Abstract
Central pain syndrome (CPS) is a debilitating condition that affects a large number of patients with a primary lesion or dysfunction in the CNS, most commonly due to spinal cord injury, stroke, and multiple sclerosis lesions. The pathophysiological processes underlying the development and maintenance of CPS are poorly understood. We have recently shown, in an animal model of CPS, that neurons in the posterior thalamic nucleus (PO) have increased spontaneous and evoked activity. We also demonstrated that these changes are due to suppressed inhibitory inputs from the zona incerta (ZI). The anterior pretectal nucleus (APT) is a diencephalic nucleus that projects on both the PO and ZI, suggesting that it might be involved in the pathophysiology of CPS. Here we test the hypothesis that CPS is associated with abnormal APT activity by recording single units from APT in anesthetized rats with CPS resulting from spinal cord lesions. The firing rate of APT neurons was increased in spinal-lesioned animals, compared with sham-operated controls. This increase was due to a selective increase in firing of tonic neurons that project to and inhibit ZI and an increase in bursts in fast bursting and slow rhythmic neurons. We also show that, in normal animals, suppressing APT results in increased PO spontaneous activity and evoked responses in a subpopulation of PO neurons. Taken together, these findings suggest that APT regulates ZI inputs to PO and that enhanced APT activity during CPS contributes to the abnormally high activity of PO neurons in CPS.
INTRODUCTION
Many patients with insults to the spinal cord or brain suffer from excruciating, unrelenting chronic pain, a condition called central pain syndrome (CPS). CPS affects over half of spinal cord injury patients, almost 30% of multiple sclerosis patients, and 8% of stroke patients and therefore millions of people worldwide (Andersen et al. 1995; Bonica 1991; Osterberg et al. 2005; Siddall et al. 2003).
There is no effective treatment for CPS. An understanding of the pathophysiological mechanisms of CPS is needed for the development of effective long-term treatments. Although the underlying insults and their location vary among CPS patients there is general agreement that CPS involves insults to the spinothalamocortical tract and abnormal inhibition in the thalamus (Boivie 2005; Bowsher 1995; Canavero and Bonicalzi 2007; Head and Holmes 1911). We have recently reported that CPS resulting from spinal cord injury is associated with suppressed inputs from the inhibitory nucleus zona incerta (ZI) to the posterior thalamus (PO), resulting in higher spontaneous firing rates and evoked responses in PO (Masri et al. 2009). ZI projects exclusively to higher-order thalamic nuclei (Bartho et al. 2002; for review see Mitrofanis 2005), many of which are involved in the pathophysiology of pain disorders (Dostrovsky and Craig 2009).
PO activity is regulated by inhibitory inputs from two additional nuclei: the reticular thalamic nucleus and the anterior pretectal nucleus (APT). The reticular nucleus innervates both first-order and higher-order thalamic nuclei (Jones 2007). We have recently shown that this nucleus is unlikely to be directly involved in CPS resulting from spinal cord injury (Masri et al. 2009). APT, like ZI, exclusively innervates higher-order thalamic nuclei (Bokor et al. 2005; Wanaverbecq et al. 2008) and several lines of evidence indicate that APT is involved in somatosensory functions, including nociception (for review see Berkeley and Mash 1978; Brandao et al. 1991; Neto et al. 1999; Porro et al. 1999; Prado 1989; Prado and Faganello 2000; Prado and Roberts 1985; Rees and Roberts 1987, 1993; Roberts and Rees 1986; Rosa and Prado 1997; Rosa et al. 1998; Terenzi et al. 1995; Villarreal and Prado 2007; Villarreal et al. 2003, 2004). For example, APT is part of the descending pathways that modulate responses to noxious input in the spinal cord (for review see Rees and Roberts 1993). Peripheral noxious stimulation activates APT neurons (Neto et al. 1999; Porro et al. 1999; Rees and Roberts 1989; Villarreal et al. 2003), electrical and chemical stimulations of APT produce long-lasting antinociceptive effects in both acute and persistent pain models (Chiang et al. 1989; Prado 1989; Prado and Roberts 1985; Rees and Roberts 1987; Rhodes and Liebeskind 1978; Roberts and Rees 1986; Villarreal et al. 2004; Wang et al. 1992; Wilson et al. 1991), and APT inactivation increases responses to noxious stimuli (Villarreal et al. 2003, 2004). In a chronic pain model, APT inactivation resulted in increased autotomy, suggesting an increase in spontaneous pain (Rees et al. 1995). Along with its projections on PO, APT also innervates the ventral subregion of ZI (Giber et al. 2008; May et al. 1997; Terenzi et al. 1995), the ZI subregion that projects to PO (Giber et al. 2008; Trageser et al. 2006). Given the potential for APT to regulate both PO and ZI and the dramatic changes recorded in PO and ZI of animals with CPS (Masri et al. 2009), we hypothesized that APT activity would be abnormal in CPS. Specifically, the changes in PO and ZI could be caused by decreased inhibitory drive from APT to PO and/or increased inhibitory drive from APT to ZI. The experiments described here test these hypotheses.
METHODS
All procedures were approved by the University of Maryland School of Medicine Animal Care and Use Committee. Experiments were conducted according to institutional guidelines, federal regulations, and the guidelines of the International Association for the Study of Pain.
Spinal lesions
Seven adult female Sprague–Dawley rats, weighing 250–300 g, were used in the spinal lesions portion of this study. Three of these rats underwent spinal lesion and four rats underwent sham lesion surgery (Masri et al. 2009; Wang and Thompson 2008). Surgeries were conducted under strict aseptic conditions. Rats were anesthetized with ketamine/xylazine (100/8 mg/kg, administered intraperitoneally [ip]) and placed on a thermoregulated heating pad to maintain body temperature. A laminectomy was performed to expose the spinal cord between C6 and T2. A quartz-insulated platinum electrode (5 μm tip) was targeted to the anterior spinothalamic tract on one side of the spinal cord. Current (10 μA for 10 s, repeated four times) was passed through the electrode to produce an electrolytic lesion (∼0.6 mm3). Sham surgery was performed without laminectomy. The analgesic buprenorphine (0.05 mg/kg) was administered every 12 h for 24 h postoperatively.
Behavioral testing
Rats were habituated to the testing room for 30 min before behavioral testing, which consisted of measuring mechanical hindpaw withdrawal thresholds bilaterally using calibrated von Frey filaments (Stoelting, Wood Dale, IL). Filaments were applied to the dorsal surface of the hindpaw based on studies demonstrating that threshold changes are more reliably and consistently detected at this site (Ren 1999). Mechanical withdrawal threshold was defined as the force at which the animal withdrew to three of the five stimuli delivered. Rats underwent behavioral testing for 3 days in the week before surgery, to establish baseline thresholds, and on day 28 postsurgery.
