriguing is cathelin, which inhibits cathepsin L and papain, but lacks all the three cystatin inhibitory motifs.14,15 Yet another sub-group of family-2 proteins is represented by cystatin-related epididymal spermatogenic (CRES) proteins.16 The CRES proteins contain only the C-terminal PW sequence and lack the Q-X-V-X-G. These differences are reflected on Table 1.
Role of Cystatins in tumorigenesis
It is established that cysteine proteinases play key roles in the progression of a variety of tumors. Tumor progression leading to metastasis involves the destruction and remodeling of the extracellular matrices during local invasion, angiogenesis, intravasation, and extravasation.17,18 These processes are facilitated by matrix metalloproteases,19 serine,20 aspartic,21 and cysteine proteases.20 The prototype cysteine protease which plays a key role in tumor cell invasion is cathepsin B.22,23 Even though the cathepsins are generally regarded as intracellular proteases in normal cells, they are expressed extracellularly on the surface of tumor cells. The enzymes have been shown to be concentrated in the leading edge of motile metastatic cells where they dissolve the ECM proteins to pave the way for the movement of the cells.24,25 It is in this micro-environment that the activity of cysteine proteases are inhibited by cystatins, particularly Cyst C, which is also present extracellularly.26
The expression of these proteinases and their natural inhibitors in vivo are under tight regulatory controls for obvious reasons.27–29 For example, a number of studies have suggested an inverse relationship between the stage of tumor progression and the levels of cystatins in the tumor microenvironment. As tumors progress towards the metastatic end stage, the levels of the cystatins in both the cytosol and extracellular spaces are drastically reduced.30,31 In addition, studies have demonstrated a direct correlation between high cystatin C levels and improved tumor prognosis.26
The mechanisms by which cystatins modulate tumorigenicity are not only attributed to their inhibitory roles against cysteine proteinases. Studies by Sokol et al.32 identified Cyst C as a novel antagonist of TGF-β signaling. They determined that Cyst C physically interacts with TGF-β receptor II, thereby abrogating the binding of TGF-β. It is known that TGF-β has growth suppressing properties, particularly in normal epithelial cells,17–19 but that during tumor progression the tumor suppressing properties of TGF-β is frequently subverted, and it becomes a powerful progression factor for the transformed cells.33,34 The ability of Cyst C to down-regulate the growth promoting properties of TGF-β is very provocative in view of the fact that ahsg (a family-3 cystatin) has also been shown to down regulate tumorigenesis, presumably by a similar mechanism. Swallow et al.,35 in elegant experiments using ahsg mutant mice, showed that this family-3 cystatin down-regulates colon carcinogenesis by antagonizing TGF-β activity. Moreover, two other notable studies, by Zhang et al.36 and Hsu et al.37 have demonstrated that cystatin M and fetuin-B (family-3 cystatin) also act as tumor suppressor proteins in lung and skin tumors, respectively.
Assuming that cystatins generally suppress tumorigenicity, it is difficult to explain why sera of patients with melanoma and colorectal cancers contain more Cyst C than normal volunteers.28,38 Experimentally, when syngeneic tumor cells were inoculated subcutaneously into Cyst C wild-type and null mice, no difference in the latency and growth of the primary tumors were observed in the two groups of mice. However, lung colonization by the same syngeneic cells following experimental metastasis protocol was suppressed in Cyst C-deficient mice in comparison with their wild-type counterparts.39 We have recently demonstrated that fetuin-A is a potent promoter of tumor cell growth both in vitro and in vivo.40 The role of fetuin-A in cell growth, particularly in vitro, has been controversial, to say the least.41 Its ability to associate with a number of adhesive proteins and growth factors fueled this controversy because it was difficult to delineate whether the growth promotion was due to fetuin-A per se or the co-purifying proteins. We have demonstrated that the highly malignant Lewis lung carcinoma cells have reduced growth potential in fetuin-A null C57BL/6 mice while they rapidly and substantially colonize the lungs, livers, and subcutaneous tissues of the wild type animals.40 The data suggest that in highly tumorigenic cells, the growth promoting potential of fetuin-A far exceeds its ability to downregulate the effects of TGF-β. Therefore, depending on the particular circumstances, cystatin family members may provide both favorable and unfavorable microenvironments for tumor growth.
Stabilization of Matrix Metalloproteinases by Cystatins
Matrix metalloproteinases (MMPs) are homologous Zn2+-dependent proteinases that participate in the physiological and pathological processes, which require tissue remodeling. 42 Their expression profiles are highly regulated and defects in their regulatory pathways are likely to give rise to diseases ranging from cancer, arthritis, and cardiovascular disorders to periodontal diseases.43–46 Matrix metalloproteinases, like other proteinases, are secreted as zymogens and assume their activities in the extracellular milieu after activation processes.47 For some MMPs such as MMP-9, the activation process is mediated by other MMPs.48 Once activated, several proteinases including matrix metalloproteinases have the capacity for autolytic inactivation when they are in their active state.49,50 There are key mechanisms that have evolved in vivo to regulate the activities of these enzymes and prevent their autolytic inactivation. Tissue inhibitors of matrix metalloproteinases (TIMPs), the natural inhibitors of MMPs, interact with the enzymes in vivo and inhibit their activities. In the absence of these inhibitors, the activated enzymes if left unchecked, may turn against themselves and undergo autolysis. Therefore, under physiological conditions, which favor the active state of the enzymes and limited expression of the TIMPs, matrix metalloproteinases are unstable and can easily undergo autolysis unless some other protective mechanisms are in place.
