Abstract
Introduction
The objective was to investigate the potential implication of the IL18 gene promoter polymorphisms in the susceptibility to giant-cell arteritis (GCA).
Methods
In total, 212 patients diagnosed with biopsy-proven GCA were included in this study. DNA from patients and matched controls was obtained from peripheral blood. Samples were genotyped for the IL18-137 G>C (rs187238), the IL18-607 C>A (rs1946518), and the IL18-1297 T>C (rs360719) gene polymorphisms with polymerase chain reaction, by using a predesigned TaqMan allele discrimination assay.
Results
No significant association between the IL18-137 G>C polymorphism and GCA was found. However, the IL18 -607 allele A was significantly increased in GCA patients compared with controls (47.8% versus 40.9% in patients and controls respectively; P = 0.02; OR, 1.32; 95% CI, 1.04 to 1.69). It was due to an increased frequency of homozygosity for the IL18 -607 A/A genotype in patients with GCA (20.4%) compared with controls (13.4%) (IL18 -607 A/A versus IL18 -607 A/C plus IL18 -607 C/C genotypes: P = 0.04; OR, 1.59; 95% CI, 1.02 to 2.46). Also, the IL18-1297 allele C was significantly increased in GCA patients (30.7%) compared with controls (23.0%) (P = 0.003; OR, 1.48; 95% CI, 1.13 to 1.95). In this regard, an increased susceptibility to GCA was observed in individuals carrying the IL18-1297 C/C or the IL18-1297 C/T genotypes compared with those carrying the IL18-1297 T/T genotype (IL18-1297 C/C plus IL18-1297 T/C versus IL18-1297 T/T genotype in GCA patients compared with controls: P = 0.005; OR, 1.61; 95% CI, 1.15 to 2.25). We also found an additive effect of the IL18 -1297 and -607 polymorphisms with TLR4 Asp299Gly polymorphism. The OR for GCA was 1.95 for combinations of genotypes with one or two risk alleles, whereas carriers of three or more risk alleles have an OR of 3.7.
Conclusions
Our results show for the first time an implication of IL18 gene-promoter polymorphisms in the susceptibility to biopsy-proven GCA. In addition, an additive effect between the associated IL18 and TLR4 genetic variants was observed.
Introduction
Giant cell, arteritis (GCA) is a large- and medium-sized blood vessel systemic vasculitis characterized by the granulomatous involvement of the aorta and especially its cranial branches [1]. GCA is now considered the most common systemic vasculitis in elderly individuals from Western countries [2,3]. Dendritic cells localized at the adventitia-media border of normal medium-sized arteries play a critical role in the initiation of this vasculitis [4]. The inflammatory activity of vascular lesions in GCA is mediated by adaptive immune responses, with CD4 T cells undergoing clonal expansion in the vessel wall and releasing interferon (IFN)-γ [4]. In the experimental mouse model of GCA, systemic administration of ligands for Toll-like receptor (TLR)2 or TLR4 in human artery-SCID chimeras led to differentiation of adventitial dendritic cells into chemokine-producing effector cells with high-level expression of both CD83 and CD86 and mediated T-cell recruitment through release of interleukin (IL)-18 [4]. GCA is also known to be associated with upregulation of IFN-γ, which is critically involved in modulating the process of intimal hyperplasia, leading to the severe ischemic complications observed in this vasculitis [5]. Interestingly, IFN-γ activity is promoted by IL-18, a proinflammatory cytokine, member of the IL-1 cytokine family, which has been shown to exert innate and acquired immune responses [6,7]. IL-18 is expressed by a wide range of immune cells [8] and can mediate both Th1 and Th2 driven immune responses [9,10]. Of potential implication in GCA, IL-18 in combination with IL-12, induces IFN-γ production in Th1 cells, B cells, and natural killer cells, promoting Th1-type immune responses [11,12]. However, IL-18 may also stimulate Th2 immune responses in the absence of IL-12 [13,14].
GCA is a complex polygenic disease [15]. Besides a strong association of GCA with genes that lie within the major histocompatibility complex (MHC) [16-21], many other studies have shown the implication of genetic variants in key components of immune and inflammatory pathways in GCA susceptibility or clinical expression of this vasculitis [21-34].
IL18 gene is located on chromosome 11q22.2-22.3 [35] and several polymorphisms within the IL18 promoter gene have been associated with different inflammatory and autoimmune diseases [36-43].
An important step forward in our understanding of the pathogenesis of autoimmune diseases may be to establish the presence of shared mechanisms that may lead to a variety of very different complex autoimmune diseases. Taking all these considerations together, in this study we sought to establish the potential role of three polymorphisms (-137, -607, -1297) within the promoter of the IL18 gene in the susceptibility to biopsy-proven GCA.
