Abstract
T lymphocytes expressing a chimeric antigen receptor (CAR) targeting the CD19 antigen (CAR.19) may be of value for the therapy of B-cell malignancies. Because the in vivo survival, expansion and anti-lymphoma activity of CAR.19+ T cells remain suboptimal even when the CAR contains a CD28 costimulatory endodomain, we generated a novel construct that also incorporates the interleukin-15 (IL15) gene and an inducible caspase-9-based suicide gene (iC9/CAR.19/IL15). We found that compared to CAR.19+ T cells, iC9/CAR.19/IL15+ T cells had: (i) greater numeric expansion upon antigen stimulation (10-fold greater expansion in vitro, and 3 to 15 fold greater expansion in vivo) and reduced cell death rate (Annexin-V+/7-AAD+ cells 10% ± 6% for iC9/CAR.19/IL15+ T cells and 32% ± 19% CAR.19+ T cells); (ii) reduced expression of the programmed death 1 (PD-1) receptor upon antigen stimulation (PD-1+ cells <15% for iC9/CAR.19/IL15+ T cells versus >40% for CAR.19+ T cells); (iii) improved anti-tumor effects in vivo (from 4.7 to 5.4-fold reduced tumor growth). In addition, iC9/CAR.19/IL15+ T cells were efficiently eliminated upon pharmacologic activation of the suicide gene. In summary, this strategy safely increases the anti-lymphoma/leukemia effects of CAR.19-redirected T lymphocytes and may be a useful approach for treatment of patients with B-cell malignancies.
INTRODUCTION
T lymphocytes expressing a chimeric antigen receptor (CAR) can be adoptively transferred to target a range of human malignancies including non-Hodgkin and Hodgkin lymphomas(1–5). CARs most commonly combine the antigen-binding specificity of a monoclonal antibody with the effector endodomain of the CD3/T-cell receptor complex (ζ chain), and redirect the specificity of T lymphocytes toward surface antigens expressed by tumor cells(6). CARs that target B-lineage restricted antigens such as CD19(7, 8), CD20(9), and the light chain of human immunoglubulins(10), or CD30 expressed by Reed-Sternberg cells(2, 4) have been cloned and validated in preclinical lymphoma/leukemia models, and some are currently in Phase I clinical trials(1, 3, 5, 11). However, it is evident from both clinical trials(1, 12, 13) and preclinical models(3, 10, 14) that the expansion and persistence of CAR-modified T cells in vivo are hampered by the lack of costimulatory signals following engagement with target antigens, since many tumor cells down regulate their expression of the costimulatory molecules required for optimal and sustained T-cell function, proliferation and persistence(3, 5).
This limitation has been partially resolved by the construction of “second generation” CARs in which a costimulatory endodomain derived from molecules such as CD28(10, 14, 15) or 4-1BB(16, 17) have been incorporated within the chimeric receptors. T cells expressing these enhanced CARs retain their cytotoxic function, but, upon antigen engagement, they produce IL2 which helps sustain their activation and expansion(10, 14, 15), and augments anti-tumor activity(3, 10, 14). To further potentiate the costimulation of CAR-modified T cells, “third generation” CARs have been developed which contain multiple costimulatory endodomains such as combinations of CD28 and 4-1BB(18–21) or CD28 and OX40(22), which may have superior activity compared to those encoding single costimulatory endodomains(18–20, 22).
We now describe an alternative strategy. We have engineered CAR-modified T cells to receive not only costimulation through the CD28 pathway but also to ectopically produce IL15, a cytokine crucial for T-cell homeostasis and survival(23, 24). Since these changes may increase the risk of direct toxicity and uncontrolled proliferation(25), we have also included a suicide gene that can be pharmacologically activated to eliminate transgenic cells as required(26, 27).
MATERIALS AND METHODS
Cell lines
The following cell lines were used: Daudi and Raji (CD19+ Burkitt lymphoma cell lines), HDLM-2 (CD30+CD19− Hodgkin lymphoma cell line), Karpas-299 (CD30+CD19− anaplastic lymphoma cell line) and K562 (chronic erythroid leukemia cell line). All cells were purchased from ATCC and maintained in culture in RPMI 1640 (Gibco-BRL, San Francisco, CA) supplemented with 10% FBS (Hyclone, Waltham, MA) and and 2 mM L-glutamine (Gibco-BRL).
Plasmid construction and retrovirus production
The cassette encoding the single chain antibody targeting CD19(28), the CD28 endodomain(10) and the ζ chain of the T-cell receptor complex(10) was cloned into the SFG retroviral backbone to generate the CAR.19 retroviral vector (Supplemental Fig. 1A). We then generated a second retroviral vector encoding the same CD19-specific CAR in combination with the human IL15 gene(27) and the inducible caspase-9 suicide gene that induces apoptosis upon specific binding with the small molecule dimerizer CID AP20187(26). The three genes were linked together using 2A sequence peptides derived from foot-and-mouth disease virus(27), and cloned into the SFG retroviral vector to generate the iC9/CAR.19/IL15 retroviral vector (Supplemental Fig. 1A). The vectors encoding FireFly Luciferase (FFLuc) and the fusion protein eGFP-FireFly luciferase (eGFP-FFLuc) used for in vivo imaging have been described previously(4, 10). Transient retroviral supernatants was produced as previously described(10).
Generation of CAR-modified T cells
Peripheral blood mononuclear cells (PBMC) were obtained from four healthy donors and three patients with chronic lymphocytic leukemia (B-CLL) as per local IRB approved protocols. PBMC or CD3+ enriched T cells (Miltenyi, Bergisch Gladbach, Germany) for samples collected from B-CLL patients(10) were activated with OKT3 (Ortho Biotech, Bridgewater, NJ) and CD28 (Becton Dickinson, Mountain View, CA) antibodies and recombinant human interleukin-2 (IL2) (100 U/mL) (Proleukin; Chiron, Emeryville, CA) in complete media [RPMI 1640 (Gibco-BRL) 45%, Click medium (Irvine Scientific, Santa Ana, CA) 45%, supplemented with 10% FCS (Hyclone) and 2 mM L-glutamine (GIBCO-BRL)](10). Activated T cells were transduced with retroviral supernatants on day 3 in plates coated with recombinant fibronectin fragment (FN CH-296; Retronectin; Takara Shuzo, Otsu, Japan)(10). After transduction, T cells were expanded using IL2 and then used for the experiments described below.
Coculture experiments
Cytokine production
Seven days after transduction, control non-transduced (NT), CAR.19+ and iC9/CAR.19/IL15+ T cells (1 × 106 cells per well) were cocultured in 24 well plates with B-CLL cells (enriched-CD19+ cells) at an effector:tumor cell ratio (E:T) of 1:1. Culture supernatants were collected after 24, 48 and 72 hrs of culture to measure the production of IL2, IL15 and IFNγ using specific Elisas (R&D Systems, Inc, Minneapolis, MN).
T-cell expansion
To evaluate the T-cell growth, control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells were maintained in culture and stimulated once a week with CD19+ B-CLL cells (E:T ratio 1:2) without any addition of exogenous cytokines. Cells were cultured for five weeks, and counted by trypan blue exclusion every week.
T-cell division and death
To measure the cell division of T cells upon antigen stimulation, we labeled control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells with carboxyfluorescein diacetate succinimidyl ester (CFSE) (eBioscience, Inc. San Diego, CA)(29). We then stimulated the T cells with CD19+ B-CLL cells (E:T ratio of 2:1) and measured the CFSE dilution by FACS analysis after 5 days of culture. To measure T-cell death upon antigen stimulation we used the Annexin-V/7-AAD staining and FACS analysis(27).