Extracellular recordings
Following confirmation of decreased mechanical withdrawal thresholds 28 days after spinal lesion/sham surgery, rats were anesthetized with urethane (1.5 g/kg, ip) and infused with local anesthetics at surgical sites, head-fixed in a stereotaxic apparatus, and placed on a thermoregulated heating pad to maintain body temperature at 37°C. Depth of anesthesia was monitored every 15 min by testing reflexes to pinching of the skin and cornea stimulation. Supplemental doses of urethane (0.15 g/kg) were given when necessary. Urethane was selected because it is the only anesthetic that has no, or negligible, effects on glutamatergic and GABAergic transmission and therefore produces only minimal disruption of signal transmission in the neocortex (Sceniak and Maciver 2006).
A craniotomy was performed over the APT contralateral to the spinal lesion site or over PO in naive animals. Extracellular recordings of single units were obtained with quartz-insulated platinum electrodes (1 μm diameter tip; 2–4 MΩ). Spike waveforms were digitized through a Plexon (Dallas, TX) data acquisition system and sampled at 40 kHz. Electrodes were advanced to APT or PO based on stereotaxic coordinates—APT: anteroposterior (AP), −4.8; mediolateral (ML), 1.9; PO: AP, −3.6; ML, 2.6 (Paxinos and Watson 1998). Animals were maintained within the stereotaxic frame throughout the recordings. Once a well-isolated unit was identified in APT, spontaneous activity was recorded for 3 min.
In PO thalamus, responses were recorded to 50 deflections of the contralateral vibrissae by air puffs delivered through a tube (0.5 mm diameter) by a computer-controlled Picospritzer. Spontaneous activity was computed from a 100 ms period preceding stimulus onset. Air puffs were delivered at 1 Hz with a pressure of 60 psi, resulting in deflections of multiple whiskers of <30°. An orientation of the air tube was chosen that deflected the maximal number of whiskers and was not changed for the duration of each recording session.
At the end of each experiment, electrolytic lesions (20 μA of negative current for 20 s, performed twice) were made to confirm the recording sites. Animals were then deeply anesthetized with sodium pentobarbital (60 mg/kg) and perfused transcardially with buffered saline followed by buffered 4% paraformaldehyde. Coronal brain and spinal sections (80 μm thick) were obtained and Nissl-stained to identify recording and lesion sites.
APT inactivation
Seven adult female Sprague–Dawley rats weighing 250–300 g were used in the APT inactivation portion of this study. APT lesions were performed in four of these rats; the remaining three rats served as naive controls and did not receive lesions. Lesions were made by advancing a quartz-insulated platinum electrode into the APT following stereotaxic coordinates (AP, −4.8; ML, 1.9) and passing negative current through the electrode at 40 μA for 10 s, repeated four times at 5.9, 5.5, and 5.1 mm from the pial surface, resulting in lesions 0.07 to 0.5 mm3 in volume. Verification of lesion sites was performed as described earlier. Only animals with clear lesions in the APT were included in the analyses. PO recordings were obtained within 15 min of creating lesions in the APT and the recording session lasted ≤12 h. No significant differences were observed in any of the analyzed parameters over the course of an experiment.
Data analysis
Statistical analyses were performed with Intercooled Stata (Stata, College Station, TX) and GraphPad Prism (GraphPad Software, San Diego, CA). Between-group statistical comparisons were assessed with the nonparametric Mann–Whitney U test. Proportional data were analyzed using the χ2 test. The significance level was set at P < 0.05 for all tests.
BEHAVIORAL DATA.
To test whether hindpaw withdrawal thresholds changed over time after surgery, data from spinal-lesioned rats and sham-operated rats were analyzed separately with the Wilcoxon matched-pairs test.
VIBRISSA EVOKED RESPONSES.
Time stamps of well-isolated units and of stimulus triggers were exported to Matlab (The MathWorks, Natick, MA) for analyses using custom-written algorithms. Peristimulus time histograms (PSTHs; 1 ms bins) were constructed from these time stamps. Significant stimulus-evoked responses were defined as PSTH bins with a response magnitude that significantly exceeded (99% confidence interval) spontaneous activity levels, computed from a 100 ms period preceding the stimuli.
RESULTS
Behavioral confirmation of CPS in spinal-lesioned animals
We use an established rodent model of CPS in which electrolytic lesions of the spinal cord result in mechanical hyperalgesia below the lesion site (Endo et al. 2008; Masri et al. 2009; Wang and Thompson 2008). The time course and clinical features of this hyperalgesia are consistent with CPS symptoms in human patients (reviewed in Canavero and Bonicalzi 2007). Animals with such spinal lesions develop mechanical and thermal hyperalgesia within 14 days of spinal lesion surgery (Masri et al. 2009). Consistent with previous reports, all animals in the present study that underwent spinal lesion surgery developed a significant reduction in hindpaw withdrawal thresholds to mechanical stimuli by 28 days following surgery. Mechanical thresholds decreased from 93.3 g (SD = 24.2 g, median = 80, range 80 to 140) to 66.7 g at 28 days postsurgery (SD = 16.3 g, median = 60, range 60 to 100; P = 0.03, the Wilcoxon matched-pairs test; n = 3 rats). Sham surgery had no effect on mechanical withdrawal thresholds of either the ipsilateral or contralateral hindpaw (presurgery mean = 90.0 ± 21.4 g, median = 100, range 60 to 120; 28 days postsurgery mean = 95.0 ± 14.1 g, median = 100, range 60 to 100; P = 0.6; n = 4).
Neuronal activity in APT following CPS
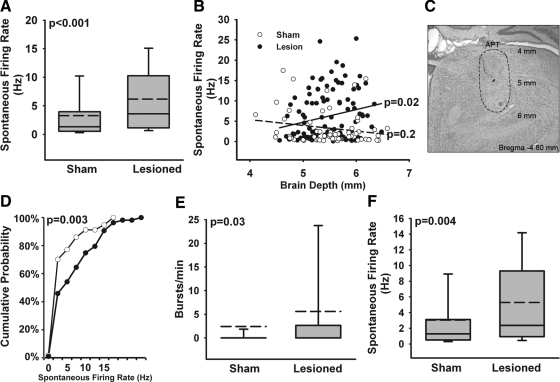
Within 1 wk after hyperalgesia was confirmed (at 28 days postlesion), we obtained recordings from 164 well-isolated single units in APT of urethane-anesthetized rats (57 from sham-operated control and 107 from spinal-lesioned animals). Post hoc histological analysis of electrode tracts and lesion sites (see methods) confirmed that all neurons were located within APT, throughout both dorsal and ventral APT. Recorded cells were located between 4.8 and 5.2 mm posterior to bregma. There was no difference in location of cells recorded from sham-operated and spinal-lesioned animals. Consistent with a previous report (Bokor et al. 2005), APT neurons exhibited a heterogeneous pattern of spontaneous firing, with a high degree of variability, ranging from 0.2 to 17.5 Hz in sham-operated controls and 0.06 to 25.3 Hz in spinal-lesioned rats. We describe the heterogeneity of responses in detail in the following text.
