Abstract
Adult neurogenesis has captivated neuroscientists for decades, with hopes that understanding the programs underlying this phenomenon may provide unique insight toward avenues for brain repair. Interestingly, however, despite intense molecular and cellular investigation, the evolutionary roles and biological functions for ongoing neurogenesis have remained elusive. Here I review recent work published in the Journal of Neuroscience that reveals a functional role for continued neurogenesis toward forming short-term olfactory memories.
The mammalian brain shows remarkable plasticity, ranging from structural changes in synapses to circuit remodeling and continued neurogenesis. In the adult brain two main areas support ongoing neurogenesis: the subgranular layer of the dentate gyrus and the subventricular zone (SVZ) of the lateral ventricle (Zhao et al. 2008). Neurons born in the SVZ migrate anteriorly and differentiate into mainly inhibitory interneurons of the olfactory bulb (OB). These newborn interneuron subtypes include both periglomerular and granule cells (GCs), both of which are known to form dendrodendritic synapses onto the principal excitatory cells of the olfactory circuit, the mitral and tufted cells (Lledo and Saghatelyan 2005). A popular working model for dendrodendritic signaling in the OB is one of lateral inhibition, in which interneurons sculpt mitral cell firing properties prior to relaying odor information to cortical targets. This inhibitory refinement of mitral cell firing is thought to ultimately provide contrast enhancement between different odor-detection circuits and drive synchronous activity among cohorts of mitral cells.
Although the functions of interneurons in the OB are becoming better understood, relatively little is known about the role of adult neurogenesis in the olfactory system. Clues have emerged showing that the rates (or levels) of OB neurogenesis seem to be dynamically modulated by diverse sets of innate and external factors. For example, whereas pregnancy, odor enrichment, and context-dependent training increase the number of newborn neurons, naris occlusion, aging, and disease states such as Parkinson's and Alzheimer's result in a decrease (Ma et al. 2009). Despite extensive cellular and developmental characterization of individual adult-born neurons, little is known about the circuit level modifications or behavioral changes that accompany this phenomenon. To assess such functions, detailed studies are required to reveal not only the morphological and electrophysiological network dynamics, but also the behavioral changes that result from manipulations that alter the rates of neurogenesis.
In a recent study published in the Journal of Neuroscience by Breton-Provencher and colleagues (2009) titled “Interneurons Produced in Adulthood Are Required for the Normal Functioning of the Olfactory Bulb Network and for the Execution of Selected Olfactory Behaviors,” the authors convincingly demonstrate for the first time that the phenomenon of continued adult neurogenesis in the rodent olfactory system is important to the maintenance of circuit-based feedback inhibition onto mitral cells and the biological process of forming spontaneous short-term odor memories.
To investigate the functional significance of continued neurogenesis in the olfactory bulb, the authors used a pharmacological approach to chronically block the division of progenitor cells born in the SVZ. This was accomplished by continuously infusing arabinofuranosyl cytidine (Ara-C) into the lateral ventricle using a mini-osmotic pump. Ara-C is a potent antimitotic drug commonly used as a chemotherapeutic for leukemia and lymphoma for its selective inhibition of cell division. After suppressing olfactory neurogenesis for 1 month, the time required for neural progenitors to reach maturation and become synaptically integrated into OB circuits, the authors conducted a battery of cellular, electrophysiological, and behavioral assays to elucidate the network effects of neurogenic ablation.
Long-term interference of neurogenesis has been shown to lower the numbers of inhibitory interneurons in the OB, arguing a role for structural maintenance (Imayoshi et al. 2008). However, the short-term circuit effects have remained unknown. Interestingly, results from this study show that acute ablation of OB neurogenesis does not affect the anatomy of the preexisting bulbar circuitry. Using immunohistochemistry, bromodeoxyuridine (BrdU) labeling, and viral reporter expression to visually track neurons born prior to Ara-C treatment, the authors found that the overall integrity of the treated OB tissue was normal and that both the numbers and the morphologies of preexisting GCs remained unchanged. This is an interesting observation, given that >30,000 progenitors migrate into the bulb daily (Alvarez-Buylla et al. 2001). Perhaps acute loss of newborn interneurons in the OB triggers a compensatory mechanism for increased resident neuron survival or, alternatively, 28 days of Ara-C infusion may simply not be sufficient to cause a detectable decrease in overall cell numbers.