Anti-tumor effects
To evaluate elimination of tumor cells, control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells were cocultured with Daudi cells (CD19+) (E:T ratio of 5:1). After 3 days culture, cells were collected and residual tumor cells enumerated by FACS analysis. For samples obtained from B-CLL patients, anti-tumor effects were evaluated against autologous B-CLL cells (E:T ratio of 2:1). In these experiments, B-CLL cells were labeled with CFSE and enumerated by FACS after 3–4 days of coculture(10). In some experiments, we used wild type Karpas cells (CD30+CD19−) or CD19+ transgenic Karpas as the targets.
Activation of the suicide gene
CID AP20187 (ARIAD Pharmaceuticals, Cambridge, MA) was kindly provided by Dr. Spencer (Baylor College of Medicine) and added at the indicated concentrations to T-cell cultures. The elimination of transgenic cells coexpressing the inducible suicide gene was evaluated 24–48 hours after incubation using Annexin-V/7-AAD staining and FACS analysis(27).
Immunophenotyping
Cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or Peridinin-chlorophyll proteins (PerCP)-conjugated monoclonal antobodies (mAbs). To stain the tumor cells we used CD19, CD20 and CD30 from Becton Dickinson (Mountain View, CA). For the T lymphocytes we used CD3, CD4, CD8, CD56, CD45RA, CD45RO, CD62L, CD27, CD28, CCR7, Bcl-2 and PD-1 from Becton Dickinson (Mountain View, CA). To detect the expression of CAR.19, we used a mAb Fc-specific cyanine-Cy5-conjugated provided by Jackson ImmunoResearch (West Grove, PA), which recognized the IgG1-CH2CH3 component of the artificial receptor(10). Apoptosis was measured using Annexin-V and 7AAD staining (Becton Dickinson). Cells were analyzed by FACScan (Becton Dickinson), equipped with the filter set for triple fluorescence signals.
Chromium release assay
We used 4 hour 51Cr-release assays to evaluate the cytotoxic activity of control and CAR+ T lymphocytes(10). The labeled targets tested included Daudi (CD19+ target), HDLM-2 (CD19− target) and K562 (natural killer cell target).
Xenogeneic lymphoma models
To assess the persistence and anti-tumor effect of CAR+ T cells in vivo, we used a SCID-lymphoma human xenograft model. Mouse experiments were performed in accordance with Baylor College of Medicine Animal Husbandry guidelines according to an IACUC approved protocol.
Trafficking and expansion of CAR+ cells
In the first set of experiments to evaluate engraftment, Daudi and Raji cells were labeled with FFLuc. SCID mice (8–10 week old; Harlan Sprague Dawley Inc. Indianapolis, IN) were sublethally irradiated (250 rad) and injected intravenously (i.v) with either Daudi (3 ×106) or Raji (2 × 105). Tumor engraftment was measured using the in vivo imaging system as previously described(4, 10, 27). Briefly, mice were injected intraperitoneally (i.p.) with D-luciferin (150 mg/kg), and analyzed using the Xenogen-IVIS Imaging System. Signal intensity was measured as total photon/sec/cm2/sr (p/s/cm2/sr) as previously described(27, 29, 30) In the second set of experiments to evaluate the in vivo trafficking and persistence of CAR+ cells, either control or CAR.19+ or iC9/CAR.19/IL15+ cells were labeled with eGFP-FFLuc gene(4, 27). Seven days post engraftment with unlabeled Daudi or Raji cells, mice received i.v. 10 × 106 T cells. No exogenous cytokines were administered to the mice. Trafficking, persistence and expansion of labeled T cells were measured using the Xenogen-IVIS Imaging System(4, 10, 27).
Anti-tumor effect of CAR+ T cells
To measure the anti-tumor effects of CAR+ T cells, mice were engrafted either i.p. or subcutaneously (s.c.) with Daudi cells (1 × 106 cells) labeled with the FFLuc gene. Ten days later, when the tumor was consistently measurable by light emission, mice received either control NT or CAR.19+ or iC9/CAR.19/IL15+ T cells (10 × 106; 2 doses, 1 week apart). For these experiments we used unlabeled T cells. We evaluated tumor growth using the Xenogen-IVIS Imaging System(4, 10, 27).
In vivo validation of the suicide gene
To evaluate the functionality of the suicide gene, mice bearing tumor cells and receiving iC9/CAR.19/IL15+ T cells labeled with the eGFP-FFluc gene, were treated with CID (50 µg) i.p. 2–3 doses every other day(27). CID treatment was initiated when the T-cell bioluminescent signal was exponentially increasing, indicating active expansion of the transgenic cells. Mice were then imaged as described above.
Statistical analysis
Student’s t test was used to determine the statistical significance of differences between samples, and P < .05 was accepted as indicating a significant difference. For the bioluminescence experiments, intensity signals were summarized using mean ± SD at baseline and multiple subsequent time points for each group of mice. Changes in intensity of signal from baseline at each time point were calculated and compared using the Wilcoxon signed-ranks test(27, 29).
RESULTS
T lymphocytes transduced with iC9/CAR.19/IL15 vector release IL15 following antigen stimulation and have greater expansion than T cells transduced with the CAR.19 vector
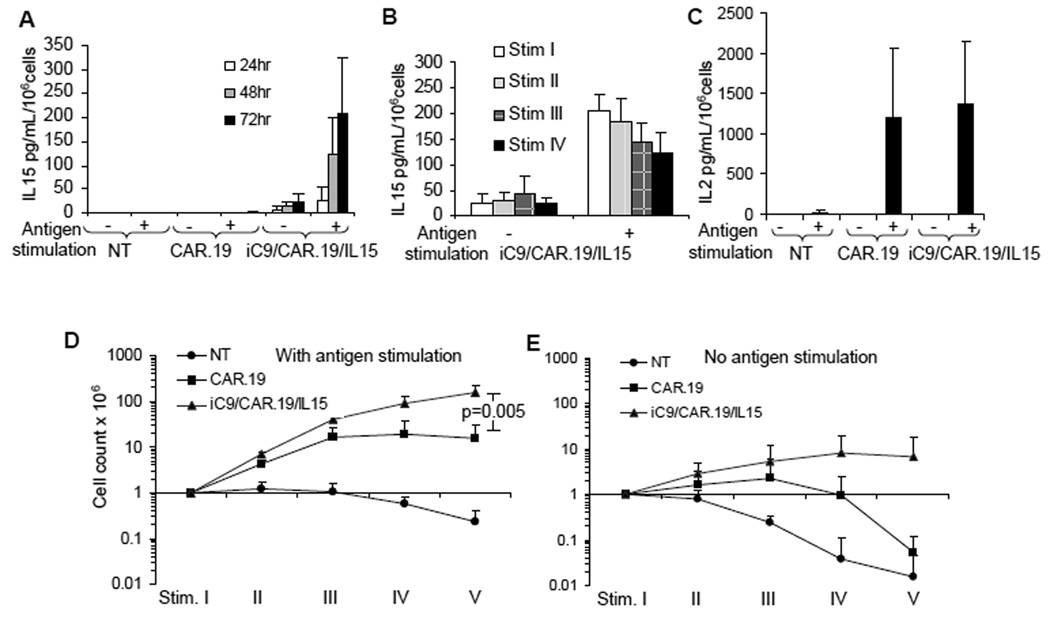
Activated T lymphocytes were equally transduced with one of two retroviral vectors encoding either CAR targeting the CD19 antigen and incorporating the CD28 endodomain (CAR.19) or the same CAR coexpressed with IL15 and the inducible suicide gene caspase-9 (iC9/CAR.19/IL15) (Supplemental Fig. 1B). We then measured IL15 production by iC9/CAR.19/IL15+ T cells and determined whether production was antigen dependent. Control NT, CAR.19+ and iC9/CAR.19/IL15+ T lymphocytes were cultured with or without CD19+ target cells (B-CLL)(10). Culture supernatants were collected at multiple time points to measure IL15 release. As shown in Fig. 1A, IL15 was undetectable in supernatants collected from stimulated or unstimulated control NT and CAR.19+ T cells. By contrast, iC9/CAR.19/IL15+ T cells produced small amounts of IL15 in the absence of antigen stimulation [25 pg/mL/106 cells (range 3 – 47 pg/mL)], which significantly increased 72 hours after antigen stimulation [240 pg/mL/106 cells (range 110 – 380 pg/mL)] (p<0.001). Importantly, when iC9/CAR.19/IL15+ T cells were maintained in culture for more than 4 weeks by weekly stimulation with CD19+ B-CLL cells, we found that the production of IL15 was sustained upon each antigen stimulation (Fig. 1B). Since CAR.19 contains the costimulatory endodomain CD28, we also measured IL2 production by genetically modified T cells(10, 14, 15). As shown in Fig. 1C, incorporation of IL15 within the iC9/CAR.19/IL15 vector did not compromise the production of IL2 by iC9/CAR.19/IL15+ T cells following antigen stimulation (mean 1527 pg/mL/106 cells, range 740 to 2000 for CAR.CD19+ cells and 1643 pg/mL/106 cells, range 631 to 2000 for iC9/CAR.19/IL15+ T cells) (p=0.8).