Studies in our laboratory and others, have determined that apart from TIMPs, members of the cystatin superfamily can stabilize and protect matrix metalloproteinases without affecting their activities towards their natural substrates, such as gelatin.50,51 Purified MMP-2 and MMP-9 in their active state are rapidly inactivated within hours unless they are in the presence of any of the cystatin family members or TIMPs. The MMPs, particularly MMP-9, interact with cystatins with dissociation constants in the range of 25 nM to 1.9 μM. However, the ability of fetuin-A to protect MMP-9 from autolysis requires a molar ratio of at least 8:1 (fetuin-A:MMP-9). Interestingly, the molar ratio of fetuin-A: MMP-9 in vivo, far exceeds the minimal ratio of [8:1].50 This is also true for other members of the family.50
Cystatins do occur in all body compartments and fluids where matrix metalloproteinases are present. These include blood, urine, saliva, and cerebrospinal, seminal, and synovial fluids. The enzymes in these compartments are protected from autolytic degradation but maintain the capacity to digest their preferred substrates including a myriad of proteins that they modify in vivo such as galectin-3.52 We therefore envision an in vivo scenario where MMPs, TIMPs, cystatins, and other proteases interact with each other to regulate extracellular remodeling and to proteolytically modify a host of housekeeping proteins in both normal and pathophysiological conditions. For example, Mai et al.53 recently demonstrated that cathepsin B is present on the surface of tumor cells where it co-localizes with annexin-2 tetramer. Annexin-2 tetramer also serves as a receptor for tPA, plasminogen, tenascin C, and plasmin.54,55 Additionally, other studies have also demonstrated that annexin-2 on the cell surface can serve as receptor for fetuin-A.56,57 Therefore matrix metalloproteinases, which normally co-localize with fetuin-A,58–60 are likely to be part of this annexin-2 tetramer complex on the surface of tumor cells. The cystatins in these complexes may inhibit the activity of cathepsin-B while allowing the activity of matrix metalloproteinases.
Role of Cystatin C as a New Marker of Glomerular Filtration rate
For many years, serum creatinine has been the benchmark marker for the renal function assay also known as glomerular filtration rate (GFR).61,62 The ideal marker for GFR should be produced endogenously and at a constant rate, regardless of age, sex, weight, and disease state. It should be filtered and excreted by the kidney only without renal tubular secretion and reabsorption, and once in urine it should be stable to allow for later analysis. Although creatinine meets some of these conditions, it has a number of limitations. Creatinine is produced in muscle, and the amount produced depends on muscle mass. It does not bind plasma proteins, and is freely filtered by the kidney although it is also secreted by the renal tubules. One major drawback for creatinine as a marker for GFR is that it is not a sensitive marker of early GFR, necessitating the collection of timed urinary samples and using a formula that requires measurement of serum and urinary forms of creatinine. Rhabdomyolisis and eating uncooked meats can dramatically raise serum creatinine, which can skew the readings of urinary creatinine. Because of these drawbacks related to creatinine, other endogenous markers for GFR have been evaluated. So far, the most promising marker which can potentially replace creatinine is cystatin C.
Cyst C has proven to be a suitable marker because it satisfies most of the above established criteria. Simonsen et al.63 first noted the excellent correlation between Cyst C secretion and GFR when compared with the gold standard exogenous markers of GFR, such as51 Cr-EDTA. Cyst C is present in high concentrations in serum, saliva, and seminal, synovial, and cerebrospinal fluids.64 It is produced and secreted at a constant rate by most nucleated cells and is freely filtered by the glomerular because of its small size. Unlike creatinine, serum Cyst C is not secreted by renal tubular epithelial cells, although they reabsorb and catabolyze it so that Cyst C does not return to the bloodstream.65
Immunomodulatory Properties of Cystatins
Fetuin-A and its human homologue (ahsg) are defined as negative acute-phase proteins. Normal circulating levels of the proteins ~0.5 mg/ml fall significantly (by approximately 40%) during injury and infection.66,67 These cystatins have been shown to be immunomodulators that can mediate bacterial phagocytosis by neutrophils68 and promotion of endocytosis by mouse macrophages.69,70 Studies by Wang et al.71 demonstrated that spermine, a ubiquitous biorganic polyamine that accumulates at sites of injury or inflammation, inhibits macrophage cytokine synthesis only in the presence of fetuin-A. Thus, during inflammation, fetuin-A down-regulates the production of pro-inflammatory cytokines and prevents excessive inflammation.71,72 Another study by this group determined that fetuin-A modulates macrophage deactivation by opsonizing both endogenous (spermine) and therapeutically administered cationic cytokine synthesis inhibitors (CNI-1493) that restrain the innate immune response.73
Apart from the fetuin-A, family-2 cystatin members have also been shown to be important immunomodulatory proteins. For example, during inflammatory processes, cystatin C release is down-regulated, contributing to increased cysteine protease activities in the macrophage microenvironment.74 Cyst C is a powerful inhibitor of cathepsins S and L. High levels of Cyst C are detectable in class II-positive lysosomes of immature dendritic cells (DCs) and Langerhans cells. The maturation process of DCs leads to reduced levels of cyst C, and allows the up-regulation of cysteine proteases cathepsins L and S. For this reason, it has been suggested that Cyst C plays a role in the intracellular control of invariant chain (li) degradation and antigen presentation.75 One of the initial steps of an antigen-specific T cell response to external antigens, is the formation of peptide-MHC class II complexes in antigen presenting cells (APCs) such as macrophages, DCs, and T cells. In this process, cysteine proteases play a key role in two important processes. Firstly, they degrade the proteins within the endosomal-lysosomal compartments of APCs. The resulting peptides then bind to MHC class II molecules that are later targeted to the cell surface. Secondly, cysteine proteases are involved in the cleavage of MHC class II-associated li, leading to the formation of clip associated MHC-molecules. The generation of clip that binds to the antigen-binding groove of MHC class II molecules subsequently allows the binding of peptides to the MHC class II molecule. It has been documented that cathepsin S promotes the generation of clip fragment in B cells and DCs,76 while cathepsin L is active in thymus epithelial cells77,78 and cathepsin F in macrophages.