Materials and methods
Patients
In total, 212 patients diagnosed with biopsy-proven GCA and 405 controls were included in this study. All of the patients fulfilled the 1990 American College of Rheumatology criteria for the classification of GCA [44]. Inclusion criteria [45] and clinical features of the patient population were described previously [46]. Also, definitions for specific features of the disease, such as polymyalgia rheumatica (PMR), visual ischemic complications, or other severe ischemic manifestations, have been previously described [47,48]. In all cases, biopsy-proven GCA patients were initially treated with prednisone, 40-60 mg/day, for 3 to 4 weeks. Methyl-prednisolone boluses (1 g daily for 3 days) followed by 60-mg prednisone/day for 3 to 4 weeks were used in most patients who had visual ischemic complications or strokes. The prednisone dose was progressively tapered until discontinuation. Apart from visual complications or strokes that were irreversible in some cases, other typical features of the disease such as headache, asthenia, jaw claudication, or PMR improved after corticosteroid therapy. A decrease of erythrocyte sedimentation rate was observed in all cases after the onset of corticosteroid therapy.
Patients and controls are Caucasians, with at least two previous generations born in the corresponding regions, and were included in this study after written informed consent. We obtained approval for the study from the local ethical committees.
IL18 polymorphisms selection
Several variations within the IL18 gene promoter region are responsible for changes in the transcription rate [49,50]. In the present study, we selected two functional IL18 promoter polymorphisms (IL18 -137 and -607), which were suggested to alter the IL18 promoter activity. To investigate further into genetic variants within the IL18 promoter region, we observed in the database [51] a variant in this region that could have a potential role in IL-18 expression (IL18-1297 or rs360719). We also studied this polymorphism based on the minor allele frequency and its ability to bind the transcription factor Oct-1. Location of the polymorphisms site was based on the GenBank Accession Nos. [Genbank:AB015961] and [Genbank:BC007461] as the reference sequence. Interestingly, we recently confirmed that the IL18-1297 gene polymorphism has a functional association with systemic lupus erythematosus [52].
IL18 genotyping methods
DNA was obtained from peripheral blood mononuclear cells, by using standard methods. The genotyping of the three IL18 polymorphisms was performed by using predesigned TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA), as previously described [52].
Statistical analysis
We used the χ2 test for Hardy-Weinberg equilibrium and statistical analysis to compare allelic and genotypic distributions. Genotype distribution was assessed by using the χ2 test. Odds ratio (ORs) and 95% confidence intervals (95% CIs) were calculated according to Woolf's method by using the Statcalc program (Epi Info 2002; Centers for Disease Control and Prevention, Atlanta, GA, USA). P values < 0.05 were considered statistically significant. LD was calculated by using Haploview v 4.0. A logistic regression model was used to estimate gene-gene interaction between the IL18 and TLR4 SNPs and for the additive effects of the three SNPs. Fisher's Exact test was used to test for the difference in IL18 and TLR4 -risk allele counts between Cases and Controls. Logistic regression analyses were performed by using the software STATA (v.10.1).
Results
IL18 gene polymorphisms are associated with susceptibility to GCA
The case/control ratio was 1:2, approximately. The estimated power of this study for an estimated OR between 1.5 and 2.0 was 77% to 99.5%.
No evidence of departure from Hardy-Weinberg equilibrium was observed in controls.
Table 1 shows the allele and genotype frequencies of the IL18 -137 G>C, -607 C>A, and -1,297 T>C polymorphisms in biopsy-proven GCA patients and healthy subjects.
Table 1.
IL18 gene polymorphisms in a series of biopsy-proven GCA and matched controls
|
IL18
Polymorphisms |
GCA patients Number (%) |
Healthy controls Number (%) |
||
|---|---|---|---|---|
| -137 (G->C) (rs187238) | Number = 212 | Number = 403 | P value | OR (95% CI) |
| G/G | 106 (50.0) | 224 (55.6) | Reference | - |
| G/C | 94 (44.3) | 159 (39.4) | 0.20 | 1.25 (0.87-1.79) |
| C/C | 12 (5.7) | 20 (5.0) | 0.53 | 1.27 (0.56-2.84) |
| G | 306 (72.2) | 607 (75.3) | Reference | - |
| C | 118 (27.8) | 199 (24.7) | 0.23 | 1.18 (0.89-1.55) |
| -607 (C->A) (rs1946518)a | Number = 212 | Number = 405 | P value | OR (95% CI) |
| C/C | 53 (24.9) | 129 (31.9) | Reference | - |
| C/A | 116 (54.7) | 220 (54.3) | 0.21 | 1.28 (0.85-1.94) |
| A/A | 43 (20.4) | 56 (13.8) | 0.02 | 1.87 (1.09-3.21) |
| C | 221 (52.2) | 478 (59.1) | Reference | - |
| A | 203 (47.8) | 332 (40.9) | 0.02 | 1.32 (1.04-1.69) |
| -1297 (T->C) (rs360719)b | Number = 212 | Number = 405 | P value | OR (95% CI) |
| T/T | 99 (46.7) | 237 (58.5) | Reference | - |
| T/C | 96 (45.3) | 150 (37.0) | 0.02 | 1.53 (1.07-2.20) |
| C/C | 17 (8.0) | 18 (4.4) | 0.02 | 2.26 (1.063-4.82) |
| T | 294 (69.3) | 624 (77.0) | Reference | - |
| C | 130 (30.7) | 186 (23.0) | 0.003 | 1.48 (1.13-1.95) |
aGenotype distribution for the IL18 -607 (C->A) polymorphism: P = 0.054.
bGenotype distribution for the IL18 -1297 (T->C) (rs360719): P = 0.011.