To test the hypothesis that CPS is associated with abnormal APT activity, we compared the spontaneous firing of APT neurons recorded from animals with CPS to those recorded from sham-operated rats. Neurons from CPS rats had a significantly higher mean spontaneous firing rate (CPS: mean = 6.2 ± 6.0 Hz, median = 3.6, range 0.06 to 25.3; Sham: mean = 3.3 ± 4.3 Hz, median = 1.3, range 0.2 to 17.5; P < 0.001, two-tailed Mann–Whitney U test; Fig. 1A). This finding is consistent with the hypothesis that CPS is associated with increased inhibitory drive from APT to ZI and inconsistent with the hypothesis of decreased inhibitory drive from APT to the posterior thalamus.
Fig. 1.
Anterior pretectal nucleus (APT) activity is increased during central pain syndrome (CPS). A: spontaneous firing rate of APT neurons in sham-operated controls (n = 57) and in spinal-lesioned rats with behaviorally confirmed CPS (n = 107). A, E, F: boxes represent the 25th and 75th percentile of distribution; dashed lines represent mean values; whiskers show the 10th and 90th percentiles. B: spontaneous firing rate plotted against recording depth and fitted with a linear regression line for sham-operated (dashed; r2 = 0.04) and lesioned (solid; r2 = 0.05) rats. Spontaneous firing rate increases with recording depth for lesioned, but not sham, rats. C: a micrograph of a coronal section depicting a representative lesion (asterisk) marking the location of a recorded neuron indicated in B (grey). The outline demarcates the borders of APT and the numerals indicate depth from the pial surface. D: cumulative probability plot showing the distribution of spontaneous firing rates of APT neurons in sham-operated and lesioned rats. Spinal lesion (black circles) results in a significant rightward shift in the distribution of spontaneous firing rate. E: incidence of bursts of action potentials is increased in APT of CPS animals, compared with sham-operated controls. F: spontaneous firing rate of nonbursting cells in APT in control (n = 44) and CPS animals (n = 60). Nonbursting cells in APT show increased spontaneous firing rate during CPS, compared with controls.
APT is a heterogeneous nucleus, forming at least two regions with distinct anatomical, physiological, and behavioral characteristics. Projections from the brain stem trigeminal and dorsal column nuclei preferentially terminate in ventral APT, a region that projects to areas involved in motor functions (Berman et al. 1977; Terenzi et al. 1995; Yoshida et al. 1992). Villarreal et al. (2004) found that stimulating dorsal APT was more effective than stimulating the ventral APT in inducing antinociception of acute pain, whereas stimulation of ventral APT was more effective for antinociception of persistent pain. We sought to determine whether the increase in APT activity in CPS occurred preferentially in one of these APT subregions. Figure 1B shows spontaneous firing rates plotted against brain depth. Linear regression fits show a significant deviation from zero for neurons from spinal-lesioned animals (r2 = 0.05; P = 0.02) such that a greater increase in activity is seen in ventral APT compared with dorsal APT. The same analysis for sham animals is not significant (r2 = 0.04; P = 0.2). The directionality of the increase in APT activity is consistent with prior evidence that APT is heterogeneous and supports the notion that the ventral APT is more involved in somatosensory processes than the dorsal APT. A cumulative probability plot of spontaneous firing rate shows a significant rightward shift in lesioned compared with sham-operated animals (P = 0.003, Kolmogorov–Smirnov test; Fig. 1D), indicating that not only is the firing in lesioned animals increased but that a greater proportion of neurons show increased firing rate following spinal lesions.
Besides its involvement in pain processing, APT is also involved in the processing of nonnoxious somatosensation (for review see Rees and Roberts 1993; Terenzi 1995). We tested the effects of spinal lesions on innocuous, sensory-evoked responses in APT by deflecting the contralateral vibrissae with air puffs or by tactile stimulation of various body parts. None of the neurons recorded responded to these stimuli. The failure to find neurons in APT that respond to low-threshold cutaneous and whisker stimuli agrees with the report by Rees and Roberts (1989) that APT neurons do not respond to hair movements, light pressure, or limb flexion.
Bursting activity in APT also showed a high degree of variability, with sham-operated rats displaying 0 to 206 bursts/min and spinal-lesioned rats 0 to 214 bursts/min (Fig. 1E). The percentage of APT neurons exhibiting at least one burst was significantly higher in CPS rats compared with that of sham-operated controls (44 vs. 23%; P = 0.01, χ2 test). This increase in the population of bursting neurons was also reflected in a significant increase in burst rate (CPS: mean = 5.6 ± 14 bursts/min, median = 0, range 0 to 71; n = 107; Sham: mean = 2.4 ± 11, median = 0, range 0 to 68; P = 0.004, two-tailed Mann–Whitney U test; n = 57; Fig. 1E).
To test whether the increase in spontaneous firing affects both bursting and nonbursting APT neurons, we computed the mean spontaneous firing rate separately for each group. Spontaneous activity was significantly higher in the nonbursting neurons from spinal-lesioned animals: CPS: mean = 5.3 ± 5.5 Hz, median = 2.4, range 0.06 to 25; n = 60; Sham: mean = 3.0 ± 4.1 Hz, median = 1.3, range 0.2 to 15; P = 0.02; n = 44; Fig. 1F). By contrast, in bursting neurons spinal lesions had no effect on either spontaneous firing rate or on any of the burst properties (sham-operated, n = 13; spinal-lesioned, n = 47; Table 1).
Table 1.
Firing properties of bursting APT neurons from spinal-lesioned and control rats
| Spontaneous Firing Rate, Hz | Bursts/min | Percentage Spikes in Bursts | Mean Burst Duration, ms | Mean Spikes in Burst | Mean ISI in Burst, ms | Mean Frequency in Burst, Hz | |
|---|---|---|---|---|---|---|---|
| Sham (n = 13) | 4.1 ± 5.2 | 4.1 ± 5.2 | 12.3 ± 13.9 | 17 ± 7 | 4.8 ± 1.3 | 4.4 ± 1.3 | 248 ± 62 |
| Lesion (n = 47) | 7.3 ± 6.4 | 7.3 ± 6.4 | 12.7 ± 18.3 | 20 ± 10 | 5.2 ± 1.7 | 4.8 ± 1.3 | 232 ± 71 |
| P value | 0.08 | 0.2 | 0.5 | 0.3 | 0.4 | 0.4 | 0.4 |
Values are means ± SD, n = 13 sham, 47 lesion. See text for burst criteria.
Effects on specific neuronal classes
Bokor et al. (2005) showed that APT neurons can be classified into three populations based on their firing properties. These are 1) fast bursting cells that periodically fire high-frequency bursts of action potentials (>350 Hz), 2) tonic cells that fire monotonous or irregularly spaced action potentials, and 3) slow rhythmic cells that fire short bursts of action potentials (at <150 Hz) at a rhythm locked to the slow cortical oscillations of anesthetized animals (0.7 to 8 Hz).