The inhibition of immunologically relevant cysteine proteases by cystatins secreted by parasites such as filarial nematodes, could lead to drastic changes in antigen processing and presentation by APCs of the host. Apart from their ability to inhibit proteases, cystatins emanating from parasitic nematodes can also affect the production of cytokines by host macrophages, resulting in anti-inflammatory responses. For example, it was demonstrated by Schonemeyer et al.79 that Onchocerca volvulus cystatin induces early TNF-alpha response in human peripheral blood mononuclear cells (PBMC), followed by a down-regulation of the IL-12 production and a massive increase in IL-10 production by monocytes. High levels of IL-10 are characteristic for filarial worm infection, and increased IL-10 production of unstimulated or antigen-stimulated PBMC of lymphatic filariasis patients coincides with T Cell hypoactivity.
Members of the three cystatin families have been shown to up-regulate the release of nitric oxide (NO) from IFN-gamma activated macrophages.80,81 This feature applies only to natural cysteine protease inhibitors, as synthetic inhibitors are unable to up-regulate NO productions.82 The inducible NOS (iNOS) is induced by IFN-gamma and accounts for rapid production of large quantities of NO.83 The increased NO production by chicken cystatin was demonstrated to be potent enough to cure mice from potentially lethal visceral Leishmaniasis.84 Interestingly, NO has been demonstrated to induce a strong inhibition of lymphocyte proliferation in vitro and to modulate cytokine gene expression in various cell types.85,86 It is important to note that cystatin induced up-regulation of NO release by activated macrophages is stimulated via the synthesis of TNF-alpha and IL-10 and not due to the conserved inhibitory domains (QXVXG). We conclude that while the cystatins produced by the parasites ensure their intracellular survival in the host cells, the host cysteine proteases speed up the antigen presentation events culminating in the elimination of the parasite by the host immune system.
Role of Cystatins in Neurodegenerative Diseases
Cerebrovascular amyloid deposition (CAA) is a disease characterized by amyloid protein deposition in the blood vessels of the brain. Severe forms of the disease may cause cerebrovascular disorders such as lobar cerebral hemorrhage and leukoencephalopathy and may present with dementia such as that observed in Alzheimer’s disease (AD).87 Several cerebrovascular amyloid proteins have been characterized and include amyloid-β-protein type (Aβ), cystatin C, prion protein, variant transthyretins (ATTR) in meningovascular amyloidoses, mutated gelsolin (AGEl) in familial amyloidosis of Finnish type, disease associated prion protein (PrP(Sc)) in a variant of the Gerstmann-Straussler-Scheinker syndrome.87,88 Among the several types of CAA, that of the Aβ type is the most commonly found in elderly individuals and in patients with AD and mutations found in the genes encoding amyloid precursor proteins are associated with hereditary CAA.87
Studies have shown cystatin C to be immunohistochemically co-localized with Aβ in sporadic CAA89,90 and to co-immunoprecipitate with amyloid-beta precursor protein.91 However, in such cases examined to date, cystatin C has not been shown to be an intrinsic component of the amyloid fibrils and more definitive studies must be conducted to elucidate the significance of the immunohistochemical co-localization of cystatin C. The association of cystatin C with Aβ in AD patients may have a hitherto unexplored role. As we have discussed above, members of the cystatin superfamily can stabilize and protect matrix metalloproteinase from autolytic degradation. These enzymes have also been shown to associate with Aβ proteins and can specifically cleave them.92–94 This cleavage or degradation may modulate or increase the clearance of the amyloid bodies, thereby reducing their detrimental effects in AD patients. In support of this notion, Mendes Sousa et al.95 have demonstrated an up-regulation of gelatinase-associated lipocalin and matrix metalloproteinase-9 in familial amyloid polyneuropathy. Interestingly, lipocalin—which is distantly related to the cystatin family—has been shown to stabilize and protect MMP-9 from autolytic degradation.96
Apart from simply being co-localized with Aβ in the brain, there are studies showing that cystatin C can also polymerize and give rise to amyloid bodies. In hereditary cerebral hemorrhage with amyloidosis icelandic-type (HCHWA-1), an autosomal dominant disorder in icelanders, cystatin C is directly involved in the pathogenesis of CAA. This disorder is associated with a (68Leu—Gln) mutation and a loss of 10 amino acids in the N-terminus of the cystatin C.97,98
Concerning the formation of Aβ fibrils in the brain, it is generally believed that globular amyloidogenic proteins partially unfold and then switch into alternatively folded conformations to self assemble into fibrils (amyloid deposits).99,100 More specifically, cystatins and other amyloidogenic proteins have been shown to participate in domain-swapping, which leads to formation of stable dimers and eventually stable fibrils in amyloidogenic deposits.101,102 Domain-swapping, as the name implies, is a process in which a domain in a protein breaks its non-covalent bonds with the rest of the molecule and has its place taken by the same domain of a second molecule. The theoretical consideration of domain-swapping in cystatins began with the studies of Ekiel and Abrahamson,103 where they demonstrated that human cystatin C had the propensity to form inactive dimers under pre-denaturing conditions. Subsequent studies involving human stefins confirmed the initial observations.104,105 For each of the cystatins, NMR chemical shift changes indicated that dimerization involved no structural rearrangement of the main fold. There were, however, rearrangements in the active site regions of the molecules. The rather slow kinetics and high activation energy of dimerization were suggestive of domain-swapping instead of simple association.104,106
It is possible that the behavior of domain-swapping is shared by other members of the cystatin superfamily including fetuin-A, which can also form aggregates under favorable conditions as demonstrated in our laboratory (unpublished information). Moreover, conditions that favor domain-swapping are also ideal for amyloidogenesis.101 For normal cystatin molecules, the phenomenon does not occur readily under physiological conditions but human cystatin C with the L68Q mutation, dimerizes readily under physiological conditions. Thus, certain mutations destabilize the monomeric structures, making it possible for the mutant proteins to form dimers readily by the domain-swapping mechanism.101,102
Other than amyloidogenesis, cystatin C has been shown to be associated with multiple sclerosis (MS), another neurodegenerative disease. Studies have demonstrated that the level of cystatin C is highly reduced in the cerebrospinal fluid of individuals suffering from MS compared with healthy controls.107 The reduced levels of cystatin C in CSF of these patients was accompanied by increased activity of cysteine proteases in the fluid compared with normal subjects, pointing to the role of this cysteine proteinase inhibitor in the pathogenesis of MS. A possible role of cystatin C in MS therefore is to modulate the activities of cathepsin B and possibly other cysteine proteases and is not an active participant in the pathogenesis of the disease.