Genotype frequencies for the IL18 -607 (C->A) (rs1946518) polymorphism: IL18 -607 A/A homozygous versus IL18 -607 C/A plus IL18 -607 C/C: P = 0.04; OR, 1.59; 95% CI, 1.02-2.46.
Genotype frequencies for the IL18-1297 (T->C) (rs360719) polymorphism: IL18-1297 C/C plus IL18-1297 T/C genotypes compared to IL18-1297 T/T genotype: P = 0.005; OR, 1.61; 95% CI, 1.15-2.25.
No significant association between the IL18 -137 G>C and GCA was observed. However, when the IL18 -607 C>A was assessed, we found that the frequency of allele A was significantly increased in biopsy-proven GCA patients compared with controls (47.8% versus 40.9%, respectively; P = 0.02; OR, 1.32; 95% CI, 1.04-1.69). It was due to a significantly increased frequency of homozygosity for the IL18 -607 A/A genotype in the group of patients with biopsy-proven GCA compared with controls (20.4% versus 13.8 in patients and controls, respectively; IL18 -607 A/A homozygous versus IL18 -607 C/A plus IL18 -607 C/C: P = 0.04; OR, 1.59; 95% CI, 1.02-2.46) (Table 1).
Interestingly, a significant association between biopsy-proven GCA and the IL18-1297 T>C was also found. In this regard, the IL18-1297 allele C frequency was significantly increased in biopsy-proven GCA patients (30.7%) compared with controls (23.0%) (P = 0.003; OR, 1.48; 95% CI, 1.13-1.95) (Table 1). Moreover, the genotype distribution of the IL18-1297 T>C polymorphism disclosed statistically significant differences between biopsy-proven GCA patients and controls (P = 0.011). It was due to a reduced frequency of individuals carrying the IL18-1297 T/T genotype in the group of biopsy-proven GCA patients (46.7%) compared with the controls (58.5%). In this regard, an increased susceptibility to GCA was observed in individuals carrying the IL18-1297 C/C or the IL18-1297 C/T genotypes (IL18-1297 C/C) plus T/C genotypes versus T/T genotype in GCA patients compared with controls: P = 0.005; OR, 1.61; 95% CI, 1.15-2.25). We did not perform a haplotype analysis because the most associated SNP rs360719 (-1297) is not located in a haplotype block, but is a singleton.
IL18 gene polymorphisms are not associated with clinical manifestation of GCA patients
In a further step, we stratified GCA patients according to the presence of PMR, visual ischemic complications, and severe ischemic manifestations. However, no significant differences were observed when GCA patients were compared according to the presence or absence of these specific clinical features of the disease (data not shown).
Additive effects of the IL18 and TLR4 risk alleles in GCA
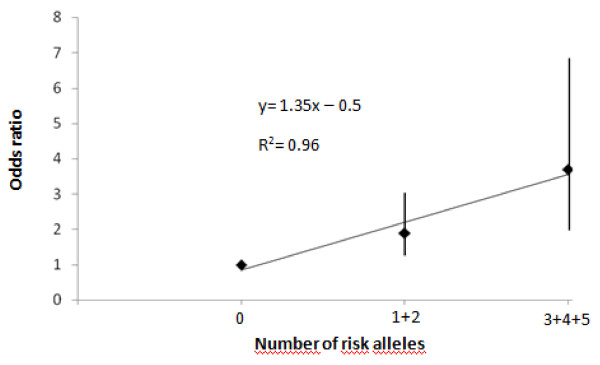
We recently reported an association between the TLR4 Asp299Gly polymorphism and GCA in our population [46]. In the present study, we investigated the potential combined effect of the risk IL18 and TLR4 alleles on GCA susceptibility by using an additive logistic regression model. The distribution of the different combinations of IL18 and TLR4 risk alleles in GCA patients and controls is shown in Additional file 1. The overall difference in risk allele counts between GCA patients and controls was statistical significance, P = 0.01. We observed an additive effect of risk alleles on susceptibility to GCA. Figure 1 shows the OR for GCA according to the presence of one or two and three or more risk alleles among these three genetic variants, by using the individuals with zero risk allele as the reference group. As shown in the figure, the risk of GCA increases as a function of the number of risk alleles, in an additive manner. Thus, the OR for GCA is 1.9 (CI, 1.1-2.3) for carriers of one or two risk alleles, and 3.7 (CI, 1.9-7.2) for carriers of three or more risk alleles.
Figure 1.
Combined effects of the risk alleles of (IL18 -607 and -1297) and TLR4 (Asp299Gly) on susceptibility to GCA. Linear regression analysis showed an additive effect of the risk alleles of the IL18 and TLR4 on GCA susceptibility. The ORs with 95% CI are shown as a function of number of risk alleles of GCA.
