Abstract
Responses of vestibular-only translation sensitive (VOTS) neurons in vestibular nuclei of two squirrel monkeys were studied at multiple frequencies to three-dimensional translations and rotations. A novel frequency-dependent spatiotemporal analysis examined in each neuron whether complex models, with unrestricted response dynamics in three-dimensional (3D) space, provided significantly better fits than restricted models following simple, cosine rule. Subsequently, the statistically selected optimal model was used to predict the maximum translation direction, expressed as a unitary vector, Vtmax, and its associated sensitivity and phase across frequencies. Simple models were sufficient to quantify the 3D translational responses of 66% of neurons. Most VOTS neurons, complex or simple, exhibited flat-gain or low-pass response dynamics. The Vtmax of simple neurons was fixed, whereas that of complex neurons changed with frequency. The spatial distribution of Vtmax in simple neurons, which fell within 30° of either the horizontal plane or/and the sagittal plane, was closely aligned with Vtmax of vestibular afferents. In contrast, the frequency-dependent Vtmax of most complex neurons migrated from the dorsoventral axis at higher frequency toward the horizontal plane, especially the interaural axis, at lower frequency. When the maximum rotation direction was estimated from responses of the same VOTS neurons to 1.2 Hz yaw, pitch, and roll rotations, complex neurons were more likely to respond to rotations activating vertical canals. Responses to 0.15–0.3 Hz linear accelerations produced by inertial or gravitational forces were indistinguishable in most complex neurons but significantly different in most simple neurons. These observations suggest that simple and complex VOTS neurons constitute distinctive vestibular pathways where complex neurons, exhibiting a novel spatiotemporal filtering mechanism in processing otolith-related signals, are well suited to drive tilt-related responses, whereas simple neurons probably mediate pure translation related responses.
INTRODUCTION
Animals rely on sensory inputs for reconstructing a perceptual representation of the external surrounding, the internal self, and the relationship between them. Since the sensory information is often coded in a reference frame that is different from that of the eventual motor act, various spatial transformation strategies that produce spatial maps conforming to neither sensory nor motor topography are adopted by the brain to deal with complex systems (Georgopoulos 1986; Masino and Knudsen 1990; Soechting and Flanders 1992). Furthermore, these centrally generated spatial tuning properties are often frequency dependent, reflecting the interaction of dynamics on spatial transformation (Chacron et al. 2005; Johnson et al. 1999; Levi et al. 2005).
The signal processing in central vestibular pathways provides a case study for understanding how spatial information is shaped to meet functional demands, especially for eliciting the vestibuloocular reflex (VOR) to compensate for head movements. Past studies of responses to rotations suggest that, through the convergence of proper semicircular canal (SCC) inputs at the vestibular nuclei, the sensory reference frame is converted into a motor frame (Ezure and Graf 1984; Graf et al. 1993). In other cases, visual following systems seem optimized to operate in the SCC-based coordinate system (Graf et al. 1988; Simpson et al. 1988; Wylie et al. 1998). This adaptation of a frame exogenous to the geometry of the sensory epithelium clearly involves interaction with other sensorimotor systems and probably improves the efficiency of the motor functions.
Past efforts to understand the spatial coding of linear accelerations by vestibular central mechanisms showed that signals present in vestibular neurons are far more diverse than those of otolith afferents (Bush et al. 1993; Chan et al. 1999; Chen-Huang and Peterson 2006; Dickman and Angelaki 2002; Schor and Miller 1982; Xerri et al. 1987; Yakushin et al. 2006; Zhou et al. 2006). In contrast to responses of otolith afferents (Angelaki and Dickman 2000; Fernandez and Goldberg 1976a; Fernandez et al. 1972), where the temporal phase to stimulus directions in two-dimensional (2D) planes is fixed and the modulation is described by the cosine rule with clearly identifiable maximum and null directions, some vestibular neurons exhibit complex tuning properties characterized by a gradual shift in the response phase and only a small change in sensitivity. In other words, the sensitivity of translation-related responses becomes broadly tuned, whereas the phase changes gradually with stimulus direction. This complex tuning response has been successfully modeled as resulting from the summation of translation-related inputs that are in spatial and temporal quadrature (Angelaki 1992) or spatiotemporal convergence (STC).
What roles, if any, such complex vestibular responses play in shaping motor or perceptual functions are unclear. If the majority (∼60–80%) of vestibular-only neurons exhibit complex behavior to translations in the horizontal plane, as estimated in previous studies (Angelaki and Dickman 2000; Bush et al. 1993), the profound implication is that most vestibular signals arriving at their final motor targets, including extraocular motoneurons during the VOR, would drastically depart from the spatiotemporal tuning properties of otolith afferents. On the other hand, our previous study of vestibular neurons to three-dimensional (3D) translations at a single frequency estimated that only 30% of vestibular neurons exhibited complex tuning (Chen-Huang and Peterson 2006). We also showed that maximum translation response vectors (Vtmax) of most simple tuning neurons lay within 20° of either the horizontal or sagittal plane, whereas those of complex neurons lay >20° from both planes. Our results suggest that the complex behavior observed in otolith-related vestibular neurons probably arises from summing inputs from afferents with diverse response dynamics, innervating different otolith maculae.