In other studies of patients with inflammatory neurological disorders, such as Guillain-Barre syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy, levels of cystatin C were shown to be consistently lower in the CSF compared with control subjects.108 In the neurodegenerative disorder known as progressive myoclonus epilepsy of Unverricht-Lundborg type (EPMI), a significant reduction in cystatin B activity was recorded in lymphoblastoid cells of EPMI.109 Concomitant to the decreased level of cystatin B, levels of cathepsin B, L, and S shot up significantly, as expected. Furthermore, mice with disruptions in the cystatin-B gene (loss of function mutation) display myoclonic seizures, progressive ataxia and cerebellar pathology, which closely parallels EPMI in humans.110
Conclusion
In summary, cystatins are at the heart of a number of normal and pathological conditions, and should not be regarded merely as inhibitors of C1 cysteine proteases. Particularly their roles in tumorigenesis and neurodegenerative diseases should be given increased attention, since we now have animal models for mechanistic studies. It is also our hope that in future more proteins with cystatin-like domains will be identified and their physiological roles described.
Acknowledgments
This work was supported by NIH grants IU54 CA091408 (J.O. and G.C.).
Biographies
JOSIAH OCHIENG is a professor of Biochemistry and Cancer Biology, Meharry Medical College. His research interests are in the area of molecular determinants of cancer metastasis.
GAUTAM CHAUDHURI is a professor of Biochemistry, Cancer Biology and Microbiology. He is interested in the molecular mechanisms of breast cancer growth regulation and the regulation of Leishmaniasis.
Notes
- 1.Turk V, Bode W. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 1991 Jul 22;285(2):213–9. doi: 10.1016/0014-5793(91)80804-c. [DOI] [PubMed] [Google Scholar]
- 2.Cornwall GA, Hsia N. A new subgroup of the family 2 cystatins. Mol Cell Endocrinol. 2003 Feb 28;200(1–2):1–8. doi: 10.1016/s0303-7207(02)00408-2. [DOI] [PubMed] [Google Scholar]
- 3.Ni J, Abrahamson M, Zhang M. Cystatin E is a novel human cysteine proteinase inhibitor with structural resemblance to family 2 cystatins. J Biol Chem. 1997 Apr 18;272(16):10853–8. doi: 10.1074/jbc.272.16.10853. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Anderson JB, DeWeese-Scott C, et al. MMDB: Entrez’s 3D-structure database. Nucleic Acids Res. 2003 Jan 1;31(1):474–7. doi: 10.1093/nar/gkg086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimba N, Kariya E, Tate S, et al. Structural comparison between wild-type and P25S human cystatin A by NMR spectroscopy. Does this mutation affect the alpha-helix conformation? J Struct Funct Genomics. 2000;1(1):26–42. doi: 10.1023/a:1011380315619. [DOI] [PubMed] [Google Scholar]
- 7.Brown WM, Dziegielewska KM. Friends and relations of the cystatin superfamily— new members and their evolution. Protein Sci. 1997 Jan;6(1):5–12. doi: 10.1002/pro.5560060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown WM, Saunders NR, Mollgard K, et al. Fetuin—an old friend revisited. Bioessays. 1992 Nov;14(11):749–55. doi: 10.1002/bies.950141105. [DOI] [PubMed] [Google Scholar]
- 9.Winderickx J, Hemschoote K, De Clercq N, et al. Tissue-specific expression and androgen regulation of different genes encoding rat prostatic 22-kilodalton glycoproteins homologous to human and rat cystatin. Mol Endocrinol. 1990 Apr;4(4):657–67. doi: 10.1210/mend-4-4-657. [DOI] [PubMed] [Google Scholar]
- 10.Devos A, De Clercq N, Vercaeren I, et al. Structure of rat genes encoding androgen-regulated cystatin-related proteins (CRPs): a new member of the cystatin superfamily. Gene. 1993 Mar 30;125(2):159–67. doi: 10.1016/0378-1119(93)90323-u. [DOI] [PubMed] [Google Scholar]
- 11.Hu B, Coulson L, Moyer B, et al. Isolation and molecular cloning of a novel bone phosphoprotein related in sequence to the cystatin family of thiol protease inhibitors. J Biol Chem. 1995 Jan 6;270(1):431–6. doi: 10.1074/jbc.270.1.431. [DOI] [PubMed] [Google Scholar]
- 12.Ni J, Fernandez MA, Danielsson L, et al. Cystatin F is a glycosylated human low molecular weight cysteine proteinase inhibitor. J Biol Chem. 1998 Sep 18;273(38):24797–804. doi: 10.1074/jbc.273.38.24797. [DOI] [PubMed] [Google Scholar]
- 13.Sotiropoulou G, Anisowicz A, Sager R. Identification, cloning, and characterization of cystatin M, a novel cysteine proteinase inhibitor, down-regulated in breast cancer. J Biol Chem. 1997 Jan 10;272(2):903–10. doi: 10.1074/jbc.272.2.903. [DOI] [PubMed] [Google Scholar]
- 14.Kopitar M, Ritonja A, Popovic T, et al. A new type of low-molecular mass cysteine proteinase inhibitor from pig leukocytes. Biol Chem Hoppe Seyler. 1989 Oct;370(10):1145–51. doi: 10.1515/bchm3.1989.370.2.1145. [DOI] [PubMed] [Google Scholar]
