Abstract
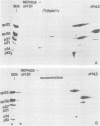
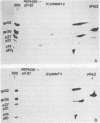
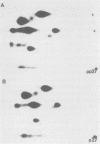
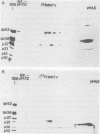
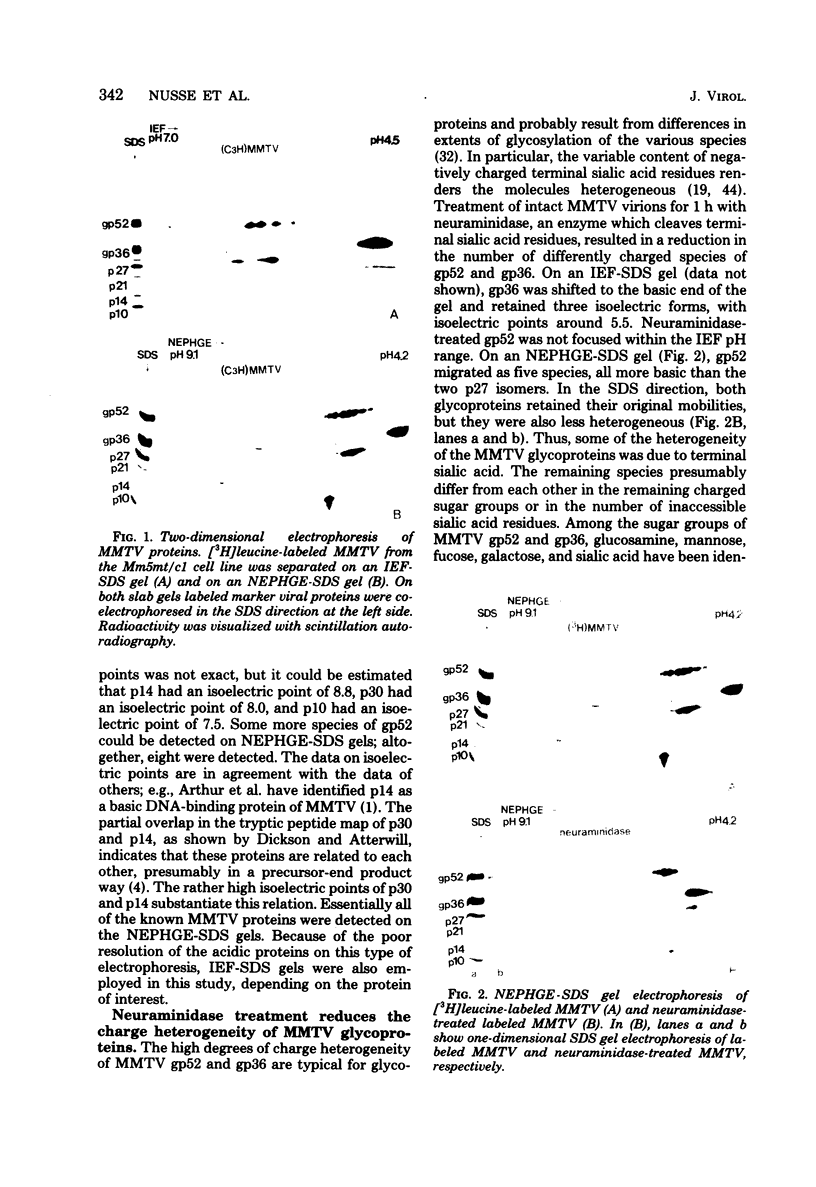
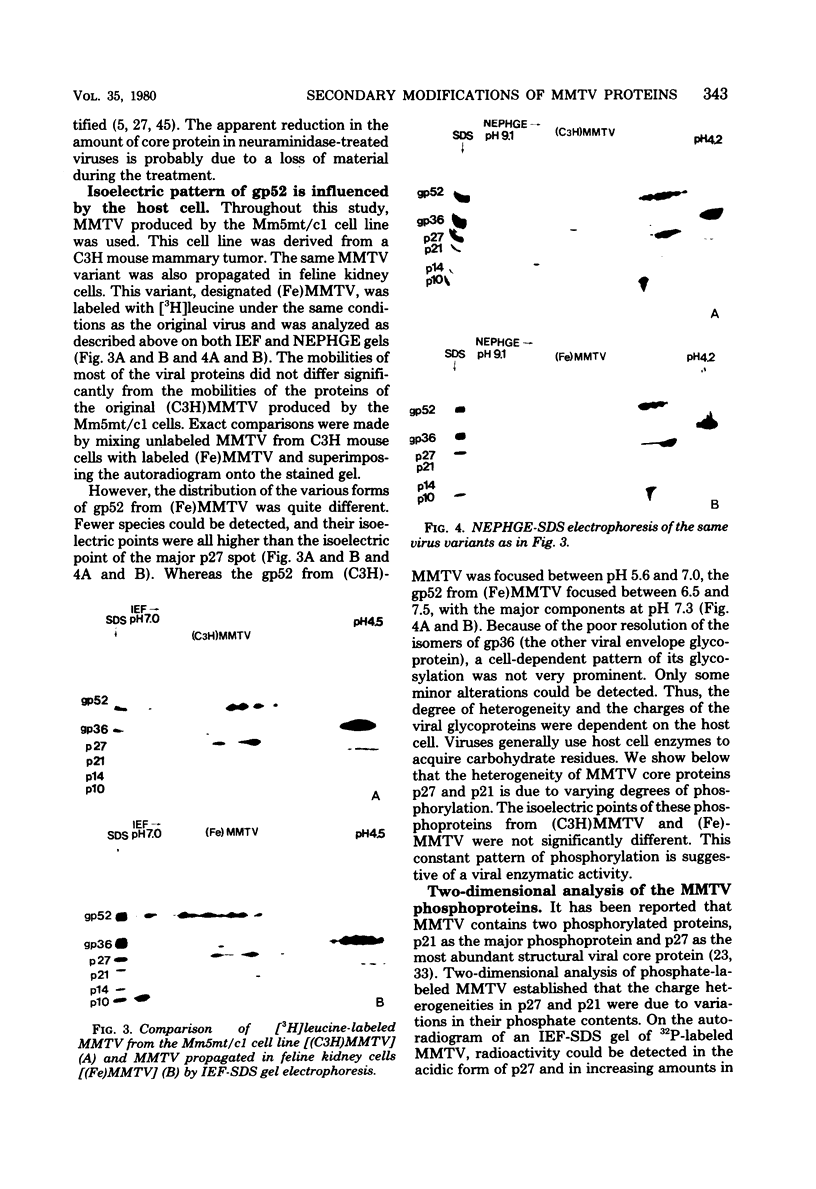
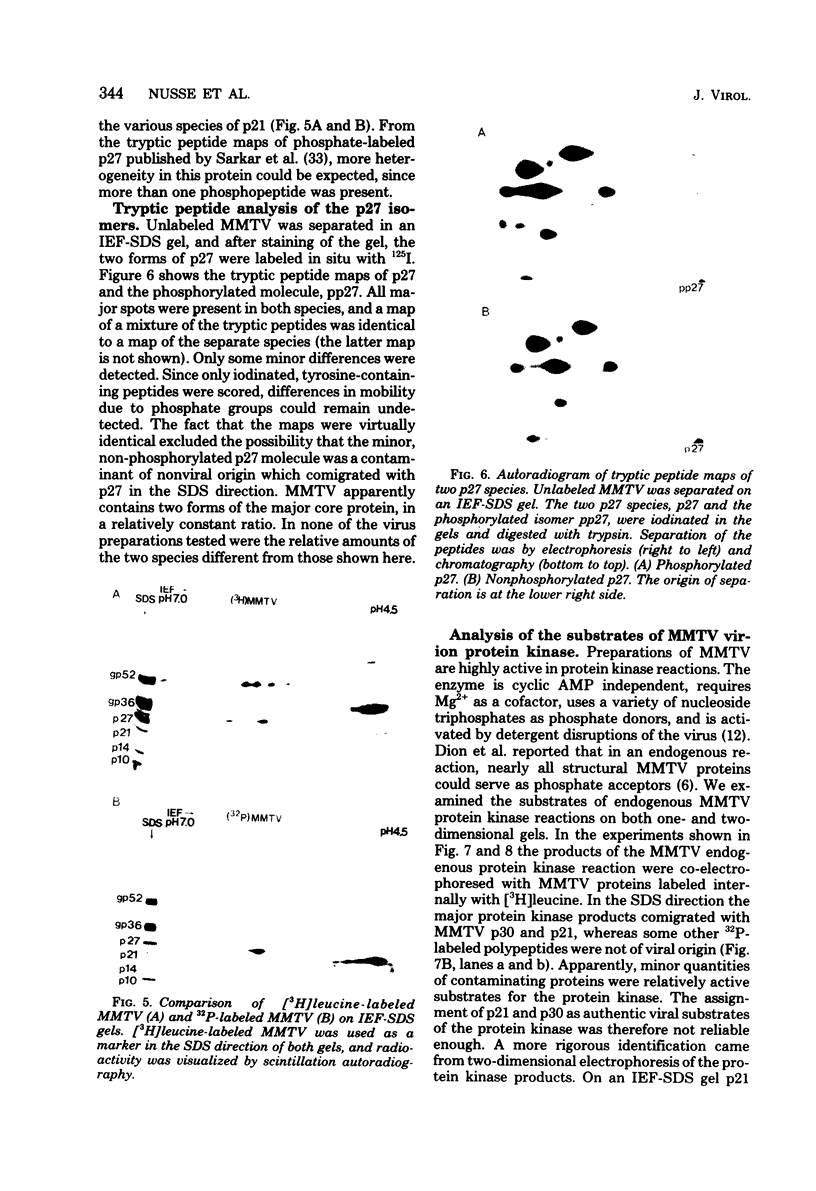
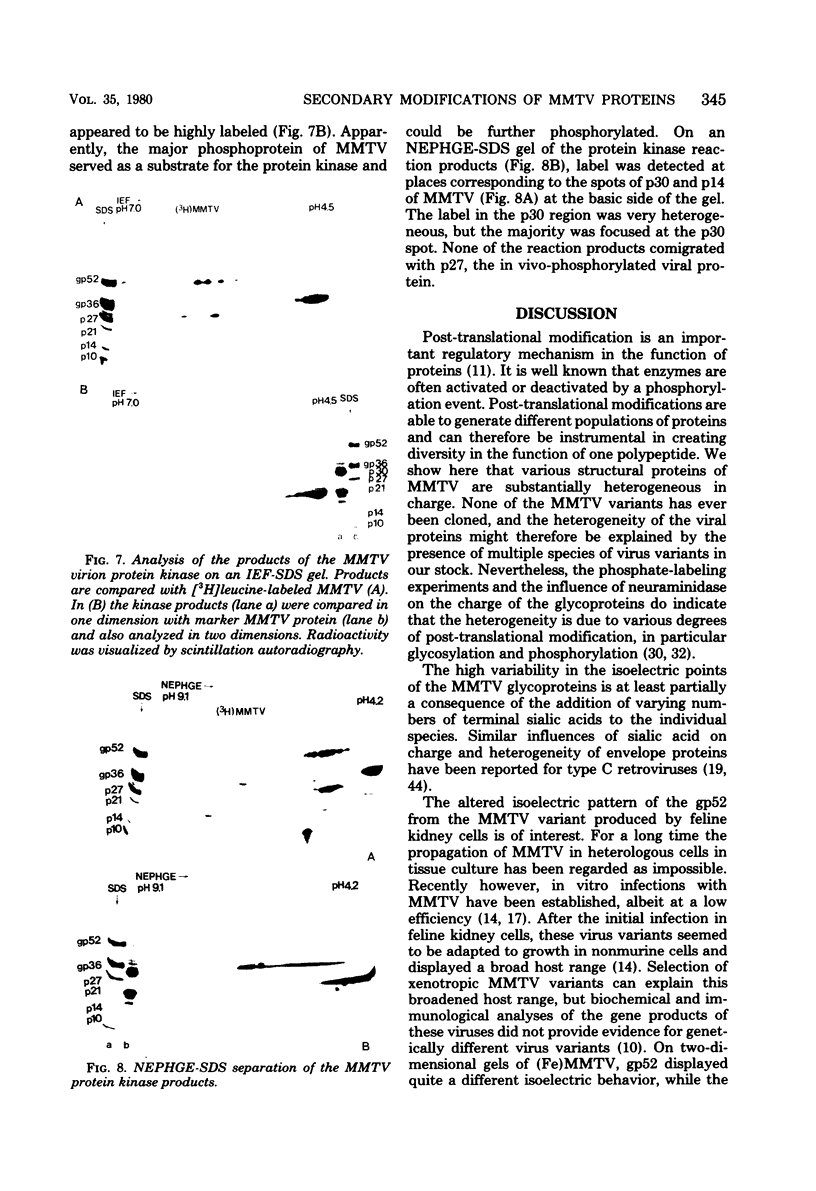
The structural proteins of mouse mammary tumor virus (MMTV) were analyzed by two-dimensional electrophoresis on isoelectric focusing and sodium dodecyl sulfate gels. Many of the viral proteins displayed heterogeneity in charge due to variable contents of carbohydrates (in particular, sialic acid) and phosphate residues. Neuraminidase treatment of the virions influenced the isoelectric pattern of the envelope glycoproteins. The glycoproteins of an MMTV variant which was attenuated by replication in feline kidney cells had different isoelectric points. This suggested that the acquisition of an altered carbohydrate configuration had changed the host range of the virus. The major MMTV structural core protein, p27, consisted of two species, which had identical iodinated tryptic peptide compositions but differed in phosphate contents. Another MMTV phosphoprotein, p21, was separated into four different phosphorylated species. Phosphorylation of p21 could be performed in vitro by the MMTV virion-associated protein kinase. This enzyme also has a high affinity for MMTV p30 as a substrate. Possible functions of this enzyme are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Long C. W., Smith G. H., Fine D. L. Immunological characterization of the low-molecular-weight DNA binding protein of mouse mammary tumor virus. Int J Cancer. 1978 Oct 15;22(4):433–440. doi: 10.1002/ijc.2910220411. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dahl H. H., Dickson C. Cell-free synthesis of mouse mammary tumor virus Pr77 from virion and intracellular mRNA. J Virol. 1979 Mar;29(3):1131–1141. doi: 10.1128/jvi.29.3.1131-1141.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Composition, arrangement and cleavage of the mouse mammary tumor virus polyprotein precursor Pr77gag and p110gag. Cell. 1979 Aug;17(4):1003–1012. doi: 10.1016/0092-8674(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Intracellular synthesis of mouse mammary tumor virus polypeptides: indication of a precursor glycoprotein. J Virol. 1975 Aug;16(2):250–258. doi: 10.1128/jvi.16.2.250-258.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Fout G. S., Pomenti A. A. In vivo and in vitro phosphorylation of murine mammary tumour virus proteins. J Gen Virol. 1979 Sep;44(3):669–678. doi: 10.1099/0022-1317-44-3-669. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Pomenti A. A., Farwell D. C. Vicinal relationships between the major structural proteins of murine mammary tumor virus. Virology. 1979 Jul 15;96(1):249–257. doi: 10.1016/0042-6822(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Plowman J. K., Kelley S. P., Arthur L. O., Hillman E. A. Enhanced production of mouse mammary tumor virus in dexamethasone-treated, 5-iododeoxyuridine-stimulated mammary tumor cell cultures. J Natl Cancer Inst. 1974 Jun;52(6):1881–1886. doi: 10.1093/jnci/52.6.1881. [DOI] [PubMed] [Google Scholar]
- Forchhammer J., Turnock G. Glycoproteins from murine C-type virus are more acidic in virus derived from transformed cells than from nontransformed cells. Virology. 1978 Jul 1;88(1):177–182. doi: 10.1016/0042-6822(78)90121-6. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Lerner R., Howard D., Teramoto Y. A., Schlom J. Strain-specific markers for the major structural proteins of highly oncogenic murine mammary tumor viruses by tryptic peptide analyses. J Virol. 1978 Sep;27(3):688–699. doi: 10.1128/jvi.27.3.688-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Twiddy E., Gilden R. V. Protein kinase associated with RNA tumor viruses and other budding RNA viruses. Virology. 1972 Feb;47(2):536–538. doi: 10.1016/0042-6822(72)90297-8. [DOI] [PubMed] [Google Scholar]
- Hizi A., Wunderli W., Joklik W. K. Purification and partial characterization of a protein kinase from the Prague-C strain of Rous sarcoma virus. Virology. 1979 Feb;93(1):146–158. doi: 10.1016/0042-6822(79)90283-6. [DOI] [PubMed] [Google Scholar]
- Howard D. K., Schlom J. Isolation of host-range variants of mouse mammary tumor viruses that efficiently infect cells in vitro. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5718–5722. doi: 10.1073/pnas.75.11.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Differences between the envelope glycoproteins and glycopeptides of avian tumor viruses released from transformed and from nontransformed cells. Virology. 1972 Nov;50(2):359–372. doi: 10.1016/0042-6822(72)90387-x. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lasfargues E. Y., Lasfargues J. C., Dion A. S., Greene A. E., Moore D. H. Experimental infection of a cat kidney cell line with the mouse mammary tumor virus. Cancer Res. 1976 Jan;36(1):67–72. [PubMed] [Google Scholar]
- Ledbetter J. A. Two-dimensional analysis of murine leukemia virus gag-gene polyproteins. Virology. 1979 May;95(1):85–98. doi: 10.1016/0042-6822(79)90403-3. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Smith S. W., Racevskis J., Sarkar N. H. The relative hydrophobicity of oncornaviral structural proteins. Virology. 