Discussion
In the present study, we examined for the first time the contribution of three polymorphisms in the promoter region of the IL18 gene for the susceptibility to GCA. Our results support a potential role of the IL18 -607 C>A (rs1946518) and the IL18-1297 T>C (rs360719) gene polymorphisms in the predisposition to biopsy-proven GCA. Individuals carrying the IL18 -607 A/A showed an increased risk of having GCA compared with controls. A protective effect against the development of GCA was found in individuals carrying the IL18-1297 T/T genotype. In contrast, an increased risk of GCA was observed in individuals carrying the IL18- 1297 allele C.
Proinflammatory cytokines play a major role in the pathogenesis of GCA [53], a disease associated with a high inflammatory response [54]. IL-18 is a proinflammatory cytokine that induces T-helper 1 differentiation and has cytotoxic T-lymphocyte functions. IL-18 has also emerged as a pivotal cytokine in different autoimmune diseases [55]. A number of functional polymorphisms within the proximal promoter of the IL18 gene that may interfere with transcription-factor-binding sites have been verified [49,50]. The implication of the IL18 -1297 T>C polymorphism in the susceptibility to GCA also has functional relevance because recent data from our group confirmed that the relative quantification of mRNA performed in total RNA from 23 healthy individuals carrying different genotypes for IL18 -1297 T>C (rs360719) polymorphism was associated with an increased expression in individuals carrying the C allele (CC+CT versus TT) [52]. Interestingly, Nabili et al. [56] reported and increased expression of IL18 in temporal artery biopsies of GCA patients, with no correlation with clinical manifestations or hematologic parameters. All these data are in accordance with our results and support a potential role of these gene variants in the susceptibility to GCA but not in the phenotypic expression of this vasculitis.
It has been proposed that a variety of inflammatory and autoimmune diseases may share common pathogenic mechanisms. IL18 promoter gene polymorphisms have been associated with several autoimmune diseases. With respect to this, an association of the IL18 -137 G>C [rs187238] but not the IL18 -607 C>A (rs1946518) gene polymorphism with susceptibility to type I diabetes was reported in a study [39]. However, another study of the same two promoter polymorphisms in patients with type I diabetes showed an increased frequency of IL18 -607 CA genotype compared with control subjects, but no significant difference in the IL18 -137 allele frequencies [57]. No significant association was found when the IL18 -137 G>C (rs187238) and the IL18 -607 C>A (rs1946518) gene polymorphisms were studied in patients with multiple sclerosis, Crohn disease, or ulcerative colitis [50,58].
In keeping with the results derived from a study on Spanish individuals diagnosed with rheumatoid arthritis (RA) [59], in the present study, we did not find a significant association between the IL18 -137 (rs187238) polymorphism and biopsy-proven GCA.
A protective effect mediated by the IL18 -607 A/A genotype was observed in Asian patients with RA [43]. It was not the case for Spanish individuals with RA [59]. However, according to our results, an association exists between biopsy-proven GCA and the IL18 -607 (rs1946518) gene polymorphism. Moreover, our data show an additional association of biopsy-proven GCA with IL18 -1297 T>C (rs360719).
Taken together, the different results in terms of disease susceptibility mediated by the IL18 gene polymorphisms in different autoimmune diseases support the notion that different pathogenic mechanisms are involved in the development of polygenic diseases.
Although our data show a clear association of these polymorphisms with GCA susceptibility in the Spanish population, further studies in other populations with different genetic backgrounds are needed to clarify fully the implication of IL18 promoter polymorphisms in GCA susceptibility. However, most genetic associations reported in Spanish patients with GCA also have been replicated in other populations, such as HLA-DRB1 in North American [16,17], Danish [21], French [60], and Swiss [61], and IL-6 promoter and eNOS polymorphisms, in Italians [24,28]. This evidence may indicate a high reproducibility of the genetic associations with GCA among different populations and that the potential association with IL18 may be also found in other populations. Nevertheless, the lack of genome-wide association studies or whole-genome-scan linkage studies in GCA makes necessary an independent replication study to confirm our results by using a population of a different genetic background.
When we determined the joint effect of the risk alleles of IL18 and TLR4, we observed a considerably increased risk of GCA (OR, 3.7) for those 25% GCA patients who carried three or more risk alleles compared with those who carried none. Interestingly, this OR was higher than that obtained for any IL18 or TLR4 SNPs individually (OR, 1.37 for IL18 -607; OR, 1.48 for IL18 -1297, and OR, 1.65 for TLR4 +896 G allele). The additive effect observed between IL18 and TLR4 suggests that combining information from common risk polymorphisms could improve disease prediction. These observations, as well as the findings showing that IL18 and TLR4 genetic variants are associated with other autoimmune diseases [33,39-41,50,52,62-64], support the pivotal role of innate immunity in the development of autoimmunity and GCA. Nevertheless, further studies in other populations are required to validate our findings.
Conclusions
The present study shows for the first time that IL18 gene promoter polymorphisms are associated with susceptibility to biopsy-proven GCA. In addition, an additive effect between the risk IL18 and TLR4 alleles was observed.