Using these criteria, and analyzing interspike interval (ISI) plots and autocorrelograms (see below), we categorized APT neurons into these three classes. Example ISIs and autocorrelograms of neurons in each category from sham-operated rats are shown in Fig. 2. Firing parameters for the three example neurons are as follows: 1) fast bursting: spontaneous firing rate 20 Hz; burst frequency 71 bursts/min; mean frequency within bursts 256 ± 53 Hz; 2) tonic: spontaneous firing rate 25 Hz; burst frequency 1 burst/min; mean frequency within bursts 147 ± 32; 3) slow rhythmic: spontaneous firing rate 9.4 Hz; burst frequency 5.1 bursts/min; mean frequency within bursts 147 ± 21. These measurements are consistent with those observed previously (Bokor et al. 2005), thus validating our categorization method.
Fig. 2.
Firing patterns of representative APT neurons from each of the 3 distinct neuronal populations. A, C, E: interspike intervals (ISIs; left column) and autocorrelograms (right) are shown for each representative neuron. Fast bursting neurons (A) are characterized by sharp autocorrelogram peaks and narrow ISI distributions at very short interval (<0.01 s). Tonic neurons (C) are characterized by autocorrelograms that lack a central peak and a wide ISI distribution due to the paucity of very short ISIs (see also Table 2). Slow rhythmic neurons (E) are characterized by a broad central peak in the autocorrelogram and wider ISI distribution compared with fast bursting cells. Bin size in all histograms is 1 ms. B, D, F: example raster plots of spike patterns (top) and waveforms (bottom) for each unit. Distinct firing patterns characteristic of the 3 neuron populations are evident. G: relative incidence of fast bursting, tonic, and slow rhythmic neurons is not changed in CPS.
We first asked whether spinal lesions affected the prevalence of neurons in each class. They did not: the percentage of neurons in each class was indistinguishable in CPS and sham-operated animals (P = 0.3; χ2 test; Fig. 2G).
We next asked whether the increase in firing rates following spinal lesions was reflected differentially in one or more of the APT neuronal subpopulations. Only tonic cells showed a significant, nearly twofold increase in spontaneous activity following spinal lesion (CPS: mean = 8.2 ± 5.5 Hz, median = 7.6, range 0.06 to 25; Sham: mean = 4.6 ± 4.5 Hz, median = 4.0, range 0.3 to 15; P = 0.03; two-tailed Mann–Whitney U test; Fig. 3B). Conversely, fast bursting and slow rhythmic neurons showed no change in spontaneous firing rate (fast bursting, CPS: mean = 3.7 ± 5.5 Hz, median = 1.2, range 0.2 to 20; Sham: mean = 2.0 ± 3.4 Hz, median = 1.1, range 0.2 to 18, P = 0.1; Fig. 3A; slow rhythmic, CPS: mean = 9.3 ± 5.7 Hz, median = 9.4, range 0.5 to 25; Sham: mean = 6.1 ± 5.4 Hz, median = 5.2, range 0.3 to 15; P = 0.1; Fig. 3C).
Fig. 3.
Effect of spinal lesions on APT neuron subtypes. Spontaneous firing rate (left) and bursts/min (right) of fast bursting (A), tonic (B), and slow rhythmic (C) neurons in sham-operated and spinal-lesioned rats. A–C: boxes represent the 25th and 75th percentile of distribution; dashed lines represent mean values; whiskers show the 10th and 90th percentiles. Significant increases were observed in spontaneous firing rate of tonic neurons (B, left) and in bursts/min of fast bursting (A, right) and slow rhythmic (C, right) neurons.
Because bursting has been implicated in mechanisms of CPS (Lee et al. 2005; Lenz et al. 1989; Vierck et al. 1990; Weng et al. 2003; but see Dostrovsky 2007), we asked whether spinal lesions affected the propensity of neurons in each class to fire bursts of action potentials. We defined bursts in extracellularly recorded spike trains as clusters of at least four spikes with ISIs of ≤10 ms. Consistent with the qualitative classification criteria (see preceding text), this quantitative analysis revealed that, in sham-operated rats, the fast bursting class exhibited the highest incidence of bursting neurons (35%), whereas bursting neurons were rare among both tonic (7%) and slow rhythmic (11%) neurons.
Following spinal lesions, fast bursting and slow rhythmic neurons showed an increase in bursting activity (Fig. 3). Fast bursting neurons showed an increase in burst rate (Sham: mean = 4.0 ± 14 bursts/min, median = 0, range 0 to 69; CPS: mean = 9.1 ± 18, median = 0.3, range 0 to 71; P = 0.02; Fig. 3A) but no change in the proportion of neurons exhibiting bursts (35% in sham-operated, 57% in spinal-lesioned, P = 0.08, χ2 test). Slow rhythmic neurons showed a dramatic increase in the proportion of neurons exhibiting bursts (11% in sham-operated vs. 56% in spinal-lesioned, P = 0.04). This increase in bursting population was accompanied by a >100-fold increase in overall bursting rate (0.04 ± 0.11 bursts/min in sham-operated vs. 6.1 ± 8.3 in spinal-lesioned animals; Sham: median = 0, range 0 to 0.33; CPS: median = 0.33, range 0 to 27; P = 0.008; two-tailed Mann–Whitney U test; Fig. 3C). Meanwhile, tonic neurons showed no significant change in either incidence of bursting neurons (Sham: 7%; CPS: 19%, P = 0.4) or burst rate (Sham: mean = 0.05 ± 0.2 bursts/min, median = 0.2, range 0 to 0.7; CPS: mean = 0.2 ± 0.5, median = 0, range 0 to 3; P = 0.3; Fig. 3B).
We analyzed firing properties of bursting cells within each group to determine whether they were altered by spinal lesions. Compared with sham-operated rats, no group showed a significant change in spontaneous firing rate, burst frequency, burst duration, number of spikes/burst, ISIs within bursts, or average of frequencies or peak frequencies within bursts (Table 2).
Table 2.
Firing properties of bursting cells within specific classes of APT neurons from spinal-lesioned and control rats
| Spontaneous Firing Rate, Hz | Bursts/min | Mean Burst Duration, ms | Mean Spikes in Burst | Mean ISI in Burst, ms | Mean Frequency in Burst, Hz | Mean Peak Frequency in Burst, Hz | |
|---|---|---|---|---|---|---|---|
| A. Fast bursting | |||||||
| Sham (n = 12) | 3.9 ± 5.7 | 12 ± 24 | 15 ± 6.1 | 4.6 ± 1.1 | 4.1 ± 1.0 | 261 ± 54 | 340 ± 80 |
| Lesion (n = 28) | 5.9 ± 6.5 | 16 ± 22 | 19 ± 11 | 5.2 ± 2.0 | 4.3 ± 1.2 | 257 ± 71 | 345 ± 67 |
| B. Tonic | |||||||
| Sham (n = 1) | 4.7 | 0.66 | 29 | 7.5 | 4.5 | 220 | 343 |
| Lesion (n = 7) | 9.9 ± 7.4 | 0.99 ± 0.91 | 19 ± 4.7 | 4.4 ± 0.68 | 5.7 ± 1.1 | 186 ± 42 | 311 ± 148 |
| C. Slow rhythmic | |||||||
| Sham (n = 1) | 5.2 | 0.33 | 23 | 4.0 | 7.7 | 130 | 156 |
| Lesion (n = 10) | 9.8 ± 4.1 | 11 ± 8.4 | 27 ± 8.2 | 5.7 ± 1.1 | 5.5 ± 1.0 | 190 ± 53 | 267 ± 59 |
Values are means ± SD, n = 13 sham, 47 lesion. Only means are given for sham-operated tonic and slow rhythmic neurons, where n = 1. See text for burst criteria. No parameter showed a significant difference between sham and lesion groups.