1978 May 15;86(2):398–412. doi: 10.1016/0042-6822(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Gene order of mouse mammary tumor virus precusor polyproteins and their interaction leading to the formation of a virus. Virology. 1979 Dec;99(2):358–371. doi: 10.1016/0042-6822(79)90015-1. [DOI] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Nusse R., Asselbergs F. A., Salden M. H., Michalides R. J., Bloemendal H. Translation of mouse mammary tumor virus RNA: precursor polypeptides are phosphorylated during processing. Virology. 1978 Nov;91(1):106–115. doi: 10.1016/0042-6822(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Nusse R., van der Ploeg L., van Duijn L., Michalides R., Hilgers J. Impaired maturation of mouse mammary tumor virus precursor polypeptides in lymphoid leukemia cells, producing intracytoplasmic A particles and no extracellular B-type virions. J Virol. 1979 Oct;32(1):251–258. doi: 10.1128/jvi.32.1.251-258.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Scolnick E. M., Oroszlan S., Gilden R. V. Immunochemical characterization of two major polypeptides from murine mammary tumor virus. J Virol. 1974 Jun;13(6):1200–1210. doi: 10.1128/jvi.13.6.1200-1210.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Phosphorylation of murine mammary tumor virus precursor polypeptides. J Virol. 1979 Apr;30(1):241–247. doi: 10.1128/jvi.30.1.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Synthesis and processing of precursor polypeptides to murine mammary tumor virus structural proteins. J Virol. 1978 Jan;25(1):374–383. doi: 10.1128/jvi.25.1.374-383.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Portner A., Hsu C. H., Clark S. B., Kingsbury D. W. Charge heterogeneity in polypeptides of negative strand RNA viruses. Virology. 