Abbreviations
CI: confidence interval; GCA: giant cell arteritis; IL18: interleukin 18; OR: odds ratio; SNP: single-nucleotide polymorphism; TLR4: Toll-like receptor 4.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RPM carried out genotyping, participated in the design of the study, data analysis, and helped to draft the manuscript. TRV participated in the acquisition and interpretation of data and in the design of the study. OT participated in the acquisition and interpretation of data. ICM participated in the acquisition and interpretation of data. SC has been involved in the acquisition and interpretation of data and in revising it critically for important intellectual content. JAM participated in the acquisition and interpretation of data. JLC participated in the acquisition and interpretation of data. BF has been involved in the acquisition and interpretation of data and in revising it critically for important intellectual content. MAG-G made substantial contributions to the conception and design of the study, acquisition of data, coordination, helped to draft the manuscript, and gave final approval of the version to be published. JM made substantial contributions to the conception and design of the study, acquisition of data, and coordination, helped to draft the manuscript, and gave final approval of the version to be published.
Supplementary Material
Supplementary table. Distribution of IL18/TLR4 genotype combinations in GCA patients and controls.
Contributor Information
Rogelio J Palomino-Morales, Email: rpm@ipb.csic.es.
Tomas R Vazquez-Rodriguez, Email: tomas.ramon.vazquez.rodriguez@sergas.es.
Orlando Torres, Email: otorres@ipb.csic.es.
Inmaculada C Morado, Email: imorado.hcsc@salud.madrid.org.
Santos Castañeda, Email: scastas@gmail.com.
Jose A Miranda-Filloy, Email: jose.alberto.miranda.filloy@sergas.es.
Jose L Callejas-Rubio, Email: JLCALLEJA@telefonica.net.
Benjamin Fernandez-Gutierrez, Email: bfernandez.hcsc@salud.madrid.org.
Miguel A Gonzalez-Gay, Email: miguelaggay@hotmail.com.
Javier Martin, Email: martin@ipb.csic.es.
Acknowledgements
We thank Sofia Vargas and Gema Robledo for their invaluable contribution in the collection, isolation, and storage of the DNA samples. We also thank Sara Abel Liz, Maria Soledad Folgosa Rodriguez, and Ana Maria Ramos Gandoy, nurses from the Rheumatology Division (Hospital Xeral-Calde, Lugo, Spain) for their valuable help in the collection of samples. This study was supported by a grant from Fondo de Investigaciones Sanitarias PI06-0024 (Spain) and in part by Junta de Andalucía, grupo CTS-180 (Spain). This work was partially supported by the RETICS Program, RD08/0075 (RIER), from Instituto de Salud Carlos III (ISCIII).
References
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002;347:261–271. doi: 10.1056/NEJMra011913. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Garcia-Porrua C. Epidemiology of the vasculitides. Rheum Dis Clin North Am. 2001;27:729–749. doi: 10.1016/S0889-857X(05)70232-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, Llorca J. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009;61:1454–1461. doi: 10.1002/art.24459. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Ma-Krupa W, Pryshchep O, Gröschel S, Bernardino R, Goronzy JJ. Vascular dendritic cells in giant cell arteritis. Ann N Y Acad Sci. 2005;1062:195–208. doi: 10.1196/annals.1358.023. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Goronzy JJ. The Dunlop-Dottridge Lecture: the pathogenesis of giant cell arteritis. J Rheumatol. 2000;27:517–522. [PubMed] [Google Scholar]
- Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Gracie JA, Leung BP, Wei XQ, Liew FY. Interleukin 18: a pleiotropic participant in chronic inflammation. Immunol Today. 2000;21:312–315. doi: 10.1016/S0167-5699(00)01648-0. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-18. Methods. 1999;19:121–132. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/S1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, Okamura H, Nakanishi K. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12:151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Mizutani H, Tsutsui H, Noben-Trauth N, Yamanaka K, Tanaka M, Izumi S, Okamura H, Paul WE, Nakanishi K. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1:132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Tsutsui H, Tominaga K, Hoshino K, Okamura H, Akira S, Paul WE, Nakanishi K. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci USA. 1999;96:13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Amoli MM, Garcia-Porrua C, Ollier WE. Genetic markers of disease susceptibility and severity in giant cell arteritis and polymyalgia rheumatica. Semin Arthritis Rheum. 