Figure 1. T cells transduced with the iC9/CAR.19/IL15 vector produce IL15 and expand in response to antigen stimulation.
Panel A illustrates the kinetics of IL15 release by control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells with or without antigen stimulation (CD19+ B-CLL cells). Panel B illustrates the release of IL15 by iC9/CAR.19/IL15+ T cells when these cells were maintained in culture for 4 weeks and stimulated once a week with the antigen (CD19+ B-CLL cells). Panel C illustrates the release of IL2 by control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells with or without antigen stimulation (CD19+ B-CLL cells). Panels D, E. illustrate the expansion of control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells upon weekly stimulation with CD19+ B-CLL cells. Viable cells were counted by trypan blue exclusion once a week. Data in these panels represent the mean ± SD of 4 T-cell lines.
To evaluate whether IL15 and IL2 production by iC9/CAR.19/IL15+ T cells increased their expansion compared to CAR.19+ cells (which produce only IL2 in response to antigen stimulation) (Fig. 1A, C), we maintained both CAR.19+ and iC9/CAR.19/IL15+ T cells in culture by stimulating them weekly with CD19+ B-CLL cells. As shown in Fig. 1D, iC9/CAR.19/IL15+ T cell numbers increased 10 fold compared to CAR.19+ T cells (157 × 106 ± 66 ×106 total cells vs 15 × 106 ± 16 × 106 total cells, respectively) (p=0.005) after 5 weeks of culture. By contrast, neither CAR.CD19+ T cells nor iC9/CAR.19/IL15+ T cells significantly expanded in the absence of antigen stimulation (Fig. 1E). The viability of iC9/CAR.19/IL15+ T cells in the absence of antigen stimulation was, however, preserved long-term (4–5 weeks) compared to control NT or CAR.19+ T cells (Fig. 1E). Expanded CAR.19+ and iC9/CAR.19/IL15+ T cells contained both naïve (CD45RA+) and memory (CD45RO+) CD4+ and CD8+ T lymphocytes, with circa 20% of the latter retaining CD62L and CCR7 expression (data not shown).
Transgenic expression of IL15 enhances the survival of CAR-modified T cells
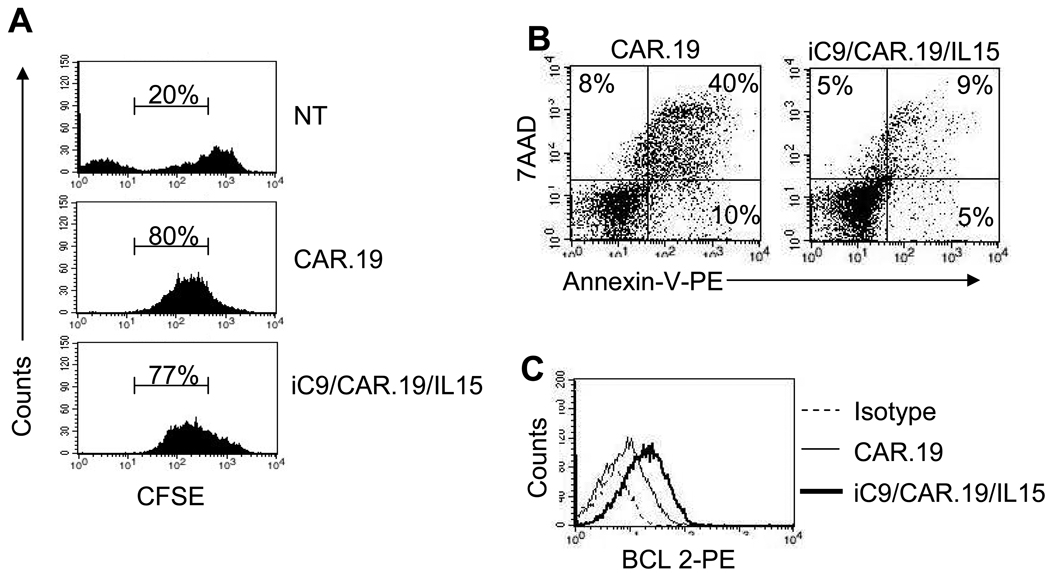
To distinguish whether the greater number of iC9/CAR.19/IL15+ T cells compared to CAR.19+ T cells following antigen stimulation was due to increased proliferation or reduced cell death, we analyzed the proliferation and apoptosis of T cells upon antigen stimulation, using CFSE and Annexin-V/7-AAD based assays, respectively. To measure cell division, T cells were labeled with CFSE and then stimulated with CD19+ B-CLL cells. As shown in Fig. 2A, after 5 days of culture CFSE dilution was comparable for CAR.CD19+ and iC9/CAR.19/IL15+ T cells (77% ± 20% and 65% ± 20%, respectively, p=0.07), suggesting similar rates of cell division. By contrast, the death rate of iC9/CAR.19/IL15+ T cells was reduced 5 days after antigenic stimulation, as assessed by Annexin-V/7-AAD staining (annexin-V+/7-AAD+ cells were 32% ± 19% and 10% ± 6% for CAR.19+ and iC9/CAR.19/IL15+ T cells, respectively) (p<0.001) (Fig. 2B). The improved viability of transgenic cells producing IL15 correlated also with an increased expression of anti-apoptotic genes, such as Bcl-2 (Fig. 2C).
Figure 2. T cells transduced with the iC9/CAR.19/IL15 vector have enhanced viability and higher expression of Bcl-2.