PO activity is increased following APT lesion
The hypothesis—which we disproved—was that CPS would be associated with decreased activity of APT neurons. This hypothesis was based on our finding that CPS is associated with a dramatic increase in spontaneous and evoked activity in PO (Masri et al. 2009) and on the assumption that APT inhibits PO neurons. The latter assumption was based on anatomical data showing that APT sends inhibitory, GABAergic projections to PO (Bokor et al. 2005) and on in vitro findings showing that stimulation of APT evokes inhibitory potentials in PO neurons (Bokor et al. 2005; Wanaverbecq et al. 2008). However, to our knowledge there is no direct evidence for the prediction that APT inhibits spontaneous or evoked activity of PO neurons in vivo.
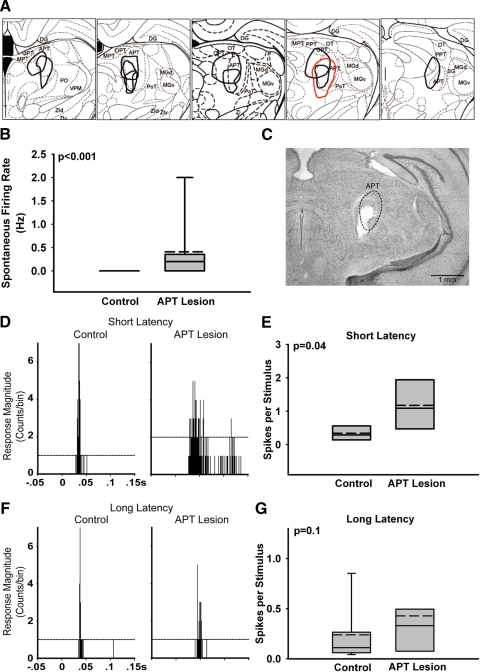
We tested this prediction by inactivating large parts of APT with electrolytic lesions (see methods). The resulting lesions were 0.07 to 0.5 mm3 and affected both the dorsal and ventral subregions of APT (Fig. 4, A and C). One lesion was located medial and dorsal; the second medial and ventral; the third was located slightly posterior compared with the first two and covered large areas of both dorsal and ventral APT.
Fig. 4.
Effects of APT inactivation on posterior thalamic nucleus (PO) response properties. A: line drawings superimposed on serial brain atlas sections (Paxinos and Watson 1998) summarizing the location and size of APT lesions (n = 3). Red trace corresponds to lesion shown in C. APT, anterior pretectal nucleus; DG, dentate gyrus; MGd, dorsal region of medial geniculate nucleus; MGv, ventral region of medial geniculate nucleus; MPT, medial pretectal nucleus; OPT, olivary pretectal nucleus; OT, nucleus of the optic tract; PoT, posterior triangular thalamic nucleus; PPT, posterior pretectal nucleus; SG, suprageniculate nucleus; VPM, ventral posteromedial thalamic nucleus; ZId, dorsal zona incerta; ZIv, ventral zona incerta. B: spontaneous activity of PO neurons is increased following APT lesions. C: photomicrograph of a coronal section from a rat that received APT lesion (5.30 mm posterior to bregma). Note that the extent of the lesion, delineated by red trace in A, covers nearly the entire APT (demarcated with a broken line). D, F: peristimulus time histograms (PSTHs) constructed from responses of PO neurons to a 50 ms air puff delivered to the vibrissae at time t = 0 in naive and in APT-lesioned animals. Horizontal dashed lines depict response magnitude levels exceeding (99% confidence interval) spontaneous activity levels (computed from a 100 ms period preceding stimulus onset). D: PSTHs from 2 representative short-latency (onset ≤30 ms) neurons from a control animal and an animal following APT lesion. E: grouped data of response magnitude of short-latency neurons from naive (n = 8) and APT-lesioned (n = 7) animals. Lesioning APT caused a significant increase in response magnitude of PO cells. F: PSTHs from 2 representative long-latency (onset >30 ms) neurons from a naive animal and APT-lesioned animal. G: grouped data of response magnitude of long-latency neurons from naive (n = 9) and APT-lesioned animals (n = 21). APT lesion did not affect response magnitude in long-latency PO neurons.
We tested the effects of APT lesions on the spontaneous activity of PO neurons and on their responses to sensory stimulation (see methods). To allow for meaningful comparisons between data from control and APT-lesioned animals, we focused on neurons in the dorsolateral portion of the medial posterior nucleus (POm), a region containing vibrissae-responsive neurons, and included only neurons that responded to vibrissae stimulation. We compared data from neurons recorded from rats with intact APT (n = 17 neurons) with data from neurons recorded from rats in which APT was lesioned 15 min before the recordings (n = 28 neurons).
As we and others reported previously, PO neurons fire spontaneously at low rates and respond to vibrissae stimulation weakly and at long latencies (Diamond et al. 1992; Lavallee et al. 2005; Masri et al. 2008; Trageser and Keller 2004). As seen in Fig. 4B, the spontaneous firing rate in PO increased following APT lesion (Naive: median = 0.0 Hz, range 0 to 0; Lesion: median = 0.2 Hz, range 0 to 3; P < 0.001; two-tailed Mann–Whitney U test).
Previous work in our lab (Trageser and Keller 2004) has shown that PO contains two neuronal populations: cells that respond to vibrissae deflections at relatively short latencies (≤30 ms) and cells that respond at longer latencies (>30 ms). We also showed that these two cell populations are regulated differently by the inhibitory nucleus zona incerta (Trageser and Keller 2004). We therefore sought to determine how APT regulates short-latency (n = 8 control; n = 7 postlesion) and long-latency (n = 9 control; n = 21 postlesion) cells in PO. Inactivating APT resulted in a selective increase in response magnitude of short-latency cells (median = 0.94 spikes/stimulus, range 0.08 to 2.8; P = 0.04; Mann–Whitney U; Fig. 4, D and E) compared with control (median = 0.24 spikes/stimulus, range 0.04 to 0.74) and had no effect on long-latency cells (P = 0.1; Fig. 4, F and G). The disinhibition of short-latency cell responses following APT lesion suggests that, like the inhibitory subthalamic nucleus ZI (Trageser and Keller 2004), APT functions to selectively suppress sensory-evoked responses in this subset of PO neurons.