2003;33:38–48. doi: 10.1053/sarh.2002.50025. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Hicok KC, Hunder GG, Goronzy JJ. The HLA-DRB1 locus as a genetic component in giant cell arteritis: mapping of a disease-linked sequence motif to the antigen binding site of the HLA-DR molecule. J Clin Invest. 1992;90:2355–2361. doi: 10.1172/JCI116125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Hunder NN, Hicok KC, Hunder GG, Goronzy JJ. HLA-DRB1 alleles in polymyalgia rheumatica, giant cell arteritis, and rheumatoid arthritis. Arthritis Rheum. 1994;37:514–520. doi: 10.1002/art.1780370411. [DOI] [PubMed] [Google Scholar]
- Dababneh A, Gonzalez-Gay MA, Garcia-Porrua C, Hajeer A, Thomson W, Ollier W. Giant cell arteritis and polymyalgia rheumatica can be differentiated by distinct patterns of HLA class II association. J Rheumatol. 1998;25:2140–2145. [PubMed] [Google Scholar]
- Mattey DL, Hajeer AH, Dababneh A, Thomson W, González-Gay MA, García-Porrúa C, Ollier WE. Association of giant cell arteritis and polymyalgia rheumatica with different tumor necrosis factor microsatellite polymorphisms. Arthritis Rheum. 2000;43:1749–1755. doi: 10.1002/1529-0131(200008)43:8<1749::AID-ANR11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Rueda B, Vilchez JR, Lopez-Nevot MA, Robledo G, Ruiz MP, Fernández O, Garcia-Porrua C, Gonzalez-Escribano MF, Martín J. Contribution of MHC class I region to genetic susceptibility for giant cell arteritis. Rheumatology. 2007;46:431–434. doi: 10.1093/rheumatology/kel324. [DOI] [PubMed] [Google Scholar]
- Jacobsen S, Baslund B, Madsen HO, Tvede N, Svejgaard A, Garred P. Mannose-binding lectin variant alleles and HLA-DR4 alleles are associated with giant cell arteritis. J Rheumatol. 2002;29:2148–21453. [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Hajeer AH, Dababneh A, Garcia-Porrua C, Amoli MM, Llorca J, Ollier WE. Interferon-gamma gene microsatellite polymorphisms in patients with biopsy-proven giant cell arteritis and isolated polymyalgia rheumatica. Clin Exp Rheumatol. 2004;22(6 Suppl 36):S18–S20. [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Hajeer AH, Dababneh A, Garcia-Porrua C, Mattey DL, Amoli MM, Thomson W, Ollier WE. IL-6 promoter polymorphism at position -174 modulates the phenotypic expression of polymyalgia rheumatica in biopsy-proven giant cell arteritis. Clin Exp Rheumatol. 2002;20:179–184. [PubMed] [Google Scholar]
- Salvarani C, Casali B, Farnetti E, Pipitone N, Nicoli D, Macchioni P, Cimino L, Bajocchi G, Catanoso MG, Boiardi L. Interleukin-6 promoter polymorphism at position -174 in giant cell arteritis. J Rheumatol. 2005;32:2173–2177. [PubMed] [Google Scholar]
- Boiardi L, Casali B, Farnetti E, Pipitone N, Nicoli D, Macchioni P, Cimino L, Bajocchi G, Catanoso MG, Pattacini L, Salvarani C. Interleukin-10 promoter polymorphisms in giant cell arteritis. Arthritis Rheum. 2006;54:4011–4017. doi: 10.1002/art.22218. [DOI] [PubMed] [Google Scholar]
- Rueda B, Roibas B, Martin J, Gonzalez-Gay MA. Influence of interleukin 10 promoter polymorphisms in susceptibility to giant cell arteritis in Northwestern Spain. J Rheumatol. 2007;34:1535–1539. [PubMed] [Google Scholar]
- Salvarani C, Casali B, Boiardi L, Ranzi A, Macchioni P, Nicoli D, Farnetti E, Brini M, Portioli I. Intercellular adhesion molecule 1 gene polymorphisms in polymyalgia rheumatica/giant cell arteritis: association with disease risk and severity. J Rheumatol. 2000;27:1215–1221. [PubMed] [Google Scholar]
- Salvarani C, Casali B, Nicoli D, Farnetti E, Macchioni P, Catanoso MG, Chen Q, Bajocchi G, Boiardi L. Endothelial nitric oxide synthase gene polymorphisms in giant cell arteritis. Arthritis Rheum. 2003;48:3219–3223. doi: 10.1002/art.11307. [DOI] [PubMed] [Google Scholar]
- Amoli MM, Garcia-Porrua C, Llorca J, Ollier WE, Gonzalez-Gay MA. Endothelial nitric oxide synthase haplotype associations in biopsy-proven giant cell arteritis. J Rheumatol. 2003;30:2019–2022. [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Oliver J, Sanchez E, Garcia-Porrua C, Paco L, Lopez-Nevot MA, Ollier WE, Martin J. Association of a functional inducible nitric oxide synthase promoter variant with susceptibility to biopsy-proven giant cell arteritis. J Rheumatol. 2005;32:2178–2182. [PubMed] [Google Scholar]
- Rueda B, Lopez-Nevot MA, Lopez-Diaz MJ, Garcia-Porrua C, Martín J, Gonzalez-Gay MA. A functional variant of vascular endothelial growth factor is associated with severe ischemic complications in giant cell arteritis. J Rheumatol. 2005;32:1737–1741. [PubMed] [Google Scholar]