- 15.Ritonja A, Kopitar M, Jerala R, et al. Primary structure of a new cysteine proteinase inhibitor from pig leucocytes. FEBS Lett. 1989 Sep 25;255(2):211–4. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- 16.Cornwall GA, Orgebin-Crist MC, Hann SR. The CRES gene: a unique testis-regulated gene related to the cystatin family is highly restricted in its expression to the proximal region of the mouse epididymis. Mol Endocrinol. 1992 Oct;6(10):1653–64. doi: 10.1210/mend.6.10.1280328. [DOI] [PubMed] [Google Scholar]
- 17.Liotta LA, Rao CN, Barsky SH. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–49. [PubMed] [Google Scholar]
- 18.Mignatti P, Robbins E, Rifkin DB. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–98. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- 19.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997 Sep 3;89(17):1260–70. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 20.Cudic M, Fields GB. Extracellular proteases as targets for drug development. Curr Protein Pept Sci. 2009 Aug;10(4):297–307. doi: 10.2174/138920309788922207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eder J, Hommel U, Cumin F, et al. Aspartic proteases in drug discovery. Curr Pharm Des. 2007;13(3):271–85. doi: 10.2174/138161207779313560. [DOI] [PubMed] [Google Scholar]
- 22.Yan S, Sameni M, Sloane BF. Cathepsin B and human tumor progression. Biol Chem. 1998 Feb;379(2):113–23. [PubMed] [Google Scholar]
- 23.Koblinski JE, Ahram M, Sloane BF. Unraveling the role of proteases in cancer. Clin Chim Acta. 2000 Feb 15;291(2):113–35. doi: 10.1016/s0009-8981(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 24.Sinha AA, Gleason DF, Deleon OF, et al. Localization of a biotinylated cathepsin B oligonucleotide probe in human prostate including invasive cells and invasive edges by in situ hybridization. Anat Rec. 1993 Feb;235(2):233–40. doi: 10.1002/ar.1092350207. [DOI] [PubMed] [Google Scholar]
- 25.Visscher DW, Sloane BF, Sameni M, et al. Clinicopathologic significance of cathepsin B immunostaining in transitional neoplasia. Mod Pathol. 1994 Jan;7(1):76–81. [PubMed] [Google Scholar]
- 26.Smith KJ, Pacey GE. Reverse dual phase gas diffusion flow injection analysis. Talanta. 1993 Dec;40(12):1961–6. doi: 10.1016/0039-9140(93)80121-7. [DOI] [PubMed] [Google Scholar]
- 27.Kos J, Werle B, Lah T, et al. Cysteine proteinases and their inhibitors in extracellular fluids: markers for diagnosis and prognosis in cancer. Int J Biol Markers. 2000 Jan–Mar;15(1):84–9. doi: 10.1177/172460080001500116. [DOI] [PubMed] [Google Scholar]
- 28.Kos J, Krasovec M, Cimerman N, et al. Cysteine proteinase inhibitors stefin A, stefin B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res. 2000 Feb;6(2):505–11. [PubMed] [Google Scholar]
- 29.Zore I, Krasovec M, Cimerman N, et al. Cathepsin B/cystatin C complex levels in sera from patients with lung and colorectal cancer. Biol Chem. 2001 May;382(5):805–10. doi: 10.1515/BC.2001.097. [DOI] [PubMed] [Google Scholar]
- 30.Kothapalli R, Bailey RD, Kusmartseva I, et al. Constitutive expression of cytotoxic proteases and down-regulation of protease inhibitors in LGL leukemia. Int J Oncol. 2003 Jan;22(1):33–9. [PubMed] [Google Scholar]
- 31.Korolenko TA, Poteryaeva ON, Falameeva OV, et al. Cystein proteinase inhibitor stefin A as an indicator of efficiency of tumor treatment in mice. Bull Exp Biol Med. 2003 Jul;136(1):46–8. doi: 10.1023/a:1026084712399. [DOI] [PubMed] [Google Scholar]
- 32.Sokol JP, Schiemann WP. Cystatin C antagonizes transforming growth factor beta signaling in normal and cancer cells. Mol Cancer Res. 2004 Mar;2(3):183–95. [PubMed] [Google Scholar]
- 33.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000 May 4;342(18):1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 34.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000 Oct 13;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 35.Swallow CJ, Partridge EA, Macmillan JC, et al. Alpha2HS-glycoprotein, an antagonist of transforming growth factor beta in vivo, inhibits intestinal tumor progression. Cancer Res. 2004 Sep 15;64(18):6402–9. doi: 10.1158/0008-5472.CAN-04-1117. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Shridhar R, Dai Q, et al. Cystatin M: a novel candidate tumor suppressor gene for breast cancer. Cancer Res. 2004 Oct 1;64(19):6957–64. doi: 10.1158/0008-5472.CAN-04-0819. [DOI] [PubMed] [Google Scholar]
- 37.Hsu SJ, Nagase H, Balmain A. Identification of Fetuin-B as a member of a cystatin-like gene family on mouse chromosome 16 with tumor suppressor activity. Genome. 2004 Oct;47(5):931–46. doi: 10.1139/g04-043. [DOI] [PubMed] [Google Scholar]
- 38.Kos J, Stabuc B, Schweiger A, et al. Cathepsins B, H, and L and their inhibitors stefin A and cystatin C in sera of melanoma patients. Clin Cancer Res. 1997 Oct;3(10):1815–22. [PubMed] [Google Scholar]
- 39.Huh CG, Hakansson K, Nathanson CM, et al. Decreased metastatic spread in mice homozygous for a null allele of the cystatin C protease inhibitor gene. Mol Pathol. 1999 Dec;52(6):332–40. doi: 10.1136/mp.52.6.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kundranda MN, Henderson M, Carter KJ, et al. The serum glycoprotein fetuin-A promotes Lewis lung carcinoma tumorigenesis via adhesive-dependent and adhesive-independent mechanisms. Cancer Res. 2005 Jan 15;65(2):499–506. [PubMed] [Google Scholar]