DISCUSSION
Our goal was to elucidate the role of APT in the pathogenesis of central pain syndrome (CPS). We considered two hypotheses: 1) decreased inhibitory input from APT to PO contributes to the increased spontaneous and evoked activity of neurons in PO during CPS; and 2) increased inhibitory input from APT to ZI decreases ZI activity and, consequently, increases PO activity (Masri et al. 2009). The first hypothesis arises from the observation that APT neurons can powerfully inhibit PO neurons (Bokor et al. 2005). The prediction follows that the activity of APT neurons would be abnormally low in CPS. Contrary to this prediction, APT activity was elevated in rats with CPS, with increases in both spontaneous activity and incidence of bursting.
Given this unexpected finding we asked whether APT did indeed inhibit PO under normal conditions. Suppression of APT with electrolytic lesions resulted in increased spontaneous and sensory-evoked activity in PO neurons. These findings indicate that APT, like its counterpart ZI (Lavallee et al. 2005; Trageser and Keller 2004), exerts potent inhibition onto PO neurons.
How, then, can we reconcile the apparently contradictory findings that in CPS APT activity increases are associated, not with enhanced inhibition of PO, but rather with enhanced activity of PO neurons? We consider several possibilities.
Efficacy of APT inputs to PO
One possibility is that the increased spontaneous activity of APT neurons is insufficient to significantly affect PO neurons. We have previously shown that, during CPS, spontaneous activity of PO neurons increases 30-fold and that this increase is associated with dramatically reduced inhibition from ZI (Masri et al. 2009). It is thus possible that the less than twofold increase in the spontaneous activity of APT neurons cannot significantly counteract the dramatically enhanced activity of PO neurons in animals with CPS.
Differential effect on neuronal subtypes
APT neurons can be classified into three groups based on firing pattern, morphology, and parvalbumin expression (Bokor et al. 2005). Two key findings in the current study were that CPS was associated with a selective increase in spontaneous firing rate of tonic neurons and a selective increased burst firing of fast bursting and slow rhythmic neurons (Fig. 3).
All three neuronal APT subtypes project to both ZI and PO (Giber et al. 2008). Their target distribution, however, is not homogeneous (Fig. 5). The GABAergic APT-ZI projections are thought to be dominated by tonic neurons, whereas fast bursting neurons, the other GABAergic group in APT, project mainly to PO and account for only a small percentage of GABAergic projections from APT to ZI (Bokor et al. 2005; Giber et al. 2008). Therefore the increase in spontaneous firing rate of tonic neurons during CPS likely leads to increased GABAergic input to ZI and provides a possible explanation for the decrease in ZI activity seen during CPS (Masri et al. 2009). This preferential increase in inhibitory output to ZI and the subsequent increase in PO activity explain how changes in APT activity could affect PO through an indirect pathway.
Fig. 5.
Schematic representation of activity changes in APT neuronal subtypes during CPS. Increased activity of GABAergic tonic neurons (orange) projecting to ZI during CPS leads to a decrease in ZI activity and, consequently, to decreased inhibitory drive to PO (bright red). The increased activity of PO-projecting tonic neurons is insufficient to counter the abnormally high PO activity that results. Excitatory slow rhythmic neurons (green), which project to both ZI and PO, show increased bursting activity (represented by dashed lines) without changing their overall firing rate. Fast bursting neurons (dark red), which project almost exclusively to PO, show increased bursting during CPS, whereas their overall firing rate remains unchanged. Abnormally high PO activity leads to increased activation of cortical areas responsive to somatosensory input.
Tonic neurons project not only on ZI because they make up roughly 20% of APT projections on PO (Giber et al. 2008). It is possible that the relatively modest increase in inhibitory tonic neuron activity is not sufficient to counteract the 30-fold increase in PO activity, driven by disinhibition from ZI (Masri et al. 2009).
Most (60%) of the synapses formed by APT axon terminals in ZI are excitatory and available evidence suggests that these originate from slow rhythmic APT neurons (Bokor et al. 2005; Giber et al. 2008; May et al. 1997). During CPS the incidence of burst firing is increased in these neurons, but their overall firing rate remains unchanged (Fig. 3C). Because ZI activity is dramatically suppressed during CPS (see preceding text), it is possible that the increased bursting of these excitatory APT–ZI inputs has little or no effect on the firing rate of ZI neurons. This would be consistent with the notion that presynaptic bursts can affect the temporal fidelity of postsynaptic neurons (Sherman and Guillery 1998, 2006), without affecting their overall excitability.
Alternatively, it is possible that the excitatory slow rhythmic neurons do not project on PO-projecting ZI neurons, but rather that they drive inhibitory interneurons within ZI to produce feedforward inhibition of PO-projecting neurons. There is precedence for such feedforward regulation of the incertothalamic pathway: Urbain and Deschênes (2007) argued that excitatory inputs from motor cortex preferentially target local interneurons in ZI that, through feedforward inhibition, inhibit other ZI neurons that project to PO. It is therefore possible that, during CPS, the increase in bursting of excitatory slow rhythmic neurons activates this intraincertal circuit and consequently inhibits ZI.
Although tonic and slow rhythmic cells make up the majority of inputs to ZI, fast bursting cells preferentially target the thalamus (Bokor et al. 2005; Giber et al. 2008). The firing rate of fast bursting neurons remains unchanged during CPS, but the incidence of burst firing is increased (Fig. 3A). The fact that there is no change in the firing rate of these neurons, which are inhibitory, is consistent with the increased PO activity seen during CPS (Masri et al. 2009) and suggests that changes in APT activity could affect PO indirectly via ZI rather than through changes in PO-projecting neurons. The increase in PO activity following APT lesion confirms that APT does inhibit PO neurons under normal conditions and further supports the notion that changes in APT during CPS are not global but target specific neuron groups. As noted earlier regarding slow rhythmic neurons that project to ZI, the increased burst firing in fast bursting neurons without a change in firing rate may not significantly affect the excitability of PO neurons.
Increased activity preferentially in ventral APT
Besides the heterogeneity in neurotransmitter expression, cellular morphology, and electrophysiological properties, APT projection targets differ along its rostrodorsal and caudoventral axes (Giber et al. 2008; Scalia and Arango 1979; Terenzi et al. 1995; for review see Mitrofanis 2005; Rees and Roberts 1993). A loose topography exists such that the dorsal APT, the APT subregion associated with visual systems, projects to the lateral ventral ZI (vZI), dominated by neurons involved in communication with visual areas, whereas the ventral APT, associated with somatosensory systems, projects preferentially to the somatosensory and motor subregions of vZI (Berman 1977; Terenzi et al. 1995). Interestingly, the greatest increase in spontaneous activity occurred in the ventral/somatosensory APT (Fig. 1B). Thus the effects of the APT activity increase would be expected to have a greater effect on vZI neurons involved in somatosensory and motor areas, including PO (Mitrofanis 2005).