- Morgan AW, Robinson JI, Barrett JH, Martin J, Walker A, Babbage SJ, Ollier WE, Gonzalez-Gay MA, Isaacs JD. Association of FCGR2A and FCGR2A-FCGR3A haplotypes with susceptibility to giant cell arteritis. Arthritis Res Ther. 2006;8:R109. doi: 10.1186/ar1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda B, Miranda-Filloy JA, Martin J, Gonzalez-Gay MA. Association of CD24 gene polymorphisms with susceptibility to biopsy-proven giant cell arteritis. J Rheumatol. 2008;35:850–854. [PubMed] [Google Scholar]
- Rodríguez-Pla A, Beaty TH, Savino PJ, Eagle RC Jr, Seo P, Soloski MJ. Association of a nonsynonymous single-nucleotide polymorphism of matrix metalloproteinase 9 with giant cell arteritis. Arthritis Rheum. 2008;58:1849–1853. doi: 10.1002/art.23457. [DOI] [PubMed] [Google Scholar]
- Nolan KF, Greaves DR, Waldmann H. The human interleukin 18 gene IL18 maps to 11q22.2-q22.3, closely linked to the DRD2 gene locus and distinct from mapped IDDM loci. Genomics. 1998;51:161–163. doi: 10.1006/geno.1998.5336. [DOI] [PubMed] [Google Scholar]
- Higa S, Hirano T, Mayumi M, Hiraoka M, Ohshima Y, Nambu M, Yamaguchi E, Hizawa N, Kondo N, Matsui E, Katada Y, Miyatake A, Kawase I, Tanaka T. Association between interleukin-18 gene polymorphism 105A/C and asthma. Clin Exp Allergy. 2003;33:1097–1102. doi: 10.1046/j.1365-2222.2003.01739.x. [DOI] [PubMed] [Google Scholar]
- Imboden M, Nicod L, Nieters A, Glaus E, Matyas G, Bircher AJ, Ackermann-Liebrich U, Berger W, Probst-Hensch NM. SAPALDIA Team. The common G-allele of interleukin-18 single-nucleotide polymorphism is a genetic risk factor for atopic asthma: The SAPALDIA Cohort Study. Clin Exp Allergy. 2006;36:211–218. doi: 10.1111/j.1365-2222.2006.02424.x. [DOI] [PubMed] [Google Scholar]
- Tiret L, Godefroy T, Lubos E, Nicaud V, Tregouet DA, Barbaux S, Schnabel R, Bickel C, Espinola-Klein C, Poirier O, Perret C, Münzel T, Rupprecht HJ, Lackner K, Cambien F, Blankenberg S. Athero Gene Investigators. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation. 2005;112:643–650. doi: 10.1161/CIRCULATIONAHA.104.519702. [DOI] [PubMed] [Google Scholar]
- Kretowski A, Mironczuk K, Karpinska A, Bojaryn U, Kinalski M, Puchalski Z, Kinalska I. Interleukin-18 promoter polymorphisms in type 1 diabetes. Diabetes. 2002;51:3347–3349. doi: 10.2337/diabetes.51.11.3347. [DOI] [PubMed] [Google Scholar]
- Mojtahedi Z, Naeimi S, Farjadian S, Omrani GR, Ghaderi A. Association of IL-18 promoter polymorphisms with predisposition to Type 1 diabetes. Diabet Med. 2006;23:235–239. doi: 10.1111/j.1464-5491.2006.01786.x. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Maeno N, Kawaguchi Y, Takei S, Imanaka H, Kawano Y, Terajima-Ichida H, Hara M, Kamatani N. A promoter haplotype of the interleukin-18 gene is associated with juvenile idiopathic arthritis in the Japanese population. Arthritis Res Ther. 2006;8:R60. doi: 10.1186/ar1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Fukuda Y, Sashio H, Takeda N, Bamba H, Kosaka T, Fukui S, Sawada K, Tamura K, Satomi M, Yamada T, Yamamura T, Yamamoto Y, Furuyama J, Okamura H, Shimoyama T. IL18 polymorphism is associated with an increased risk of Crohn's disease. J Gastroenterol. 2002;37:111–116. doi: 10.1007/s005350200133. [DOI] [PubMed] [Google Scholar]
- Sivalingam SP, Yoon KH, Koh DR, Fong KY. Single-nucleotide polymorphisms of the interleukin-18 gene promoter region in rheumatoid arthritis patients: protective effect of AA genotype. Tissue Antigens. 2003;62:498–504. doi: 10.1046/j.1399-0039.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW Jr, Masi AT. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, Gonzalez-Louzao C, Rodriguez-Ledo P. Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum. 2001;30:249–256. doi: 10.1053/sarh.2001.16650. [DOI] [PubMed] [Google Scholar]
- Palomino-Morales R, Torres O, Vazquez-Rodriguez TR, Morado IC, Castañeda S, Callejas-Rubio JL, Miranda-Filloy JA, Fernandez-Gutierrez B, Martin J, Gonzalez-Gay MA. Association between Toll-like receptor 4 gene polymorphism and biopsy-proven giant cell arteritis. J Rheumatol. 2009;36:1501–1506. doi: 10.3899/jrheum.081286. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Garcia-Porrua C, Vazquez-Caruncho M. Polymyalgia rheumatica in biopsy proven giant cell arteritis does not constitute a different subset but differs from isolated polymyalgia rheumatica. J Rheumatol. 1998;25:1750–1755. [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Pineiro A, Gomez-Gigirey A, Garcia-Porrua C, Pego-Reigosa R, Dierssen-Sotos T, Llorca J. Influence of traditional risk factors of atherosclerosis in the development of severe ischemic complications in giant cell arteritis. Medicine (Baltimore) 2004;83:342–347. doi: 10.1097/01.md.0000145369.25558.b5. [DOI] [PubMed] [Google Scholar]