- 41.Nie Z. Fetuin: its enigmatic property of growth promotion. Am J Physiol. 1992 Sep;263(3 Pt 1):C551–62. doi: 10.1152/ajpcell.1992.263.3.C551. [DOI] [PubMed] [Google Scholar]
- 42.Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–5. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 43.Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996 Nov;3(11):895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 44.Rudolph-Owen LA, Chan R, Muller WJ, et al. The matrix metalloproteinase matrilysin influences early-stage mammary tumorigenesis. Cancer Res. 1998 Dec 1;58(23):5500–6. [PubMed] [Google Scholar]
- 45.Walakovits LA, Moore VL, Bhardwaj N, et al. Detection of stromelysin and collagenase in synovial fluid from patients with rheumatoid arthritis and posttraumatic knee injury. Arthritis Rheum. 1992 Jan;35(1):35–42. doi: 10.1002/art.1780350106. [DOI] [PubMed] [Google Scholar]
- 46.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995 Nov;77(5):863–8. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 47.Grant GA, Goldberg GI, Wilhelm SM, et al. Activation of extracellular matrix metalloproteases by proteases and organomercurials. Matrix Suppl. 1992;1:217–23. [PubMed] [Google Scholar]
- 48.Fridman R, Toth M, Pena D, et al. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2) Cancer Res. 1995 Jun 15;55(12):2548–55. [PubMed] [Google Scholar]
- 49.Ellerbroek SM, Wu YI, Stack MS. Type I collagen stabilization of matrix metalloproteinase- 2. Arch Biochem Biophys. 2001 Jun 1;390(1):51–6. doi: 10.1006/abbi.2001.2345. [DOI] [PubMed] [Google Scholar]
- 50.Ray S, Lukyanov P, Ochieng J. Members of the cystatin superfamily interact with MMP-9 and protect it from autolytic degradation without affecting its gelatinolytic activities. Biochim Biophys Acta. 2003 Dec 1;1652(2):91–102. doi: 10.1016/j.bbapap.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Yan L, Borregaard N, Kjeldsen L, et al. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001 Oct 5;276(40):37258–65. doi: 10.1074/jbc.M106089200. Epub 2001 Aug 2. [DOI] [PubMed] [Google Scholar]
- 52.Ochieng J, Fridman R, Nangia-Makker P, et al. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994 Nov 29;33(47):14109–14. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 53.Mai J, Waisman DM, Sloane BF. Cell surface complex of cathepsin B/annexin II tetramer in malignant progression. Biochim Biophys Acta. 2000 Mar 7;1477(1–2):215–30. doi: 10.1016/s0167-4838(99)00274-5. [DOI] [PubMed] [Google Scholar]
- 54.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem. 1994 Aug 19;269(33):21191–7. [PubMed] [Google Scholar]
- 55.Chung CY, Erickson HP. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J Cell Biol. 1994 Jul;126(2):539–48. doi: 10.1083/jcb.126.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kojima K, Yamamoto K, Irimura T, et al. Characterization of carbohydrate-binding protein p33/41: relation with annexin IV, molecular basis of the doublet forms (p33 and p41), and modulation of the carbohydrate binding activity by phospholipids. J Biol Chem. 1996 Mar 29;271(13):7679–85. doi: 10.1074/jbc.271.13.7679. [DOI] [PubMed] [Google Scholar]
- 57.Kundranda MN, Ray S, Saria M, et al. Annexins expressed on the cell surface serve as receptors for adhesion to immobilized fetuin-A. Biochim Biophys Acta. 2004 Aug 23;1693(2):111–23. doi: 10.1016/j.bbamcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Ochieng J, Warfield P, Green B. Interactions of gelatinases with soluble and immobilized fetuin-A and asialofetuin. Arch Biochem Biophys. 1995 Sep 10;322(1):250–5. doi: 10.1006/abbi.1995.1459. [DOI] [PubMed] [Google Scholar]
- 59.Ochieng J, Green B. The interactions of alpha 2HS glycoprotein with metalloproteinases. Biochem Mol Biol Int. 1996 Sep;40(10):13–20. doi: 10.1080/15216549600201482. [DOI] [PubMed] [Google Scholar]
- 60.Tajirian T, Dennis JW, Swallow CJ. Regulation of human monocyte proMMP-9 production by fetuin, an endogenous TGF-beta antagonist. J Cell Physiol. 2000 Nov;185(2):174–83. doi: 10.1002/1097-4652(200011)185:2<174::AID-JCP2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 61.Massey D. Commentary: clinical diagnostic use of cystatin C. J Clin Lab Anal. 2004;18(1):55–60. doi: 10.1002/jcla.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992 Oct;38(10):1933–53. [PubMed] [Google Scholar]
- 63.Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest. 1985 Apr;45(2):97–101. doi: 10.3109/00365518509160980. [DOI] [PubMed] [Google Scholar]
- 64.Abrahamson M, Barrett AJ, Salvesen G, et al. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem. 1986 Aug 25;261(24):11282–9. [PubMed] [Google Scholar]
- 65.Reed CH. Diagnostic applications of cystatin C. Br J Biomed Sci. 2000;57(4):323–9. [PubMed] [Google Scholar]