1978 Oct 15;90(2):214–225. doi: 10.1016/0042-6822(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Rosok M. J., Watson K. F. Fractionation of two protein kinases from avian myeloblastosis virus and characterization of the protein kinase activity preferring basic phosphoacceptor proteins. J Virol. 1979 Mar;29(3):872–880. doi: 10.1128/jvi.29.3.872-880.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Katz F. N., Lodish H. F. Glycosylation of a membrane protein is restricted to the growing polypeptide chain but is not necessary for insertion as a transmembrane protein. Cell. 1978 Dec;15(4):1447–1454. doi: 10.1016/0092-8674(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Whittington E. S., Racevskis J., Marcus S. L. Phosphoproteins of the murine mammary tumor virus. Virology. 1978 Dec;91(2):407–422. doi: 10.1016/0042-6822(78)90387-2. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Long C. W., Oroszlan S., Arthur L., Fine D. L. Isolation of separate precursor polypeptides for the mouse mammary tumor virus glycoproteins and nonglycoproteins. Virology. 1978 Mar;85(1):168–174. doi: 10.1016/0042-6822(78)90421-x. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Phosphorylation of murine type C viral p12 proteins regulates their extent of binding to the homologous viral RNA. Cell. 1977 Mar;10(3):489–496. doi: 10.1016/0092-8674(77)90036-8. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. A murine sarcoma virus-associated protein kinase: interaction with actin and microtubular protein. Cell. 1979 Jun;17(2):347–356. doi: 10.1016/0092-8674(79)90161-2. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Smith S. W., Marcus S. L., Sarkar N. H. Identification of the messenger RNAs coding for the gag and env gene products of the murine mammary tumor virus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1736–1740. doi: 10.1073/pnas.76.