Panel A. Control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells were labeled with CFSE and stimulated with CD19+ B-CLL cells. CFSE dilution was measured by FACS analysis after 5 days of culture on live cells. Data are representative of 4 T-cell lines. CFSE negative cells of the top histogram (NT) represent residual tumor cells that are not expected to be eliminated by control T cells. Panel B illustrates Annexin-V/7-AAD staining of CAR.19+ or iC9/CAR.19/IL15+ T cells measured 5 days after the stimulation with B-CLL cells. Data are representative of 4 T-cell lines. Panel D shows BCL-2 expression as detected by FACS analysis in CAR.19+ or iC9/CAR.19/IL15+ T cells 5 days after the stimulation with B-CLL cells.
iC9/CAR.19/IL15+ T lymphocytes have enhanced expansion in vivo
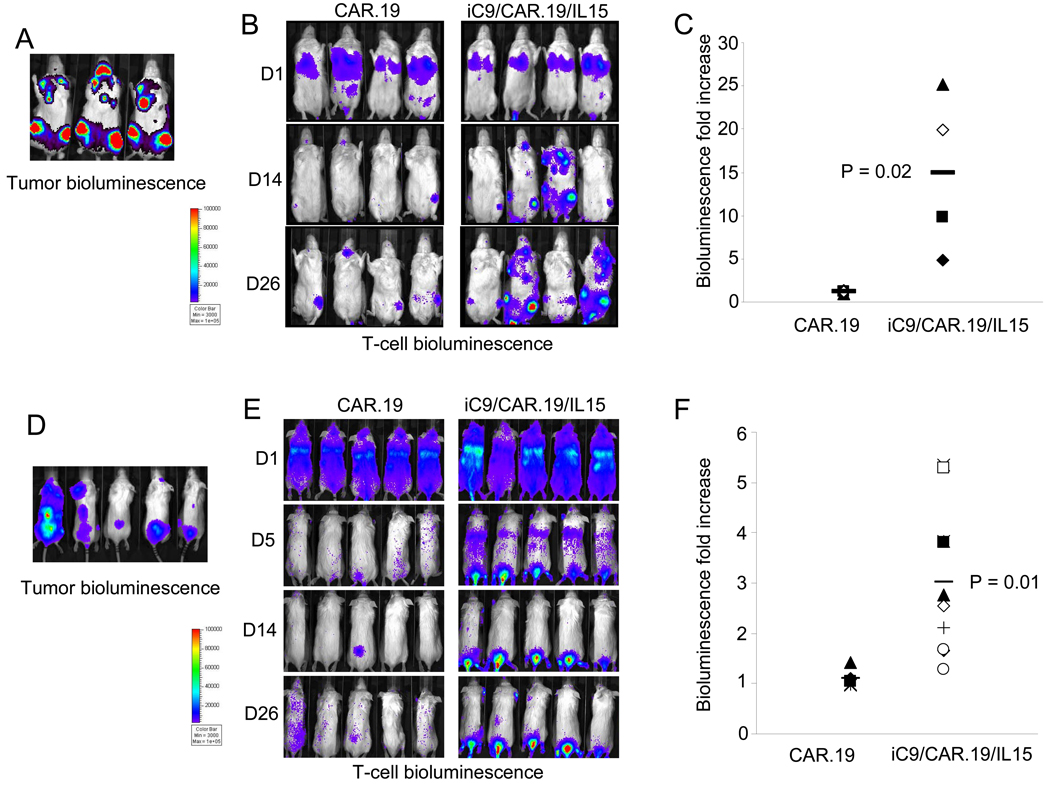
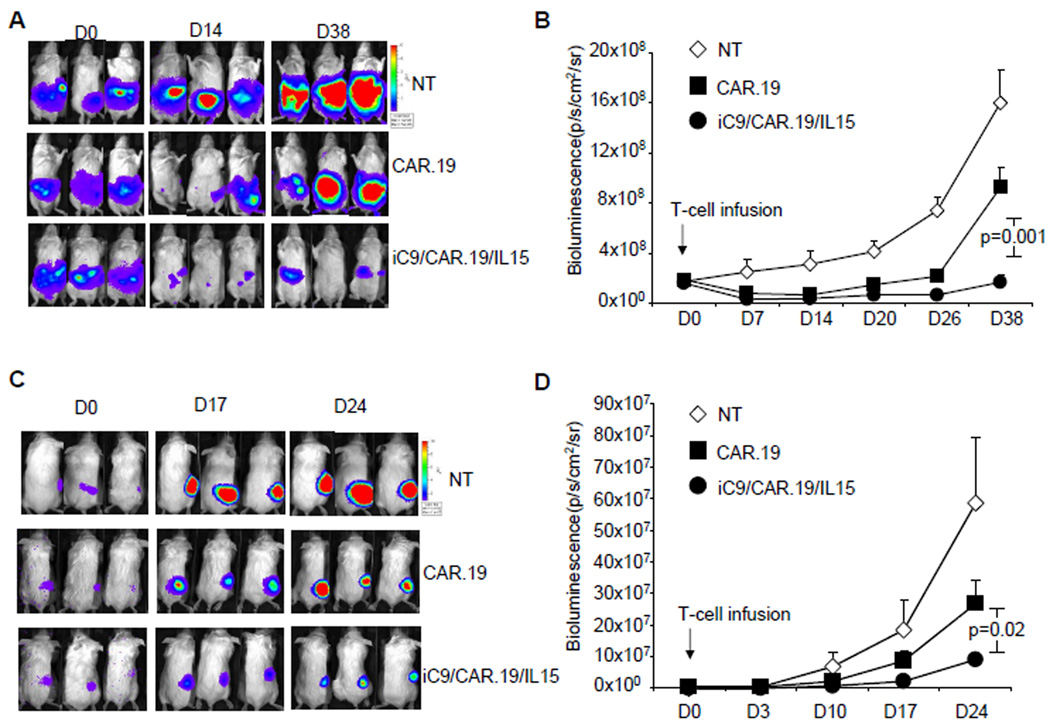
To evaluate the trafficking and persistence of our modified T cells in vivo, we used a SCID mouse lymphoma xenograft, and an extensively validated bioluminescence imaging system(4, 27, 29). We began by evaluating the tumor engraftment after i.v. inoculation either of Daudi and Raji, both CD19+ lymphoma cell lines, labeled with FFLuc. We found that 5 days after infusion, Daudi (3 × 106 cells) had engrafted diffusely in bone marrow, lymphnodes and spleen (Fig. 3A), while Raji (2 × 105 cells) had preferentially engrafted in the spinal cord (Fig. 3D). Tumor localization was confirmed by phenotypic analysis of biopsy samples (data not shown). After defining the timing and sites of tumor engraftment, we assessed T-cell trafficking to the tumor and T-cell persistence in vivo. Control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells were labeled with eGFP-FFLuc(10, 27), and infused (10 × 106 cells/mouse) in mice previously engrafted with either unlabelled Daudi or Raji cells. Figs. 3B and E illustrate that both CAR.19+ and iC9/CAR.19/IL15+ T cells localized at the tumor site since T-cell bioluminescence had superimposable anatomical localizations for the labeled tumor cells (Figs. 3A and D). T-cell bioluminescence remained barely detectable when Daudi- or Raji-engrafted tumor cells were treated with labeled control NT T cells (data not shown). Importantly, while the bioluminescence signal corresponding to CAR.19+ T cells only modestly increased by day 25 post infusion (from 4 × 105 ± 8 × 103 to 4 × 105 ± 4 × 104 for mice engrafted with Daudi, and from 1 × 105 ± 2 × 104 to 1.2 × 105 ± 3 × 104 for mice engrafted with Raji) corresponding to 1.1 and 1 fold increase, respectively (Figs. 3C and F), the signal from iC9/CAR.19/IL15+ T cells significantly increased over the following 25 days (from 5 × 105 ± 8 × 104 to 9 × 106 ± 3 × 106 for mice engrafted with Daudi and from 5 × 105 ± 8 × 104 to 2 × 106 ± 1 × 106 for mice engrafted with Raji), corresponding to 15 and 3 fold increase, respectively (p=0.02 and p=0.01) (Figs. 3C and F), showing increased expansion and persistence of these cells.
Figure 3. In vivo localization and expansion of T cells transduced either with CAR.19 or iC9/CAR.19/IL15 vectors.