- 66.van Oss CJ, Bronson PM, Border JR. Changes in the serum alpha glycoprotein distribution in trauma patients. J Trauma. 1975 May;15(5):451–5. doi: 10.1097/00005373-197505000-00013. [DOI] [PubMed] [Google Scholar]
- 67.Lebreton JP, Joisel F, Raoult JP, et al. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979 Oct;64(4):1118–29. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Oss CJ, Gillman CF, Bronson PM, et al. Opsonic properties of human serum alpha-2 HS glycoprotein. Immunol Commun. 1974;3(4):329–35. doi: 10.3109/08820137409061113. [DOI] [PubMed] [Google Scholar]
- 69.Lewis JG, Andre CM. Enhancement of human monocyte phagocytic function by alpha 2HS glycoprotein. Immunology. 1981 Mar;42(3):481–7. [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis JG, Andre CM. Effect of human alpha 2HS glycoprotein on mouse macrophage function. Immunology. 1980 Mar;39(3):317–22. [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H, Zhang M, Soda K, et al. Fetuin protects the fetus from TNF. Lancet. 1997 Sep 20;350(9081):861–2. doi: 10.1016/S0140-6736(05)62030-2. [DOI] [PubMed] [Google Scholar]
- 72.Zhang M, Caragine T, Wang H, et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997 May 19;185(10):1759–68. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Zhang M, Bianchi M, et al. Fetuin (alpha2-HS-glycoprotein) opsonizes cationic macrophagedeactivating molecules. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14429–34. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chapman HA, Jr, Reilly JJ, Jr, Yee R, et al. Identification of cystatin C, a cysteine proteinase inhibitor, as a major secretory product of human alveolar macrophages in vitro. Am Rev Respir Dis. 1990 Mar;141(3):698–705. doi: 10.1164/ajrccm/141.3.698. [DOI] [PubMed] [Google Scholar]
- 75.Pierre P, Mellman I. Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell. 1998 Jun 26;93(7):1135–45. doi: 10.1016/s0092-8674(00)81458-0. [DOI] [PubMed] [Google Scholar]
- 76.Riese RJ, Wolf PR, Bromme D, et al. Essential role for cathepsin S in MHC class IIassociated invariant chain processing and peptide loading. Immunity. 1996 Apr;4(4):357–66. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 77.Nakagawa T, Roth W, Wong P, et al. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998 Apr;280(5362):450–3. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 78.Nakagawa TY, Rudensky AY. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunol Rev. 1999 Dec;172:121–9. doi: 10.1111/j.1600-065x.1999.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 79.Schonemeyer A, Lucius R, Sonnenburg B, et al. Modulation of human T cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J Immunol. 2001 Sep 15;167(6):3207–15. doi: 10.4049/jimmunol.167.6.3207. [DOI] [PubMed] [Google Scholar]
- 80.Verdot L, Lalmanach G, Vercruysse V, et al. Cystatins up-regulate nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages. J Biol Chem. 1996 Nov 8;271(45):28077–81. doi: 10.1074/jbc.271.45.28077. [DOI] [PubMed] [Google Scholar]
- 81.Hartmann S, Schonemeyer A, Sonnenburg B, et al. Cystatins of filarial nematodes up-regulate the nitric oxide production of interferon-gamma-activated murine macrophages. Parasite Immunol. 2002 May;24(5):253–62. doi: 10.1046/j.1365-3024.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 82.Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993 Jun 15;268(17):12231–4. [PubMed] [Google Scholar]
- 83.Kolb H, Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992 May;13(5):157–60. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- 84.Das L, Datta N, Bandyopadhyay S, et al. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T cell response. J Immunol. 2001 Mar 15;166(6):4020–8. doi: 10.4049/jimmunol.166.6.4020. [DOI] [PubMed] [Google Scholar]
- 85.Denham S, Rowland IJ. Inhibition of the reactive proliferation of lymphocytes by activated macrophages: the role of nitric oxide. Clin Exp Immunol. 1992 Jan;87(1):157–62. doi: 10.1111/j.1365-2249.1992.tb06430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawabe T, Isobe KI, Hasegawa Y, et al. Immunosuppressive activity induced by nitric oxide in culture supernatant of activated rat alveolar macrophages. Immunology. 1992 May;76(1):72–8. [PMC free article] [PubMed] [Google Scholar]
- 87.Yamada M. Cerebral amyloid angiopathy: an overview. Neuropathology. 2000 Mar;20(1):8–22. doi: 10.1046/j.1440-1789.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 88.Revesz T, Ghiso J, Lashley T, et al. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 2003 Sep;62(9):885–98. doi: 10.1093/jnen/62.9.885. [DOI] [PubMed] [Google Scholar]
- 89.Fujihara S, Shimode K, Nakamura M, et al. Cerebral amyloid angiopathy with the deposition of cystatin C (gamma-trace) and beta-protein. Prog Clin Biol Res. 1989;317:939–44. [PubMed] [Google Scholar]
- 90.Maruyama K, Ikeda S, Ishihara T, et al. Immunohistochemical characterization of cerebrovascular amyloid in 46 autopsied cases using antibodies to beta protein and cystatin C. Stroke. 1990 Mar;21(3):397–403. doi: 10.1161/01.str.21.3.397. [DOI] [PubMed] [Google Scholar]