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein H., August J. T. Purification and properties of a virion protein kinase. J Biol Chem. 1976 May 25;251(10):3176–3184. [PubMed] [Google Scholar]
- Stephenson J. R., Devare S. G., Reynolds F. H., Jr Translational products of type-C RNA tumor viruses. Adv Cancer Res. 1978;27:1–53. doi: 10.1016/s0065-230x(08)60929-x. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Tanaka H. Precursor-product relationship between nonglycosylated polypeptides of A and B particles of mouse mammary tumor virus. Virology. 1977 Feb;76(2):835–850. doi: 10.1016/0042-6822(77)90263-x. [DOI] [PubMed] [Google Scholar]
- Teramoto Y. A., Cardiff R. D., Lund J. K. The structure of the mouse mammary tumor virus: isolation and characterization of the core. Virology. 1977 Mar;77(1):135–148. doi: 10.1016/0042-6822(77)90413-5. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Tsukamoto-Adey A., Weissman I. L. Cellular maturation of oncornavirus glycoproteins: topological arrangement of precursor and product forms in cellular membranes. Virology. 1977 Feb;76(2):539–553. doi: 10.1016/0042-6822(77)90236-7. [DOI] [PubMed] [Google Scholar]
- Yagi M. J., Tomana M., Stutzman R. E., Robertson B. H., Compans R. W. Structural components of mouse mammary tumor virus. III. Composition and tryptic peptides of virion polypeptides. Virology. 1978 Dec;91(2):291–304. doi: 10.1016/0042-6822(78)90377-x. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- van Beek W. P., Smets L. A., Emmelot P. Increased sialic acid density in surface glycoprotein of transformed and malignant cells--a general phenomenon? Cancer Res. 1973 Nov;33(11):2913–2922. [PubMed] [Google Scholar]