Abstract
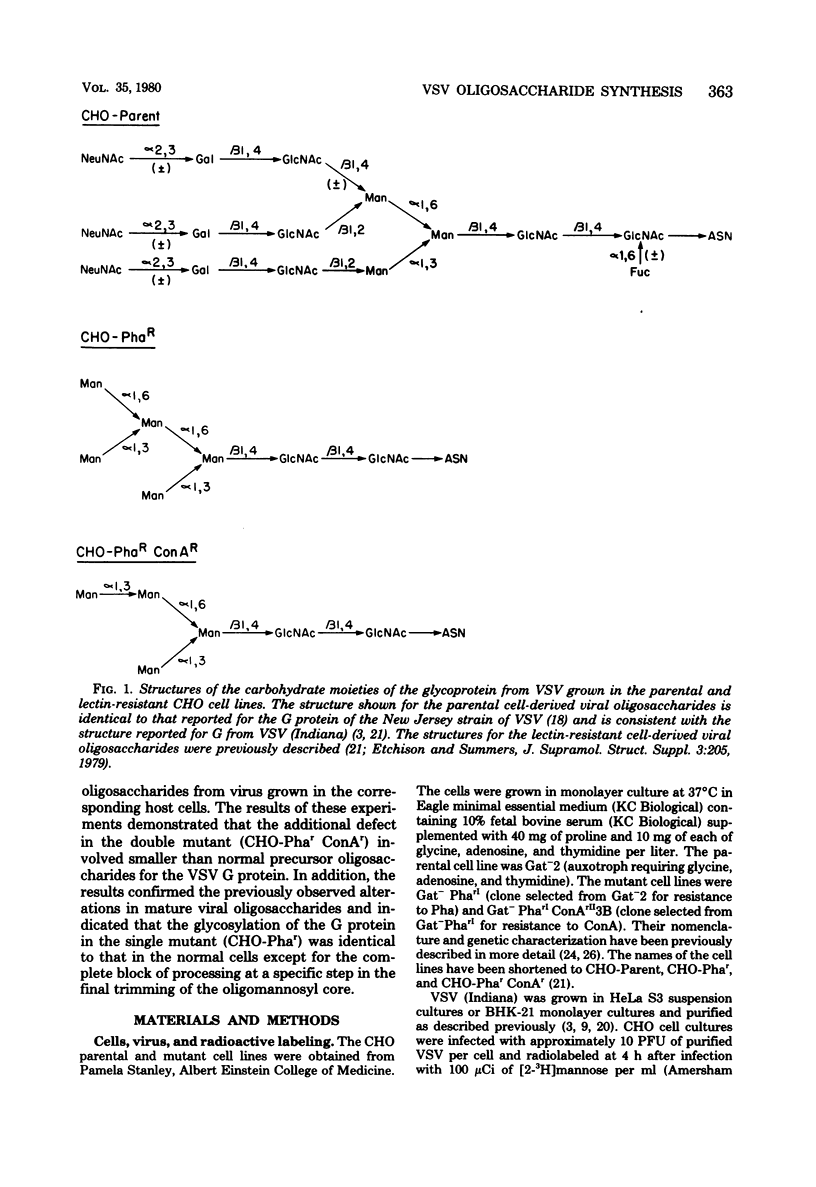
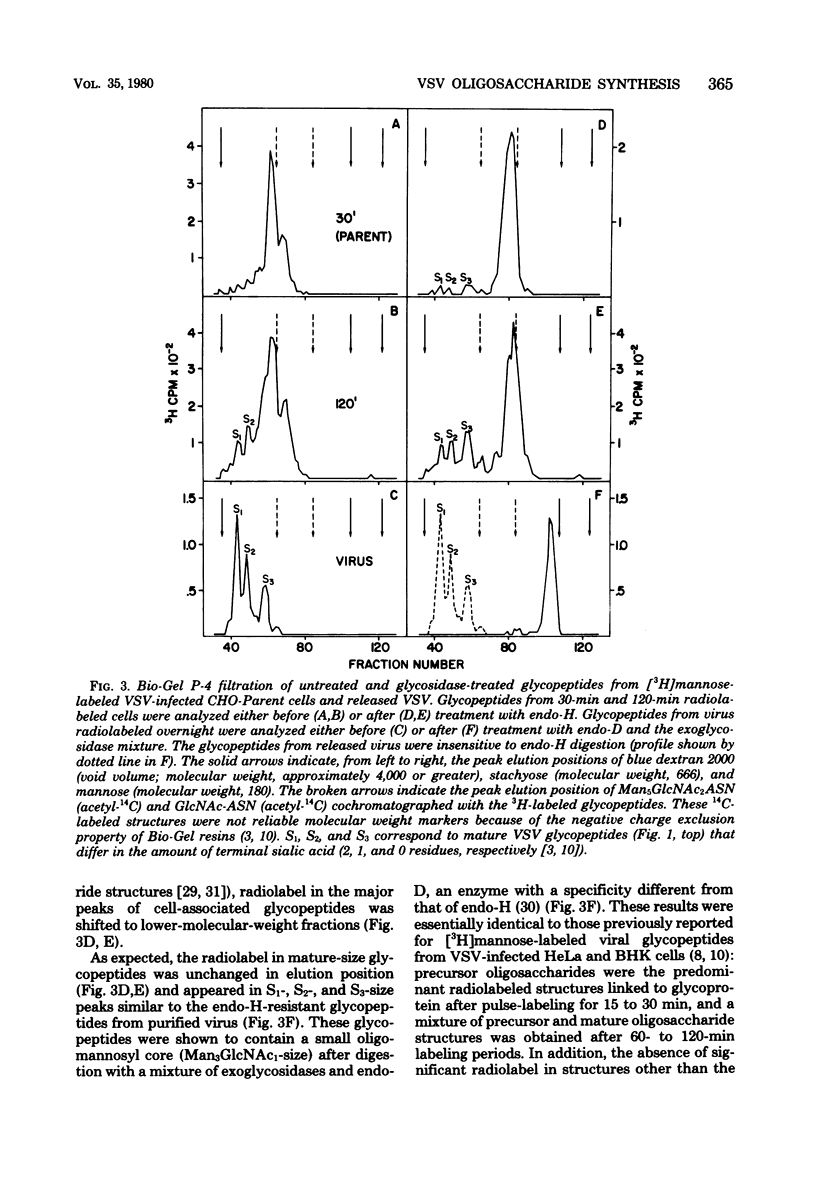
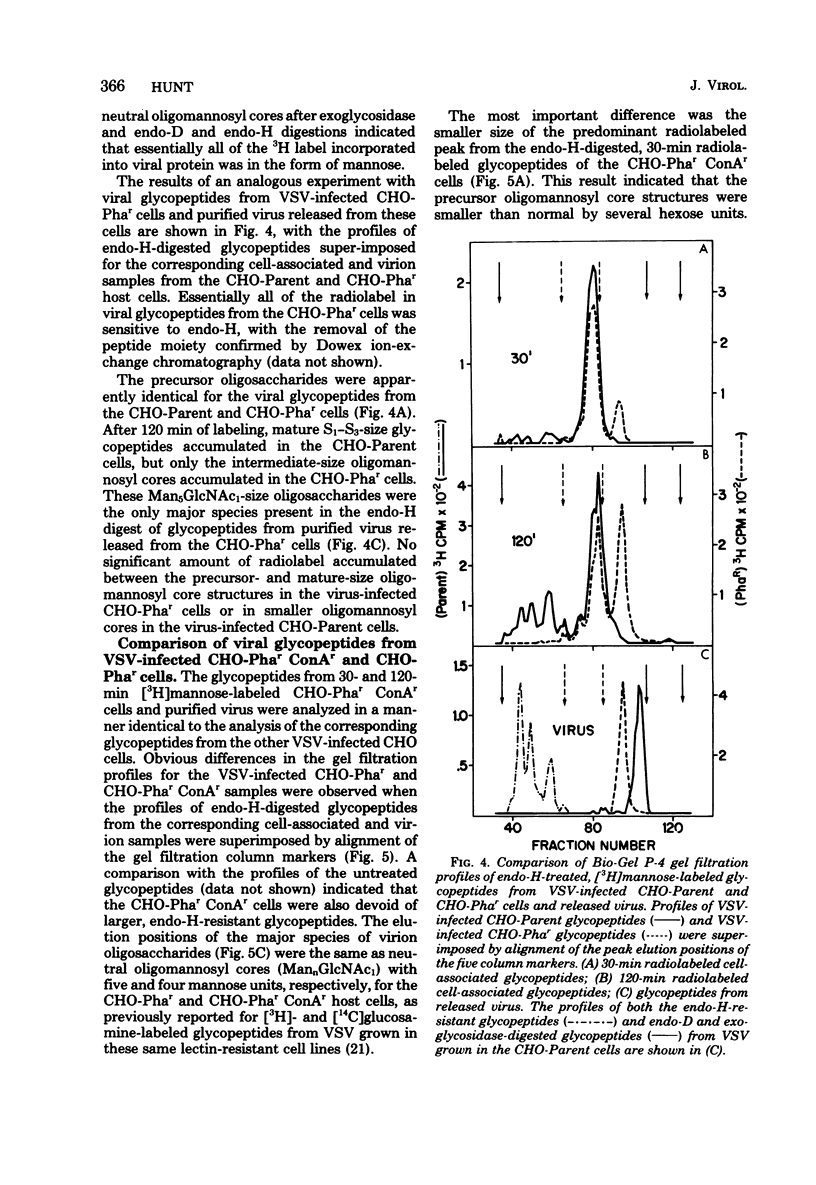
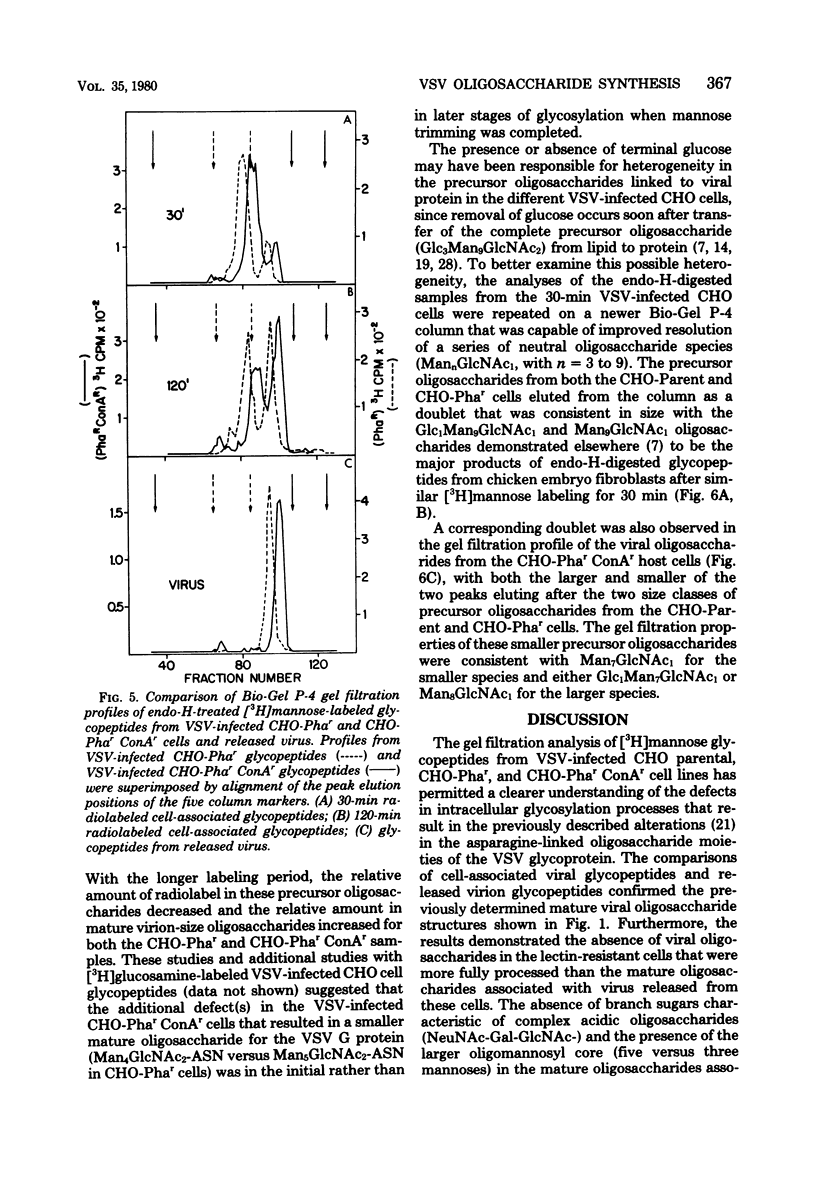
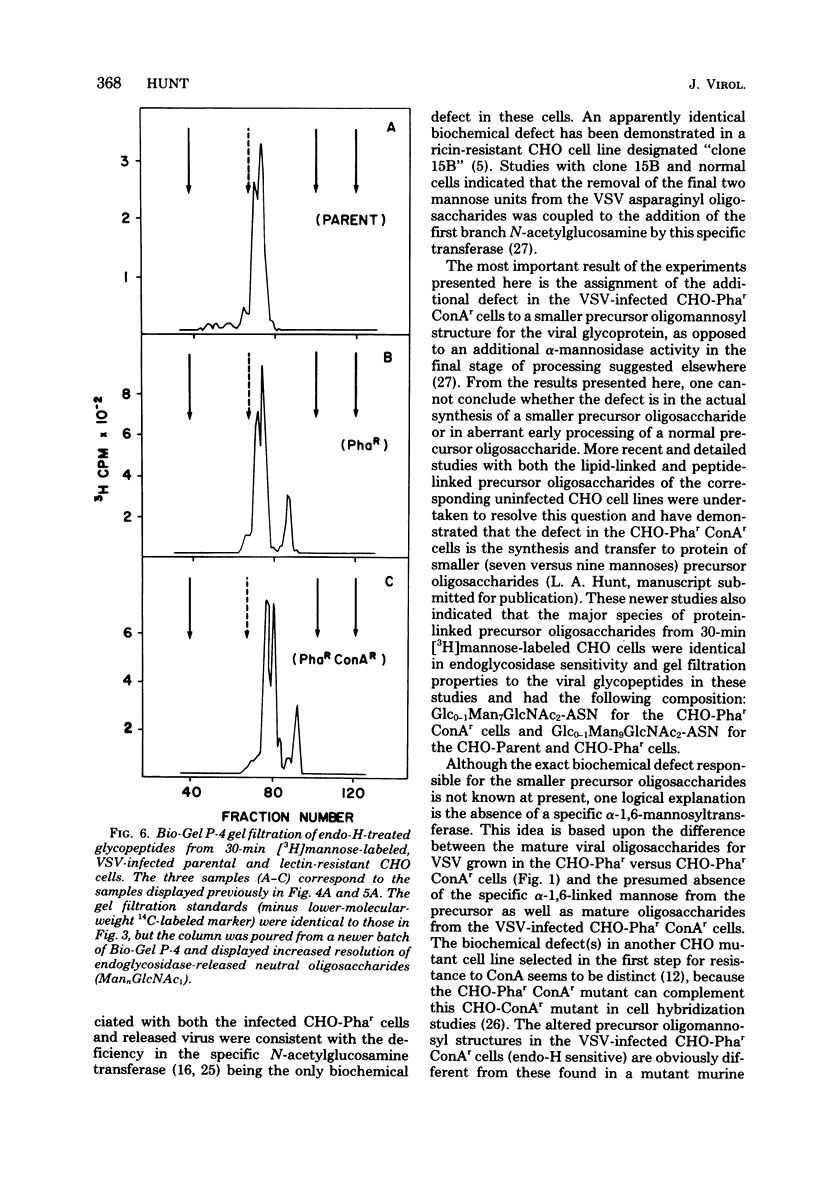
To determine the particular intracellular steps in the glycosylation of the vesicular stomatitis virus (VSV) glycoprotein that were altered in several lectin-resistant CHO cell lines, VSV-infected parental and mutant cells were pulse-labeled for 30 and 120 min with [3H]mannose and [3H]glucosamine. Cell-associated viral glycopeptides were analyzed by gel filtration combined with specific glycosidase digestions and compared with the corresponding mature virion oligosaccharides. The intracellular glycosylation of the VSV glycoprotein in a mutant cell line resistant to phytohemagglutinin was identical to that in the normal cells except for a complete block in processing at a specific step in the final trimming of the oligomannosyl core from five to three mannoses. The results demonstrated that a double-mutant cell line selected from the phytohemagglutinin-resistant cells for resistance to concanavalin A had an additional defect in one of the earliest