- 91.Vattemi G, Engel WK, McFerrin J, et al. Cystatin C colocalizes with amyloid-beta and coimmunoprecipitates with amyloid-beta precursor protein in sporadic inclusion-body myositis muscles. J Neurochem. 2003 Jun;85(6):1539–46. doi: 10.1046/j.1471-4159.2003.01798.x. [DOI] [PubMed] [Google Scholar]
- 92.Mitchell TI, Jeffrey JJ, Palmiter RD, et al. The acute phase reactant serum amyloid A (SAA3) is a novel substrate for degradation by the metalloproteinases collagenase and stromelysin. Biochim Biophys Acta. 1993 Mar 21;1156(3):245–54. doi: 10.1016/0304-4165(93)90038-a. [DOI] [PubMed] [Google Scholar]
- 93.Stix B, Kahne T, Sletten K, et al. Proteolysis of AA amyloid fibril proteins by matrix metalloproteinases-1, -2, and -3. Am J Pathol. 2001 Aug;159(2):561–70. doi: 10.1016/S0002-9440(10)61727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Backstrom JR, Lim GP, Cullen MJ, et al. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40) J Neurosci. 1996 Dec 15;16(24):7910–9. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sousa MM, do Amaral JB, Guimaraes A, et al. Up-regulation of the extracellular matrix remodeling genes, biglycan, neutrophil gelatinase-associated lipocalin and matrix metal loproteinase-9 in familial amyloid polyneuropathy. Faseb J. 2005 Jan;19(1):124–6. doi: 10.1096/fj.04-2022fje. [DOI] [PubMed] [Google Scholar]
- 96.Kjeldsen L, Johnsen AH, Sengelov H, et al. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993 May 15;268(14):10425–32. [PubMed] [Google Scholar]
- 97.Jensson O, Gudmundsson G, Arnason A, et al. Hereditary cystatin C (gamma-trace) amyloid angiopathy of the CNS causing cerebral hemorrhage. Acta Neurol Scand. 1987 Aug;76(2):102–14. doi: 10.1111/j.1600-0404.1987.tb03553.x. [DOI] [PubMed] [Google Scholar]
- 98.Levy E, Lopez-Otin C, Ghiso J, et al. Stroke in Icelandic patients with hereditary amyloid angiopathy is related to a mutation in the cystatin C gene, an inhibitor of cysteine proteases. J Exp Med. 1989 May 1;169(5):1771–8. doi: 10.1084/jem.169.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carrell RW, Gooptu B. Conformational changes and disease—serpins, prions and Alzheimer’s. Curr Opin Struct Biol. 1998 Dec;8(6):799–809. doi: 10.1016/s0959-440x(98)80101-2. [DOI] [PubMed] [Google Scholar]
- 100.Kelly JW. The alternative conformations of amyloidogenic proteins and their multistep assembly pathways. Curr Opin Struct Biol. 1998 Feb;8(1):101–6. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 101.Staniforth RA, Giannini S, Higgins LD, et al. Three-dimensional domain swapping in the folded and molten-globule states of cystatins, an amyloid-forming structural superfamily. Embo J. 2001 Sep 3;20(17):4774–81. doi: 10.1093/emboj/20.17.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Janowski R, Kozak M, Jankowska E, et al. Human cystatin C, an amyloidogenic protein, dimerizes through three-dimensional domain swapping. Nat Struct Biol. 2001 Apr;8(4):316–20. doi: 10.1038/86188. [DOI] [PubMed] [Google Scholar]
- 103.Ekiel I, Abrahamson M. Folding-related dimerization of human cystatin C. J Biol Chem. 1996 Jan 19;271(3):1314–21. doi: 10.1074/jbc.271.3.1314. [DOI] [PubMed] [Google Scholar]
- 104.Jerala R, Zerovnik E. Accessing the global minimum conformation of stefin A dimer by annealing under partially denaturing conditions. J Mol Biol. 1999 Sep;291(5):1079–89. doi: 10.1006/jmbi.1999.3045. [DOI] [PubMed] [Google Scholar]
- 105.Zerovnik E, Jerala R, Kroon-Zitko L, et al. Characterization of the equilibrium intermediates in acid denaturation of human stefin B. Eur J Biochem. 1997 Apr 15;245(2):364–72. doi: 10.1111/j.1432-1033.1997.t01-1-00364.x. [DOI] [PubMed] [Google Scholar]
- 106.Ekiel I, Abrahamson M, Fulton DB, et al. NMR structural studies of human cystatin C dimers and monomers. J Mol Biol. 1997 Aug;271(2):266–77. doi: 10.1006/jmbi.1997.1150. [DOI] [PubMed] [Google Scholar]
- 107.Bollengier F. Cystatin C, alias post-gamma-globulin: a marker for multiple sclerosis? J Clin Chem Clin Biochem. 1987 Dec;25(9):589–93. doi: 10.1515/cclm.1987.25.9.589. [DOI] [PubMed] [Google Scholar]
- 108.Nagai A, Murakawa Y, Terashima M, et al. Cystatin C and cathepsin B in CSF from patients with inflammatory neurologic diseases. Neurology. 2000;55:1828–32. doi: 10.1212/wnl.55.12.1828. [DOI] [PubMed] [Google Scholar]
- 109.Rinne R, Saukko P, Jarvinen M, et al. Reduced cystatin B activity correlates with enhanced cathepsin activity in progressive myoclonus epilepsy. Ann Med. 2002;34(5):380–5. doi: 10.1080/078538902320772124. [DOI] [PubMed] [Google Scholar]
- 110.Shannon P, Pennacchio LA, Houseweart MK, et al. Neuropathological changes in a mouse model of progressive myoclonus epilepsy: cystatin B deficiency and Unverricht-Lundborg disease. J Neuropathol Exp Neurol. 2002 Dec;61(12):1085–91. doi: 10.1093/jnen/61.12.1085. [DOI] [PubMed] [Google Scholar]