To determine the cellular and network repercussions of ablating neurogenesis in the OB, the authors targeted their analysis to the mitral cells, which are known synaptic partners of newborn neurons (Lledo and Saghatelyan 2005). Through dendrodendritic interactions, newborn GCs form reciprocal synapses with mitral cells, in which excitatory mitral-to-granule synapses localize directly adjacent to inhibitory granule-to-mitral scaffolds (Price and Powell 1970). The sum output of this interaction is that glutamate release from odor-stimulated mitral cells induces a counter release of γ-aminobutyric acid (GABA) from synaptically connected GCs back onto neighboring mitral cells, thereby sculpting patterns of mitral cell firing through feedback inhibition. Experimentally, this interaction can be probed by recording inhibitory postsynaptic currents (IPSCs) in mitral cells. Whereas the rise and decay times, as well as IPSC amplitudes of spontaneous GABAergic currents were indistinguishable between experimental groups, the authors found a significant decrease in the frequency of IPSCs in Ara-C-treated mice. Moreover, evoked release of glutamate from mitral cells also resulted in less GABAergic feedback inhibition. Comparable current kinetics and amplitudes, accompanied by a reduction in release rates, suggest normal GABAA receptor content and function and imply fewer GABA release sites in Ara-C-treated mice. Consistent with this electrophysiological signature, the authors observed a reduction in the levels of gephyrin in mitral cell dendrites, revealing fewer GABAergic synapses onto the mitral cells of Ara-C-treated mice.
Granule-to-mitral cell inhibition has been shown to be important for synchronizing the activity of mitral cell cohorts, which is believed to be critical for proper olfaction (Lagier et al. 2007). To evaluate the network-level effects of less inhibitory drive onto mitral cells, the authors recorded local field potentials from the mitral cell layer in response to olfactory nerve stimulation. Whereas controls showed normal oscillatory activity, Ara-C-treated mice showed a reduction in oscillatory peak frequencies. At the cellular level these findings demonstrate that acute suppression of adult neurogenesis in the OB does not affect the existing bulbar circuitry, but results in fewer inhibitory synapses onto the mitral cell population, and that a continuous supply of interneurons is required for the proper maintenance of inhibitory drive underlying the OB network activity (Fig. 1A).
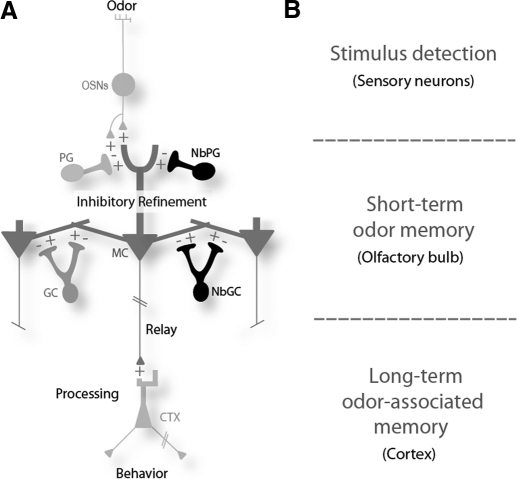
Fig. 1.
Adult neurogenesis sculpts odor processing in the bulb. A: sensory odor information enters the olfactory system by binding to odorant receptors expressed on olfactory sensory neurons (OSNs). Odorant binding excites the OSNs, generating action potentials that evoke synaptic release onto not only mitral cells (MCs), but also periglomerular cells (PGs). Mitral cell firing patterns are sculpted by 2 general classes of inhibitory interneurons that include the PGs and granule cells (GCs). After refinement, odor information is relayed to target cells in the piriform cortex (CTX), where it is processed to influence behaviors. Continued renewal of newborn interneurons (NbPGs and NbGCs) is required to maintain levels of feedback inhibition onto MCs and short-term odor memory. Plus signs represent excitatory drive from MCs onto GCs; minus signs represent inhibition by GCs onto MCs. B: a general diagram modeling the potential nodes of the olfactory circuit involved in information processing and memory formation.