stages of glycosylation, resulting in smaller precursor oligosaccharides linked to protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chapman A., Trowbridge I. S., Hyman R., Kornfeld S. Structure of the lipid-linked oligosaccharides that accumulate in class E Thy-1-negative mutant lymphomas. Cell. 1979 Jul;17(3):509–515. doi: 10.1016/0092-8674(79)90259-9. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Gottlieb C., Kornfeld S., Schlesinger S. Restricted replication of two alphaviruses in ricin-resistant mouse L cells with altered glycosyltransferase activities. J Virol. 1979 Jan;29(1):344–351. doi: 10.1128/jvi.29.1.344-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Association of vesicular stomatitis virus proteins with HeLa cell membranes and released virus. J Virol. 1976 Dec;20(3):637–645. doi: 10.1128/jvi.20.3.637-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Glycosylation of VSV glycoprotein is similar in cystic fibrosis, heterozygous carrier, and normal human fibroblasts. J Supramol Struct. 1977;7(2):213–221. doi: 10.1002/jss.400070206. [DOI] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Glycosylation of vesicular stomatitis virus glycoprotein in virus-infected HeLa cells. J Virol. 1976 Dec;20(3):646–657. doi: 10.1128/jvi.20.3.646-657.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag S. S., Cifone M., Robbins P. W., Baker R. M. Reduced synthesis of [14C]mannosyl oligosaccharide-lipid by membranes prepared from concanavalin A-resistant Chinese hamster ovary cells. J Biol Chem. 1977 May 25;252(10):3561–3564. [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Impaired intracellular migration and altered solubility of nonglycosylated glycoproteins of vesicular stomatitis virus and Sindbis virus. J Biol Chem. 1977 Dec 25;252(24):9018–9023. [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Moyer S. A., Tsang J. M., Atkinson P. H., Summers D. F. Oligosaccharide moieties of the glycoprotein of vesicular stomatitis virus. J Virol. 1976 Apr;18(1):167–175. doi: 10.1128/jvi.18.1.167-175.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S., Stanley P., Schachter H. Control of glycoprotein synthesis. Lectin-resistant mutant containing only one of two distinct N-acetylglucosaminyltransferase activities present in wild type Chinese hamster ovary cells. J Biol Chem. 1977 Jun 10;252(11):3926–3933. [PubMed] [Google Scholar]