Panel A and D. SCID mice were infused i.v either with FFLuc labeled Daudi or Raji cells, respectively. Tumor cell bioluminescence was measured 10 or 15 days after infusion. Then SCID mice engrafted either with unlabeled Daudi or Raji cells, respectively, were injected either with CAR.19+ or iC9/CAR.19/IL15+ T cells labelled with eGFP-FFLuc (Panels B, E). T-cell signal intensity increased in mice receiving iC9/CAR.19/IL15+ T cells compared to CAR.19+ T cells. Panels C and F illustrate the maximum increase in T-cell bioluminescence obtained in 5 and 10 mice per group, respectively.
T lymphocytes transduced with the iC9/CAR.19/IL15 vector have enhanced anti-tumor activity
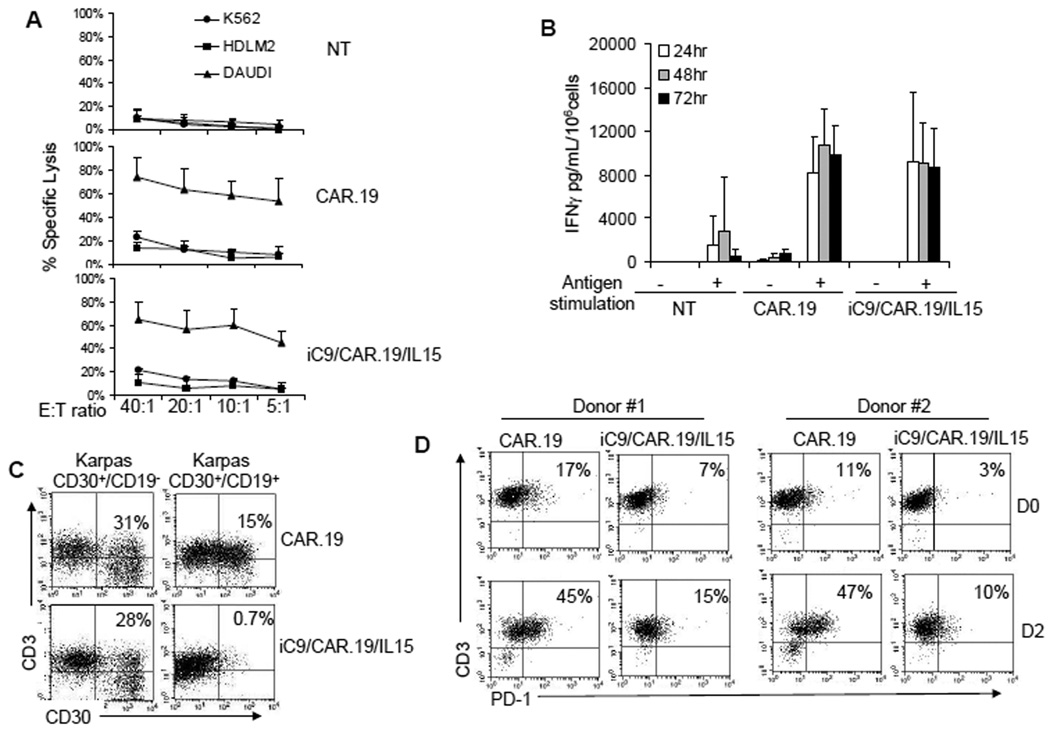
We measured the cytotoxic activity of T cells against CD19+ and CD19− tumor cell lines using standard 51Cr release assays. Both CAR.CD19+ and iC9/CAR.19/IL15+ T cells had equal and specific cytotoxic activity against CD19+ Daudi cells (63% ± 17% and 57% ± 16% specific lysis at an E:T ratio of 20:1, respectively), with less than 13% killing of HDLM-2 (CD19−) and erythroleukemia derived K562 (NK cell target) cell lines (Fig. 4A). Control T cells showed no significant cytotoxic activity against any of these target cell lines. In parallel, both CAR.19+ and iC9/CAR.19/IL15+ T cells produced equal amounts of INFγ in response to CD19+ tumor cells (mean of 8327 pg/mL/106 cells, range 5080 to 12510 for CAR.CD19+ cells and of 9147 pg/mL/106 cells, range 3025 to 18010 for iC9/CAR.19/IL15+ cells) (Fig. 4B). To further confirm that IL15 production by iC9/CAR.19/IL15+ T cells enhanced the elimination of tumor cells through the CAR, we cocultured T cells with wild type Karpas-299 tumor cells (CD30+CD19−), or with Karpas cells modified to stably express the CD19 molecule (Karpas CD30+CD19+). After 4–5 days at an initial T cell:tumor cell ratio of 5:1, residual tumor cells were quantified by FACS analysis enumerating CD30+ tumor cells. Both CAR.19+ and iC9/CAR.19/IL15+ T cells efficiently eliminated Karpas CD19+ tumor cells when T-cell anti-tumor activity was evaluated using T-cell lines maintained in short term culture (1 week) following transduction (data not shown). By contrast, when T-cell lines were maintained in culture for 4 weeks, and stimulated weekly with CD19+ B-CLL cells, only iC9/CAR.19/IL15+ T cells maintained their ability to completely eliminate tumor cells from the culture (residual tumor cells 1% ± 0.7% and 10% ± 5% for iC9/CAR.19/IL15+ T cells and CAR.19+ T cells, respectively) (p=0.001). Importantly, even after 4 weeks expansion in culture, the anti-tumor effects of both CAR.19+ and iC9/CAR.19/IL15+ T cells remained antigen-specific, since they lacked activity against wild type (CD19−) Karpas-299 cells (Fig. 4C).
Figure 4. iC/CAR.19/IL15+ T cells have enhanced anti-tumor effects and lower expression of PD-1 as compared to CAR.19+ T cells.
Panel A illustrates the cytotoxic activity of control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells. Targets were CD19+ B-cell Lymphoma cell line (Daudi), CD19− lymphoma cell line (HDLM-2) and K562 cell line. Both CAR.19+ and iC9/CAR.19/IL15+ T cells retained specific cytotoxic activity. Data illustrate the mean ± SD of 4 T-cell lines. Panel B illustrates the release of IFNγ by control, CAR.19+ and iC9/CAR.19/IL15+ T cells with or without stimulation with the antigen (CD19+ B-CLL cells). Data represents the mean ± SD of 4 T-cell lines. Panel C illustrates the antitumor effects of CAR.19+ and iC9/CAR.19/IL15+ cells kept in culture for 4 weeks. iC9/CAR.19/IL15+ T cells had enhanced capacity to eliminate tumor cells (Karpas CD30+/CD19+) as compared to CAR.19+ cells. Results are representative of 4 T-cell lines. Panel D. PD-1 was significantly overexpressed in CAR.19+ T cells as compared to iC9/CAR.19/IL15+ T cells two days upon stimulation with B-CLL leukemic cells.
To discover potential mechanisms for the sustained effector function of IL15-producing cells upon prolonged culture and repeated antigen stimulation, we evaluated the expression of PD-1, a marker of T-cell exhaustion(31), and found that iC9/CAR.19/IL15+ T cells had lower expression of PD-1 (PD-1+ T cells <15%) two days after stimulation with B-CLL cells than CAR.19+ T cells (PD-1+ T cells >40%) (Fig. 4D).