It is generally thought that inhibitory sculpting of mitral cell activity is critical for normal olfactory processing (Lagier et al. 2007; Lledo and Saghatelyan 2005). Establishing that ongoing neurogenesis in the OB is required to maintain appropriate levels of inhibitory drive onto mitral cells, the authors next set out to determine the functional circuit-level effects of neurogenic suppression by investigating odor-associated behaviors. Importantly, prior to conducting odor response tests, the authors showed that chronic Ara-C treatment had no adverse effects on general nonolfactory behaviors, including locomotion, exploration, motivation, or anxiety. Interestingly, however, the authors found that continuous OB neurogenesis is required for discrete subsets of odor-associated behaviors. For example, mice with ablated neurogenesis had lower odor concentration detection capacities, but were not affected in qualitative odor discrimination tasks. In addition, mice treated with Ara-C also exhibited differential memory deficits. Whereas long-term odor-associated memories seemed to be unaffected by Ara-C treatment, short-term odor memory was specifically compromised. In experiments in which mice were subjected to paired novel odor presentation tasks, Ara-C-treated animals failed to recognize previously presented odors at time points >30 min, whereas controls showed a clear memory of previously presented odors beyond 2 h. Taken together, these findings reveal a clear role for adult OB neurogenesis toward the functional maintenance of the OB circuitry.
Although the phenomenon of adult neurogenesis has been investigated extensively, until recently it has been difficult to ascertain its biological function. Evidence is mounting that continual renewal of OB interneurons is required for proper mitral cell function and maintenance of odor-associated behaviors. Whereas recent studies show that acute suppression of adult neurogenesis does not affect the survival or structural integrity of the preexisting mitral–granule cell network (Breton-Provencher et al. 2009; Imayoshi et al. 2008) it is clear that newborn neurons provide a steady-state level of inhibitory drive that is essential for proper olfactory processing (Breton-Provencher et al. 2009). Interestingly, one behavior that appears to be particularly sensitive to neurogenic suppression is short-term odor memory. It has long been discussed as to where odor-associated memories begin to emerge. Although it appears that long-term odor-associated memory can be largely attributed to synaptic changes in cortical targets (Roman et al. 2004), work discussed here argues for the genesis of short-term odor memory at inhibitory OB synapses in the main olfactory bulb (Fig. 1B). How could a mechanism involving inhibition and/or oscillations of local inhibitory circuits give rise to a memory? Why would this be specific to short-term odor memory? Interestingly, behavioral correlates of this phenomenon might exist. For example, Shea and colleagues (2008) have shown that execution of certain odor memory tasks relies on the novelty of an odorant and that noradrenergic modulation of mitral cell firing by inputs from the locus coreleus is gated by perceived novel stimuli. Perhaps formation of short-term odor memory in the OB involves suppressing subsequent odor responses of previously activated mitral cells by neuromodulatory control of GABAergic feedback inhibition. To ultimately understand these mechanisms at the cellular level, further circuit analysis of both short-range interactions within the bulb and long-range influences from deeper centers will be required.