- Polos P. G., Gallaher W. R. Insensitivity of a ricin-resistant mutant of Chinese hamster ovary cells to fusion induced by Newcastle disease virus. J Virol. 1979 Apr;30(1):69–75. doi: 10.1128/jvi.30.1.69-75.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Robertson J. S., Etchison J. R., Summers D. F. Glycosylation sites of vesicular stomatitis virus glycoprotein. J Virol. 1976 Sep;19(3):871–878. doi: 10.1128/jvi.19.3.871-878.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. A., Etchison J. R., Robertson J. S., Summers D. F., Stanley P. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistnat CHO cells. Cell. 1978 Mar;13(3):515–526. doi: 10.1016/0092-8674(78)90325-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Gottlieb C., Feil P., Gelb N., Kornfeld S. Growth of enveloped RNA viruses in a line of chinese hamster ovary cells with deficient N-acetylglucosaminyltransferase activity. J Virol. 1975 Jan;17(1):239–246. doi: 10.1128/jvi.17.1.239-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Lagwinska E., Schlesinger S. Enveloped virus acquires membrane defect when passaged in fibroblasts from I-cell disease patients. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2443–2447. doi: 10.1073/pnas.73.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Caillibot V., Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell. 1975 Oct;6(2):121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Stanley P., Narasimhan S., Siminovitch L., Schachter H. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine--glycoprotein N-acetylglucosaminyltransferase activity. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3323–3327. doi: 10.1073/pnas.72.9.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Siminovitch L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977 Jul;3(4):391–405. doi: 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Kobata A. The substrate specificities of endo-beta-N-acetylglucosaminidases CII and H. Biochem Biophys Res Commun. 1977 Sep 9;78(1):434–441. doi: 10.1016/0006-291x(77)91273-6. [DOI] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R. Abnormal lipid-linked oligosaccharides in class E Thy-1-negative mutant lymphomas. Cell. 1979 Jul;17(3):503–508. doi: 10.1016/0092-8674(79)90258-7. [DOI] [PubMed] [Google Scholar]