Other targets of APT
Besides PO and ZI, APT is part of the descending pain modulation system and projects to a number of areas involved with the processing of nociceptive information. These areas include the posterior hypothalamus, intralaminar thalamus, somatosensory cortex, rostral ventrolateral medulla, and possibly periaqueductal gray (Beitz 1982; Berman 1977; Foster et al. 1989; Terenzi et al. 1995; Zagon et al. 1995). It is likely that the changes we observe in APT following spinal lesions also affect these targets, along with its effects on ZI and PO, demonstrated here.
Support for a role for APT in pain also comes from studies demonstrating that stimulation of APT induces antinociception (Prado 1989; Prado and Roberts 1985; Roberts and Rees 1986) and that inhibiting APT results in exaggerated responses to painful stimuli (Villarreal et al. 2003, 2004). These effects of APT are thought to occur through descending modulation of processing in the spinal cord (Price and Mayer 1975). At first glance, these findings appear to contradict our finding that APT activity is increased in CPS. However, the studies cited earlier examined the role of APT in regulating responses to acute pain, whereas our study focused on the maladaptive plasticity that occurs in APT weeks after spinal cord injury. To our knowledge, only one other study examined the role of APT in a chronic pain condition: Rees et al. (1995) showed that, following dorsal horn rhizotomy, inhibiting APT increases autotomous behavior, suggesting an increase in spontaneous pain. However, the activity of APT was not directly tested in that study.
It is also likely that the effects of stimulating or inactivating large regions of APT are confounded by the heterogeneity of neurons in this structure (Bokor et al. 2005; also see findings from the present study). Immediately relevant to pain processing, Villarreal et al. (2004) showed that stimulating dorsal APT is more effective at producing antinociceptive effects on brief noxious stimuli, whereas stimulating ventral APT is more effective at producing antinociception to persistent noxious stimuli. Likewise, inhibiting ventral APT caused a greater increase in incisional pain than did inhibiting dorsal APT.
A role for APT in CPS
APT innervates exclusively higher order thalamic nuclei and, as such, is poised to regulate sensory information that involves communication between one cortical area to another. The findings in this study suggest that changes in APT activity are part of the maladaptive plasticity underlying the development of CPS; specifically, that the increase in spontaneous and bursting activity in APT contributes to the changes seen in ZI and PO function, resulting in aberrant pain processing that contributes to the development of CPS.
GRANTS
This work was supported by Public Health Service/National Institute of Neurological Disorders and Stroke (NINDS) Research Grant NS-066965 to A. Keller and NINDS Training Fellowship T32-NS-07375 to P. D. Murray and a Christopher and Dana Reeve Foundation grant to A. Keller. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the National Institutes of Health.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. R. Quiton and J. Lucas for valuable discussions and suggestions.
REFERENCES
- Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain 61: 187–193, 1995 [DOI] [PubMed] [Google Scholar]
- Bartho P, Freund TF, Acsády L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci 16: 999–1014, 2002 [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience 7: 133–159, 1982 [DOI] [PubMed] [Google Scholar]
- Berkeley KJ, Mash DC. Somatic sensory projections to the pretectum in the cat. Brain Res 158: 445–449, 1978 [DOI] [PubMed] [Google Scholar]
- Berman N. Connections of the pretectum in the cat. J Comp Neurol 174: 227–254, 1977 [DOI] [PubMed] [Google Scholar]
- Boivie J. Central pain. In: Wall and Melzack's Textbook of Pain, edited by McMahon S, Koltzenburg M. Oxford, UK: Churchill Livingstone, 2005, p. 1057–1074 [Google Scholar]
- Bokor H, Frere SG, Eyre MD, Slézia A, Ulbert I, Lüthi A, Acsády L. Selective GABAergic control of higher-order thalamic relays. Neuron 45: 929–940, 2005 [DOI] [PubMed] [Google Scholar]
- Bonica JJ. History of pain concepts and pain therapy. Mt Sinai J Med 58: 191–202, 1991 [PubMed] [Google Scholar]
- Bowsher D. Central pain. Pain Rev 2: 175–186, 1995 [Google Scholar]
- Brandao ML, Rees H, Witt S, Roberts MH. Central antiaversive and antinociceptive effects of anterior pretectal nucleus stimulation: attenuation of autonomic and aversive effects of medial hypothalamic stimulation. Brain Res 542: 266–272, 1991 [DOI] [PubMed] [Google Scholar]
- Canavero S, Bonicalzi V. Central Pain Syndrome: Pathophysiology, Diagnosis and Management New York: Cambridge Univ. Press, 2007 [Google Scholar]
- Chiang CY, Chen IC, Dostrovsky JO, Sessle BJ. Inhibitory effect of stimulation of the anterior pretectal nucleus on the jaw-opening reflex. Brain Res 497: 325–333, 1989 [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol 318: 462–476, 1992 [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO. The thalamus and human pain. In: Central Neuropathic Pain: Focus on Poststroke Pain, edited by Henry JL, Panju A, Yashpal K. Seattle, WA: IASP Press, 2007, p. 101–112 [Google Scholar]
- Dostrovsky JO, Craig AD. The thalamus and pain. In: Wall and Melzack's Texbook of Pain, edited by McMahon S, Koltzenburg M. Oxford, UK: Churchill Livingstone, 2009, p. 194–199 [Google Scholar]
- Endo T, Spenger C, Hao J, Tominaga T, Wiesenfeld-Hallin Z, Olson L, Xu XJ. Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain 138: 292–300, 2008 [DOI] [PubMed] [Google Scholar]
- Foster GA, Sizer AR, Rees H, Roberts MH. Afferent projections to the rostral anterior pretectal nucleus of the rat: a possible role in the processing of noxious stimuli. Neuroscience 29: 685–694, 1989 [DOI] [PubMed] [Google Scholar]
- Giber K, Slézia A, Bokor H, Bodor AL, Ludanyi A, Katona I, Acsády L. Heterogeneous output pathways link the anterior pretectal nucleus with the zona incerta and the thalamus in rat. J Comp Neurol 506: 122–140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain 34: 102–254, 1911 [Google Scholar]
- Jones EG. The Thalamus Cambridge, UK: Cambridge Univ. Press, 2007 [Google Scholar]
- Lavallee P, Urbain N, Dufresne C, Bokor H, Acsády L, Deschênes M. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J Neurosci 25: 7489–7498, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Ohara S, Dougherty PM, Lenz FA. Pain and temperature encoding in the human thalamic somatic sensory nucleus (ventral caudal): inhibition-related bursting evoked by somatic stimuli. J Neurophysiol 94: 1676–1687, 2005 [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res 496: 357–360, 1989 [DOI] [PubMed] [Google Scholar]