To date, many systemic or genetic manipulations that target changes in rates of adult neurogenesis invariably affect cell division in both the OB and hippocampus (Imayoshi et al. 2008). Here the authors demonstrate that ventricular infusion of Ara-C completely abolishes OB progenitor division while only marginally affecting the hippocampus. This is an important point, since observed changes in network function and behavioral output are being attributed to olfactory system maintenance rather than considering a complex circuit interaction involving the hippocampus. This distinction is difficult to make. Although Ara-C treatment did not affect hippocampal neurogenesis to the same extent as that observed in the OB, it remained partially intact. In addition, it must be kept in mind that although this study nicely showed a role for newborn OB neurons in short-term memory formation, Ara-C is a potent chemotherapeutic that causes significant side effects in humans, including nausea and loss of appetite. Although direct Ara-C infusion into the lateral ventricles may circumvent such issues, it remains possible that olfactory motivation side effects in treated mice could account for some of the differences observed in behavioral short-term memory experiments. To firmly rule out the possibility that newborn hippocampal neurons are not involved and that ventricular Ara-C infusion is not accompanied by significant off-target effects, the problem awaits a clever genetic manipulation that can target different populations of adult-born neurons independently and exclusively. Moreover, from this study the role for newborn periglomerular cells in forming odor memories remains unclear. Although periglomerular cells represent a much smaller population of newborn interneurons than the GCs, they indeed send direct inputs into glomeruli and thus likely influence more than just sensitivity of odor detection. Again, future studies implementing genetic techniques to selectively target periglomerular versus granule cells at different developmental stages may eventually shed light on this issue.
Additionally, to gain a clearer understanding of the circuitry that underlies olfaction, as well as the roles for ongoing neurogenesis in the adult brain, it will also be essential to further elucidate the synaptic connectivity that is made onto newborn GCs during their integration. In a companion article published in the same issue as the study being reviewed, Panzanelli and colleagues (2009) describe a dynamic pattern of synaptogenesis onto newborn neurons entering the bulb, in which they observe synaptic structures forming proximally on GC cell bodies and dendrites earlier than the establishment of dendrodendritic contacts with resident mitral cells. This follows a previous report with similar findings (Whitman and Greer 2007). It remains to be determined the exact local and centrifugal cell types that are forming these early connections, but knowing their identity will be critical toward further unraveling the biological purpose of olfactory bulb neurogenesis.
The study currently being reviewed represents a body of work that shows for the first time the cellular and electrophysiological implications for adult-born neurons toward OB network function and animal behavior. Better understanding the biology of adult neurogenesis in models such as the rodent olfactory bulb will ultimately allow us to elucidate some of the key molecular and genetic programs that govern neuron and synapse development in the context of existing brain circuits. This is an important endeavor if we ever hope to successfully repair or replace damaged or diseased nervous tissue in the aging adult brain.
GRANTS
This work was supported by a National Institute of Neurological Disorders and Stroke K99 award.
DISCLOSURES
No conflicts of interest are declared by the author.
ACKNOWLEDGMENTS
I thank I. Davison and T. Newpher for comments on the manuscript. Apologies to the authors whose works I could not cite due to space limitations.
REFERENCES
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2: 287–293, 2001 [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci 29: 15245–15257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 11: 1153–1161, 2008 [DOI] [PubMed] [Google Scholar]
- Lagier S, Panzanelli P, Russo RE, Nissant A, Bathellier B, Sassoe-Pognetto M, Fritschy JM, Lledo PM. GABAergic inhibition at dendrodendritic synapses tunes gamma oscillations in the olfactory bulb. Proc Natl Acad Sci USA 104: 7259–7264, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci 28: 248–254, 2005 [DOI] [PubMed] [Google Scholar]
- Ma DK, Kim WR, Ming GL, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann NY Acad Sci 1170: 664–673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzanelli P, Bardy C, Nissant A, Pallotto M, Sassoe-Pognetto M, Lledo PM, Fritschy JM. Early synapse formation in developing interneurons of the adult olfactory bulb. J Neurosci 29: 15039–15052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Powell TP. The synaptology of the granule cells of the olfactory bulb. J Cell Sci 7: 125–155, 1970 [DOI] [PubMed] [Google Scholar]
- Roman FS, Truchet B, Chaillan FA, Marchetti E, Soumireu-Mourat B. Olfactory associative discrimination: a model for studying modifications of synaptic efficacy in neuronal networks supporting long-term memory. Rev Neurosci 15: 1–17, 2004 [DOI] [PubMed] [Google Scholar]
- Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci 28: 10711–10719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman MC, Greer CA. Synaptic integration of adult-generated olfactory bulb granule cells: basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. J Neurosci 27: 9951–9961, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 132: 645–660, 2008 [DOI] [PubMed] [Google Scholar]