The above in vitro data were then corroborated with experiments in vivo. In two different models, SCID mice were engrafted either i.p. or s.c. with 3 × 106 Daudi cells labeled with FFLuc. Seven days later, these mice were treated with 2 weekly infusions i.p. (for mice engrafted with i.p. tumor.) or i.v (for mice engrafted with s.c. tumor) of control NT, CAR.19+ or iC9/CAR.19/IL15+ T cells (10 × 106). Tumor growth was monitored by measuring changes in tumor bioluminescence over time. As shown in Figs. 5A, B, the tumor bioluminescence of mice engrafted i.p. with Daudi cells rapidly increased in recipients of control NT T cells (rising from 1.8 × 108 ± 4 × 107 to 16 × 108 ± 2.6 × 108 by day 38). CAR.19+ T cells transiently controlled tumor growth (from 1.9 × 108 ± 3 × 107 to 9.3 × 108 ± 1.6 × 108 by day 38), while iC9/CAR.19/IL15+ T cells significantly controlled tumor expansion so that signal rose from 1.6 × 108 ± 3 × 107 to only 1.7 × 108 ± 5 × 108 by day 38 (p=0.001 when compared with mice receiving CAR.19+ T cells). Similarly, the tumor bioluminescence of mice engrafted s.c. with Daudi cells rapidly increased in mice receiving control NT T cells (from 2.6 × 105 ± 1.1 × 104 to 57 × 107 ± 20 × 107 by day 24), showed transient control in recipients of CAR.19+ T cells (from 3.3 × 105 ± 9.6 × 104 to 26 × 107 ± 7.6 × 107 by day 24) and greatest control in recipients of iC9/CAR.19/IL15+ T cells (tumor growth from 2.8 × 105 ± 7 × 104 to 5.5 × 107 ± 1.5 × 107 by day 24) (p=0.02 when compared with mice receiving CAR.19+ T cells) (Figs. 5C, D).
Figure 5. iC9/CAR.19/IL15+ T cells have enhanced anti-tumor effects in vivo as compared with CAR.19+ T cells.
To evaluate anti-tumor effects, SCID mice were engrafted in the peritoneum (Panels A, B) or subcute (Panels C, D) with Daudi cells labeled with FFLuc, and then treated either with control NT, CAR.19+ or iC9/CAR.19/IL15+ T cells 7–10 days later. Tumor growth was monitored using an in vivo imaging system. Panels A, B illustrate tumor growth in representative mice. Enhanced control of tumor growth was observed in mice receiving iC9/CAR.19/IL15+ T cells. Panels B, D summarize the bioluminescence signal as a measurement of tumor growth by day 38 and 24 after T-cell infusion. Enhanced control of tumor growth was observed in mice treated with iC9/CAR.19/IL15+ T cells. Data represent mean ± SD of 12 mice per group.
iC9/CAR.19/IL15+ T cells are eliminated after activation of the suicide gene by exposure to the small molecule CID
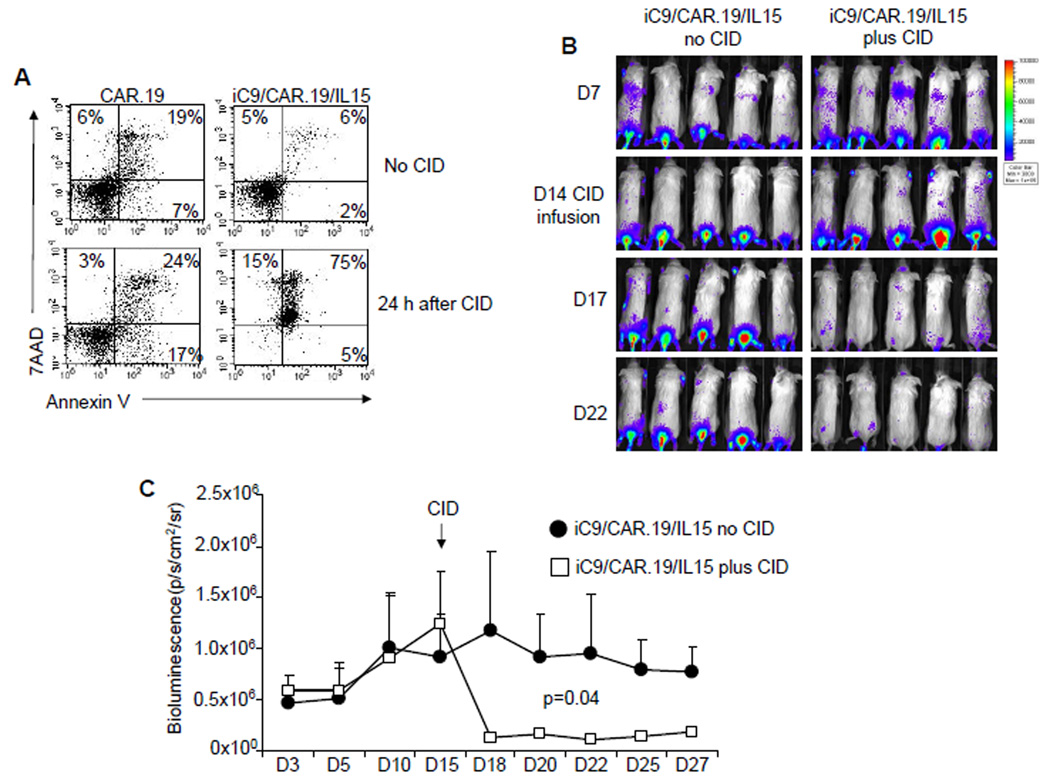
Because the production of an autocrine growth factor raises concerns about autonomous, uncontrolled T-cell growth, we incorporated in our construct a suicide gene based on the inducible caspase-9 gene(26, 27). As shown in Fig. 6A, the addition of 50 nM CID to cultures of iC9/CAR.19/IL15+ T cells induced apoptosis/necrosis of more than >95% of transgenic cells within 24 hours as assessed by annexin-V-7AAD staining(27). The suicide gene was also effective in vivo. Mice were engrafted i.v. with Raji tumor cells and then infused with eGFP-FFLuc labeled iC9/CAR.19/IL15+ T cells. These cells localized and expanded at the tumor site by day 14 after infusion as assessed by bioluminescence measurement (Fig. 6B). T-cell bioluminescence drastically reduced (from 1.2 ×106 ± 7.7 × 105 to 1.3 × 105 ± 3 × 104) following administration of the CID, consistent with a significant elimination of the transgenic cells(27).
Figure 6. Activation of the inducible caspase-9 suicide gene significantly eliminates iC9/CAR.19/IL15+ T cells.
iC9/CAR.19/IL15+ T cells undergo apoptosis upon incubation with CID AP20187 at 50 nM(27). Results are representative of 4 T-cell lines. Panel B. SCID mice engrafted i.v. with Raji cells, infused with iC9/CAR.19/IL15+ T cells expressing eGFP-FFLuc were then treated by day 14 with 2 doses of the CID AP20187 (50 µg) i.p. two days apart(27). T-cell bioluminescence reduced upon CID administration. Panel C shows the kinetics of bioluminescence in 5 mice before and after treatment with CID.