- Tone M, Thompson SA, Tone Y, Fairchild PJ, Waldmann H. Regulation of IL-18 (IFN-gamma-inducing factor) gene expression. J Immunol. 1997;159:6156–6163. [PubMed] [Google Scholar]
- Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146–152. doi: 10.1016/S0165-5728(00)00407-0. [DOI] [PubMed] [Google Scholar]
- PubMed. http://www.ncbi.nlm.nih.gov/sites/entrez
- Sánchez E, Palomino-Morales RJ, Ortego-Centeno N, Jiménez-Alonso J, González-Gay MA, López-Nevot MA, Sánchez-Román J, de Ramón E, González-Escribano MF, Pons-Estel BA, D'Alfonso S, Sebastiani GD. Italian collaborative group. Alarcón-Riquelme ME, Martín J. Identification of a new putative IL18 gene variant through a functional and association study in systemic lupus erythematosus. Hum Mol Genet. 2009;18:3739–3748. doi: 10.1093/hmg/ddp301. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Hicok KC, Hunder GG, Goronzy JJ. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann Intern Med. 1994;121:484–491. doi: 10.7326/0003-4819-121-7-199410010-00003. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Lopez-Diaz MJ, Barros S, Garcia-Porrua C, Sanchez-Andrade A, Paz-Carreira J, Martin J, Llorca J. Giant cell arteritis: laboratory tests at the time of diagnosis in a series of 240 patients. Medicine (Baltimore) 2005;84:277–290. doi: 10.1097/01.md.0000180043.19285.54. [DOI] [PubMed] [Google Scholar]
- Thompson SR, Humphries SE. Interleukin-18 genetics and inflammatory disease susceptibility. Genes Immun. 2007;8:91–99. doi: 10.1038/sj.gene.6364366. [DOI] [PubMed] [Google Scholar]
- Nabili S, Bhatt P, Roberts F, Gracie A, McFadzean R. Local expression of IL-18 in the temporal artery does not correlate with clinical manifestations of giant cell arteritis. Neuro-Ophthalmology. 2008;32:3–6. doi: 10.1080/01658100701818172. [DOI] [Google Scholar]
- Ide A, Kawasaki E, Abiru N, Sun F, Kobayashi M, Fukushima T, Takahashi R, Kuwahara H, Kita A, Oshima K, Uotani S, Yamasaki H, Yamaguchi Y, Eguchi K. Association between IL-18 gene promoter polymorphisms and CTLA-4 gene 49A/G polymorphism in Japanese patients with type 1 diabetes. J Autoimmun. 2004;22:73–78. doi: 10.1016/j.jaut.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Glas J, Torok HP, Tonenchi L, Kapser J, Schiemann U, Muller-Myhsok B, Folwaczny M, Folwaczny C. Association of polymorphisms in the interleukin-18 gene in patients with Crohn's disease depending on the CARDIS15/NOD2 genotype. Inflamm Bowel Dis. 2005;11:1031–1037. doi: 10.1097/01.MIB.0000187574.41290.b1. [DOI] [PubMed] [Google Scholar]
- Rueda B, González-Gay MA, Matarán L, López-Nevot MA, Martín J. Interleukin-18-promoter polymorphisms are not relevant in rheumatoid arthritis. Tissue Antigens. 2005;65:544–548. doi: 10.1111/j.1399-0039.2005.00408.x. [DOI] [PubMed] [Google Scholar]
- Rauzy O, Fort M, Nourhashemi F, Alric L, Juchet H, Ecoiffier M, Abbal M, Adoue D. Relation between HLA DRB1 alleles and corticosteroid resistance in giant cell arteritis. Ann Rheum Dis. 1998;57:380–382. doi: 10.1136/ard.57.6.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerne PA, Salvi M, Seitz M, Bruhlmann P, Rivier G, Frey D, Mermillod B, Vischer TL, Tiercy JM. Molecular analysis of HLA-DR polymorphism in polymyalgia rheumatica: Swiss Group for Research on HLA in Polymyalgia Rheumatica. J Rheumatol. 1997;24:671–676. [PubMed] [Google Scholar]
- Radstake TR, Franke B, Hanssen S, Netea MG, Welsing P, Barrera P, Joosten LA, van Riel PL, Berg WB van den. The Toll-like receptor 4 Asp299Gly functional variant is associated with decreased rheumatoid arthritis disease susceptibility but does not influence disease severity and/or outcome. Arthritis Rheum. 2004;50:999–1001. doi: 10.1002/art.20114. [DOI] [PubMed] [Google Scholar]
- Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, Quertinmont E, Abramowicz M, Van Gossum A, Devière J, Rutgeerts P. Deficient host-bacteria interactions in inflammatory bowel disease? The Toll-like receptor (TLR)-4 Asp299 gly polymorphism is associated with Crohn's disease and ulcerative colitis. Gut. 2004;53:987–992. doi: 10.1136/gut.2003.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A, Borghi MO, Nguyen-Oghalai T, Meroni PL. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66:1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table. Distribution of IL18/TLR4 genotype combinations in GCA patients and controls.