- Masri R, Bezdudnaya T, Trageser JC, Keller A. Encoding of stimulus frequency and sensor motion in the posterior medial thalamic nucleus. J Neurophysiol 100: 681–689, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona incerta: a role in central pain. J Neurophysiol 102: 181–191, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Sun W, Hall WC. Reciprocal connections between the zona incerta and the pretectum and superior colliculus of the cat. Neuroscience 77: 1091–1114, 1997 [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience 130: 1–15, 2005 [DOI] [PubMed] [Google Scholar]
- Neto FL, Schadrack J, Ableitner A, Castro-Lopes JM, Bartenstein P, Zieglgansberger W, Tolle TR. Supraspinal metabolic activity changes in the rat during adjuvant monoarthritis. Neuroscience 94: 607–621, 1999 [DOI] [PubMed] [Google Scholar]
- Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis: prevalence and clinical characteristics. Eur J Pain 9: 531–542, 2005 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates San Diego, CA: Academic Press, 1998 [Google Scholar]
- Porro CA, Cavazzuti M, Baraldi P, Giuliani D, Panerai AE, Corazza R. CNS pattern of metabolic activity during tonic pain: evidence for modulation by β-endorphin. Eur J Neurosci 11: 874–888, 1999 [DOI] [PubMed] [Google Scholar]
- Prado WA. Antinociceptive effect of agonists microinjected into the anterior pretectal nucleus of the rat. Brain Res 493: 147–154, 1989 [DOI] [PubMed] [Google Scholar]
- Prado WA, Faganello FA. The anterior pretectal nucleus participates as a relay station in the glutamate-, but not morphine-induced antinociception from the dorsal raphe nucleus in rats. Pain 88: 169–176, 2000 [DOI] [PubMed] [Google Scholar]
- Prado WA, Roberts MH. An assessment of the antinociceptive and aversive effects of stimulating identified sites in the rat brain. Brain Res 340: 219–228, 1985 [DOI] [PubMed] [Google Scholar]
- Price DD, Mayer DJ. Neurophysiological characterization of the anterolateral quadrant neurons subserving pain in M. mulatta. Pain 1: 59–72, 1975 [DOI] [PubMed] [Google Scholar]
- Rees H, Roberts MH. Anterior pretectal stimulation alters the responses of spinal dorsal horn neurones to cutaneous stimulation in the rat. J Physiol 385: 415–436, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees H, Roberts MH. Activation of cells in the anterior pretectal nucleus by dorsal column stimulation in the rat. J Physiol 417: 361–373, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees H, Roberts MH. The anterior pretectal nucleus: a proposed role in sensory processing. Pain 53: 121–135, 1993 [DOI] [PubMed] [Google Scholar]
- Rees H, Terenzi MG, Roberts MH. Anterior pretectal nucleus facilitation of superficial dorsal horn neurones and modulation of deafferentation pain in the rat. J Physiol 489: 159–169, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav 67: 711–716, 1999 [DOI] [PubMed] [Google Scholar]
- Rhodes DL, Liebeskind JC. Analgesia from rostral brain stem stimulation in the rat. Brain Res 143: 521–532, 1978 [DOI] [PubMed] [Google Scholar]
- Roberts MH, Rees H. The antinociceptive effects of stimulating the pretectal nucleus of the rat. Pain 25: 83–93, 1986 [DOI] [PubMed] [Google Scholar]
- Rosa MLNM, Oliveira MA, Valente RB, Coimbra NC, Prado WA. Pharmacological and neuroanatomical evidence for the involvement of the anterior pretectal nucleus in the antinociception induced by stimulation of the dorsal raphe nucleus in rats. Pain 74: 171–179, 1998 [DOI] [PubMed] [Google Scholar]
- Rosa MLNM, Prado WA. Antinociception induced by opioid or 5-HT agonists microinjected into the anterior pretectal nucleus of the rat. Brain Res 757: 133–138, 1997 [DOI] [PubMed] [Google Scholar]
- Scalia F, Arango V. Topographic organization of the projections of the retina to the pretectal region in the rat. J Comp Neurol 186: 271–292, 1979 [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Maciver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol 95: 3865–3874, 2006 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators.” Proc Natl Acad Sci USA 95: 7121–7126, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Drivers and modulators. In: Exploring the Thalamus and Its Role in Cortical Function (2nd ed.). Cambridge, MA: MIT Press, 2006, p. 253–286 [Google Scholar]
- Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103: 249–257, 2003 [DOI] [PubMed] [Google Scholar]
- Terenzi MG, Zagon A, Roberts MH. Efferent connections from the anterior pretectal nucleus to the diencephalon and mesencephalon in the rat. Brain Res 701: 183–191, 1995 [DOI] [PubMed] [Google Scholar]
- Trageser JC, Burke KA, Masri R, Li Y, Sellers L, Keller A. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol 96: 1456–1463, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci 24: 8911–8915, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N, Deschênes M. A new thalamic pathway of vibrissal information modulated by the motor cortex. J Neurosci 27: 12407–12412, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJJ, Greenspan JD, Ritz LA. Long-term changes in purposive and reflexive responses to nociceptive stimulation following anterolateral chordotomy. J Neurosci 10: 2077–2095, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal CF, Del Bel EA, Prado WA. Involvement of the anterior pretectal nucleus in the control of persistent pain: a behavioral and c-Fos expression study in the rat. Pain 103: 163–174, 2003 [DOI] [PubMed] [Google Scholar]
- Villarreal CF, Kina VA, Prado WA. Antinociception induced by stimulating the anterior pretectal nucleus in two models of pain in rats. Clin Exp Pharmacol Physiol 31: 608–613, 2004 [DOI] [PubMed] [Google Scholar]
- Villarreal CF, Prado WA. Modulation of persistent nociceptive inputs in the anterior pretectal nucleus of the rat. Pain 132: 42–52, 2007 [DOI] [PubMed] [Google Scholar]
- Wanaverbecq N, Bodor AL, Bokor H, Slézia A, Lüthi A, Acsády L. Contrasting the functional properties of GABAergic axon terminals with single and multiple synapses in the thalamus. J Neurosci 28: 11848–11861, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Thompson SM. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci 28: 11959–11969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Yuan B, Hou ZL. Role of the deep mesencephalic nucleus in the antinociception induced by stimulation of the anterior pretectal nucleus in rats. Brain Res 577: 321–325, 1992 [DOI] [PubMed] [Google Scholar]
- Weng HR, Lenz FA, Vierck C, Dougherty PM. Physiological changes in primate somatosensory thalamus induced by deafferentation are dependent on the spinal funiculi that are sectioned and time following injury. Neuroscience 116: 1149–1160, 2003 [DOI] [PubMed] [Google Scholar]
- Wilson DG, Rees H, Roberts MH. The antinociceptive effects of anterior pretectal stimulation in tests using thermal, mechanical and chemical noxious stimuli. Pain 44: 195–200, 1991 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Sessle BJ, Dostrovsky JO, Chiang CY. Trigeminal and dorsal column nuclei projections to the anterior pretectal nucleus in the rat. Brain Res 590: 81–94, 1992 [DOI] [PubMed] [Google Scholar]
- Zagon A, Terenzi MG, Roberts MH. Direct projections from the anterior pretectal nucleus to the ventral medulla oblongata in rats. Neuroscience 65: 253–272, 1995 [DOI] [PubMed] [Google Scholar]