IL15 expression improves proliferation and anti-tumor effects of CAR.19+ T cells generated from B-CLL samples
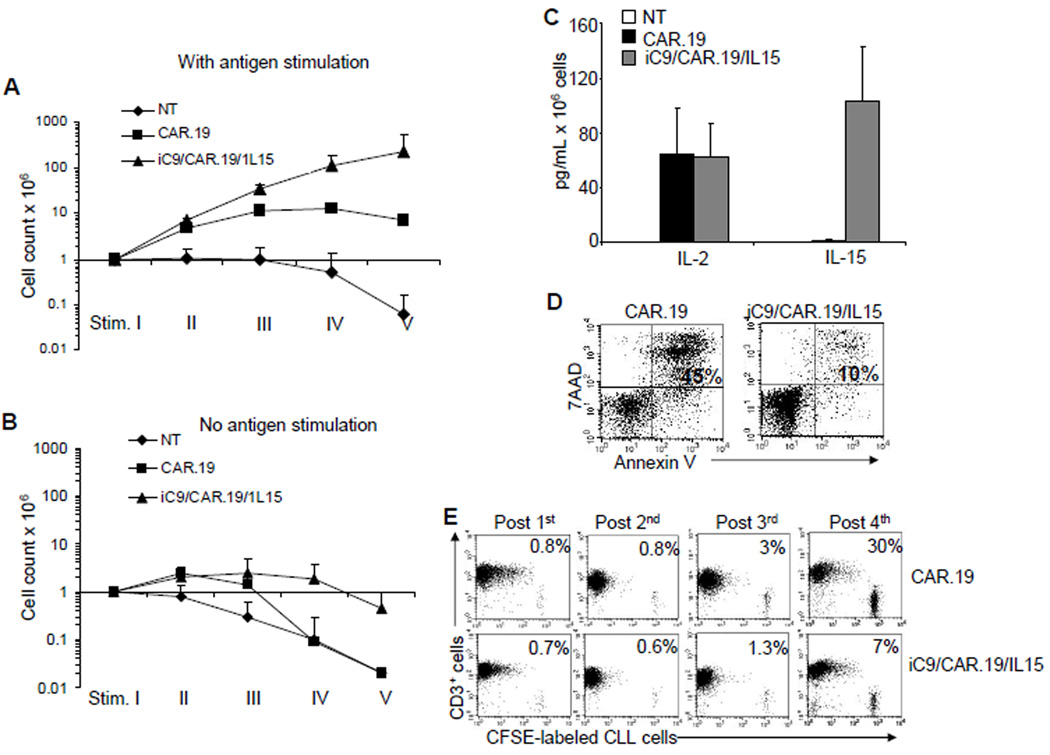
Finally, we validated the efficacy of the combination of CAR.19, IL15 and the suicide gene in cells obtained from subjects with B-CLL. As shown in Fig. 7A, the expansion of T cells isolated from B-CLL patients was enhanced following transduction with the iC9/CAR.19/IL15 vector and stimulation with autologous B-CLL cells (E:T ratio 1:1) compared to CAR.19+ T cells (228 × 106 ± 315 × 106 total cells vs 7 × 106 ± 2.7 × 106 total cells, respectively). Their expansion remained fully antigen dependent as we observed for T-cell lines generated from healthy donors (Fig. 7B). iC9/CAR.19/IL15+ T cells also released both IL15 (103 ± 39 pg/mL/106 cells) and IL2 (62 ± 24 pg/mL/106 cells) in response to autologous B-CLL cells, while CAR.19+ T cells produced only IL2 (65 ± 33 pg/mL/106 cells) (Fig. 7C). Expansion of patients’ T cells was mainly driven by the antiapoptotic effect of IL15 upon antigen stimulation (Fig. 7D). We also confirmed that iC9/CAR.19/IL15+ patient T cells retained enhanced anti-tumor activity against autologous B-CLL after long-term culture and repeated exposure to autologous tumor cells, while CAR.19+ patient T cells did not (Fig. 7E).
Figure 7. T cells isolated from B-CLL patients and expressing iC9/CAR.19/IL15 produce IL15, expand in response to autologous B-CLL and provided enhanced anti-leukemia effect.
Panels A, B. illustrate the expansion of control NT, CAR.19+ and iC9/CAR.19/IL15+ T cells obtained from B-CLL patients upon stimulation once a week with autologous B-CLL cells. Cells were counted by trypan blue exclusion once a week. Data in these panels represent the mean ± SD of 3 T-cell lines. Panel C illustrates the production of IL2 and IL15 by control, CAR.19+ and iC9/CAR.19/IL15+ T cells with or without weekly stimulation with autologous CD19+ B-CLL cells. Data in these panels represent the mean ± SD of 3 T-cell lines Panel D illustrates that IL15 protected iC9/CAR.19/IL15+ T cells from apoptosis after the stimulation with B-CLL cells. Data are representative of 3 T-cell lines. Panel E. iC9/CAR.19/IL15+ T cells retained enhanced capacity to eliminate autologous CD19+ B-CLL cells labeled with CFSE by week 4 of culture as compared to CAR.19+ T cells. Data are representative of 3 T-cell lines.
DISCUSSION
We have forced expression of IL15 in T cells redirected with a CAR that specifically targets the CD19 antigen, and demonstrated that these engineered T cells had superior survival, expansion and anti-tumor activity in vivo compared to redirected T cells that only receive CD28 costimulation through the CAR but lack IL15 production. Importantly, the incorporation of an inducible suicide gene and its pharmacologic activation efficiently eliminated these gene modified T cells further increasing the safety of the proposed approach.
In vivo persistence and expansion of adoptively transferred tumor-specific T cells is crucial to obtain sustained clinical responses(32). This is particularly important for T cells engrafted with CARs that lack costimulatory endodomains since they do not release helper cytokines upon engagement with the antigen expressed by tumor cells(10, 14, 15, 33, 34). In addition, they cannot receive appropriate activation by professional antigen presenting cells (APCs) in secondary lymphoid organs, since the native αβT-cell receptors of these redirected T cells are not generally specific for latent antigens consistently processed and presented by the host APCs(4, 13). The incorporation of the CD28 costimulatory signaling domain as part of the CAR itself(10, 14, 15, 33) can enhance activation and proliferation of these cells secondary to IL2 secretion, even without cross-presentation by APCs(10, 15). However, the overall persistence and anti-tumor effects of such CAR.19-redirectd T cells still remain limited(10, 14, 19). Alternative costimulatory endodomains may be superior to CD28, and incorporation of 4-1BB endodomain for example(16, 17) or a combination of both CD28 and 4-1BB has been reported to increase the persistence and anti-tumor efficacy over CD28 containing CARs(18–21). Nevertheless, we reasoned that the ectopic production of IL15 by CAR-modified T cells may represent a valid addition to the incorporation of costimulatory endodomains within the CAR construct since the benefits would occur through different pathways and mechanisms, since neither CD28 nor 4-1BB costimulation lead to the production of IL15, a cytokine that potently enhances the anti-tumor activity of effector T cells in vitro and in vivo(8, 35, 36). Our approach ensures that CAR-modified T cells receive both IL2 and IL15 stimulation following chimeric receptor engagement in the tumor microenvironment.
We have accommodated the IL15 gene in a single retrovirus vector in combination with the CAR.19 encoding the CD28 endodomain and an inducible suicide gene without significantly affecting CAR.19 expression, an essential requirement if tumor-specificity is to be maintained. Minimal cytokine is produced when the T cells are unstimulated, but the amount significantly increases after stimulation with tumor cells, and production is maintained by the T cells after more than 4 weeks culture. This transgenic IL15 is biologically functional, as iC9/CAR.19/IL15+ T cells have superior expansion following antigen stimulation than control CAR.19+ T cells. These benefits are largely attributable to the reduced susceptibility of iC9/CAR.19/IL15+ T cells to cell death induced upon antigen stimulation, likely because these cells have higher expression of the antiapoptotic gene Bcl-2(37) as compared to control CAR.19+ T cells. Importantly, our in vitro experiments demonstrate that iC9/CAR.19/IL15+ T cells retain enhanced anti-tumor activity when they are “chronically” exposed to tumor cells. This effect may be determined by an increased protection of iC9/CAR.19/IL15+ T cells from functional exhaustion, as indicated by a lower expression of PD-1 upon antigen-stimulation than CAR.19+ T cells, since PD-1 has been recognized as a marker of exhausted T cells in chronic infections such as HIV(31) and hepatitis C(38), and tumor infiltrating T lymphocytes(39–42). We do not yet know the transcriptional or post-transcriptional mechanisms that reduce expression of PD-1 in iC9/CAR.19/IL15+ T cells after repeated antigen stimulation. Nonetheless, our observation may have clinical implications since CAR-modified T cells that target self-antigens such as CD19, will inevitably be exposed to a large number of target cells in vivo, and PD-1 ligands may be expressed either by tumor cells itself(39, 41, 42) or by tumor-associated dendritic cells(43). The improved anti-tumor effects observed in vitro were matched by superior in vivo activity, and iC9/CAR.19/IL15+ T cells retained their tumor-homing, expanded at tumor sites and had enhanced anti-tumor activity compared to control CAR.19+ T cells.
Recently, intermittent systemic administration of recombinant IL15 has been demonstrated to induce expansion of memory CD4+ and CD8+ T cells in nonhuman primates(44). Administration of IL15 will soon be tested in patients to support the expansion of adoptively transferred tumor-specific T cells. We suggest that our proposed approach may remain advantageous as it delivers the cytokine directly to T cells at the tumor site, avoiding the toxicities that may be observed in patients receiving systemic administration of recombinant cytokines, especially when the cytokine is used at high doses(45). Our approach will also remain valuable for adoptive transfer of tumor-specific T lymphocytes in lymphodepleted patients(46) as it ensures long term availability of IL15 for tumor-specific T cells to overcome the antiproliferative effect of TGF-β(47) and regulatory T cells(48) (and manuscript in preparation) within the tumor microenvironment.
Because IL15 production by gene modified T cells occurs predominantly after they engage tumor cells through their CAR, the risks of autonomous growth should remain small. Nonetheless, given concerns with retroviral-associated oncogenesis(25), and the potential side effects reported after T-cell therapy with CAR-modified T cells(11, 49), we incorporated a suicide gene based on the inducible caspase-9 molecule within our construct. We demonstrated that the activation of this suicide gene rapidly induces apoptosis of IL15 producing T cells both in vitro and in vivo. Our previous observation that the small fraction of cells (<10%) that seems to escape apoptosis/necrosis shortly after exposure to the dimerizer drug do not express detectable levels of any transgene including cytokine(27), nor proliferate following antigen stimulation(27), may further increase the safety of the proposed approach. This suicide gene is already under evaluation in a Phase I clinical trial in which patients undergoing haploidentical transplant receive donor derived T cells gene modified with the inducible caspase-9 gene(50).
In conclusion, we show that transgenic expression of IL15 improves survival, expansion and anti-tumor effects of CD19-specific redirected T cells. The incorporation of an effective suicide gene should further assure the safety of the approach and increase its potential clinical applicability.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part from Leukemia and Lymphoma Society Specialized Center of Research (SCOR; grant no. 7018), NIH PO1CA94237, NIH P50CA126752, NIH RO1CA131027, Leukemia and Lymphoma Society Translational Research grants, Doris Duke Charitable Foundation/Clinical Scientist development award and CLL Global Research Foundation.
Footnotes
AUTHORSHIP. V.H., B.S, C.Q., M.Z., and J.V. performed the experiments. A.M. and B.S. performed the animal experiments. G.D., B.S., and V.H. designed the research and analyzed the data. V.H., B.S., and G.D wrote the manuscript. H.E.H, C.M.R. and M.K.B. critically reviewed the manuscript. All authors approved the final version of the manuscript.
DISCLOSURE. The authors declare no competing financial interests.
REFERENCE LIST
- 1.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008 Sep 15;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Pohl C, et al. Characterization of a chimeric T-cell receptor with specificity for the Hodgkin's lymphoma-associated CD30 antigen. J Immunother. 1999 Nov;22(6):473–480. doi: 10.1097/00002371-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen eceptors. Curr Opin Immunol. 2009 Apr;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30{zeta} artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007 Oct 1;110(7):2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dotti G, Savoldo B, Brenner M. Fifteen Years of Gene Therapy Based on Chimeric Antigen Receptors: "Are We Nearly There Yet?". Hum Gene Ther. 2009 Sep 26; doi: 10.1089/hum.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003 Feb 15;101(4):1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 8.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Medz. 2003 Mar;9(3):279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 9.Jensen M, Tan G, Forman S, Wu AM, Raubitschek A. CD20 is a molecular target for scFvFc:zeta receptor redirected T cells: implications for cellular immunotherapy of CD20+ malignancy. Biol Blood Marrow Transplant. 1998;4(2):75–83. doi: 10.1053/bbmt.1998.v4.pm9763110. [DOI] [PubMed] [Google Scholar]
- 10.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006 Dec 1;108(12):3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brentjens R. Unexpected toxicity of cyclophosphamide folowed by adoptively transferred CD19-targeted T cells in a patient with bulky CLL. Molecular Therapy. 2009;17(suppl 1 2009) RIHDTCNYSJLJYRSESM, Ref Type: Abstract. [Google Scholar]
- 12.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006 Oct 15;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008 Nov;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006 Nov 15;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 15.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002 Jan;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 16.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004 Apr;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 17.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric Receptors Containing CD137 Signal Transduction Do mains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol Ther. 2009 Apr 21; doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tammana S, Huang X, Wong M, Milone MC, Ma L, Levine BL, et al. 4-1BB and CD28 Signaling Plays A Synergistic Role in Redirecting Umbilical Cord Blood T Cells Against B-Cell Malignancies. Hum Gene Ther. 2009 Aug 31; doi: 10.1089/hum.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009 Nov 1;183(9):5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007 Aug;18(8):712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 22.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005 Nov;12(5):933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 24.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001 Feb;14(2):105–110. [PubMed] [Google Scholar]
- 25.Hsu C, Jones SA, Cohen CJ, Zheng Z, Kerstann K, Zhou J, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007 Jun 15;109(12):5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005 Jun 1;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007 Oct 15;110(8):2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossig C, Brenner MK. Chimeric T-cell receptors for the targeting of cancer cells. Acta Haematol. 2003;110(2–3):154–159. doi: 10.1159/000072465. [DOI] [PubMed] [Google Scholar]
- 29.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009 Jun 18;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YJ, Dubey P, Ray P, Gambhir SS, Witte ON. Multimodality imaging of lymphocytic migration using lentiviral-based transduction of a tri-fusion reporter gene. Mol Imaging Biol. 2004 Sep;6(5):331–340. doi: 10.1016/j.mibio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006 Sep 21;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 32.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003 Sep;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998 Sep 15;161(6):2791–2797. [PubMed] [Google Scholar]
- 34.Huang X, Guo H, Kang J, Choi S, Zhou TC, Tammana S, et al. Sleeping Beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol Ther. 2008 Mar;16(3):580–589. doi: 10.1038/sj.mt.6300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004 Feb 17;101(7):1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roychowdhury S, May KF, Jr, Tzou KS, Lin T, Bhatt D, Freud AG, et al. Failed adoptive immunotherapy with tumor-specific T cells: reversal with low-dose interleukin 15 but not low-dose interleukin 2. Cancer Res. 2004 Nov 1;64(21):8062–8067. doi: 10.1158/0008-5472.CAN-04-1860. [DOI] [PubMed] [Google Scholar]
- 37.Hsu C, Hughes MS, Zheng Z, Bray RB, Rosenberg SA, Morgan RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005 Dec 1;175(11):7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, et al. PD-1 Expression in Acute Hepatitis C Virus (HCV) Infection Is Associated with HCV-Specific CD8 Exhaustion. J Virol. 2006 Nov 15;80(22):11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and Its Ligands in Tolerance and Immunity. Annual Review of Immunology. 2008 Apr 9;26(1):677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009 Aug 20;114(8):1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 41.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009 Aug 20;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009 Aug 20;114(8):1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003 May;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 44.Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009 Sep 17;114(12):2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008 Apr;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005 Apr 1;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005 Apr;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 48.Ben AM, Belhadj HN, Moes N, Buyse S, Abdeladhim M, Louzir H, et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009 Jun 1;182(11):6763–6770. doi: 10.4049/jimmunol.0801792. [DOI] [PubMed] [Google Scholar]
- 49.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006 May 1;24(13):e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 50.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007 Aug;13(8):913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.