To further understand this small but significant fraction of vestibular-only neurons, a comprehensive frequency-dependent 3D spatiotemporal analysis was developed, assuming 1) neural responses to translations in a 3D space can be estimated geometrically by responses along the three orthogonal axes, i.e., interaural (IA), nasooccipital (NO), and dorsoventral (DV) axes; 2) time-varying sinusoidal stimuli produce sinusoidal neural responses at the same fundamental frequency; and 3) neural responses are linearly related to the stimulus amplitudes in the range tested as shown in afferents (Fernandez and Goldberg 1976b). Thus responses of vestibular-only translation sensitive (VOTS) neurons can be expressed by a single equation incorporating the spatial (elevation and azimuth) and temporal (frequency) parameters.
Unique features of our approach include the following:
1) Responses to translations in 3D space were examined. The advantages of 3D over 2D translational experiments have been previously demonstrated (Chen-Huang and Peterson 2006). As predicted by geometry, the tuning ratio, a measurement of spatiotemporal tuning properties (Angelaki 1992; Schor and Angelaki 1992), derived from 2D-only data sets might overestimate or underestimate the 3D ratio. Further, the likelihood of classifying simple (cosine-tuned) neurons as complex (STC) type increases if data, small in modulation and large in noise, are available only from a nonpreferred plane (Chen-Huang and Peterson 2006). Last, our previous study showed that one distinguishing feature between simple and complex VOTS neurons was whether they responded to translations in either the horizontal or sagittal plane (simple), or both planes (complex). That important finding, to be replicated here, was made possible only with 3D tests.
2) The null hypothesis that complex spatiotemporal models were significantly superior to simple models was statistically tested; whereas simple models were characterized by a single transfer function shared by all three canonical axes, complex models had two or three transfer functions. Our approach obviated the need to classify neurons based on an arbitrarily chosen “tuning ratio” that changes with frequency (Angelaki and Dickman 2000). Results of this “model-selection first” approach were compared with the conventional tuning ratio-based approach.
3) A global approach—that is, frequency-dependent spatiotemporal modeling—was developed to assess the overall spatiotemporal tuning properties of VOTS neurons across frequencies and 3D translation directions. Previous studies used different approaches in elucidating the interaction of spatiotemporal tuning properties and frequency (Angelaki and Dickman 2000; Chen-Huang and Peterson 2006; Schor et al. 1985). One major advantage of incorporating frequency as an independent variable in the evaluation of spatiotemporal tuning properties (Schor et al. 1985) over the piecewise (Angelaki and Dickman 2000; Chen-Huang and Peterson 2006), or frequency-blind, analysis is that the spatial and temporal tuning rules are linked by a single linear summation model. Here, we adopted this frequency-dependent spatiotemporal analysis that allows us to fit and predict translational responses in 3D space across frequencies within the framework of existing linear models. This was necessary since, due to complex spatiotemporal interaction, spatial tuning properties, including Vtmax and tuning ratio, change with frequency in complex neurons. Further, neurons exhibiting complex behavior globally may exhibit simple cosine tuning within a narrow frequency range. This frequency-dependent approach provided a means to evaluate the Vtmax globally across frequencies regardless of what spatiotemporal pattern was coded by the responses of each neuron.
4) Measurement noise was factored into model selections. In contrast to our previous study (Chen-Huang and Peterson 2006), in which responses from a single frequency were analyzed, the intrinsic noise of neural responses tested with a wide range of frequencies and stimulus amplitudes in this study appeared to vary greatly across test conditions. Further, model selections without noise analysis tend to skew toward complex models over simple ones since intrinsic noise in neural discharge is more likely to increase, not decrease, the uncertainty of a simple cosine fit. Thus a sound estimation of coefficient SEs was critical in selecting the optimal spatiotemporal model.
This study examined these following questions. First, with a comprehensive characterization of VOTS neurons to translations in 3D at multiple frequencies, how do the spatial tuning and response dynamics of central neurons differ from the otolith afferents? Second, how does Vtmax change vary as a function of frequency? Do VOTS neurons responding to translations in multiple planes, presumably receiving inputs from both otolith maculae, exhibit spatiotemporal tuning properties or frequency-dependent Vtmax distinctive from those neurons whose Vtmax lies near the plane of utriculus or sacculus? Last, do simple and complex VOTS neurons show other distinguishing properties, such as convergence of canal inputs, which might suggest differential functional roles? Our results strongly suggest that simple and complex VOTS neurons represent two functionally diverse populations that showed distinctive spatial distribution of Vtmax and different sensitivity to vertical canal inputs. Further, these two groups of VOTS neurons exhibited differential responses to linear accelerations produced by gravitational and inertial forces. Thus results of this study raise the possibility that complex neurons play an important role in driving tilt-related responses, whereas simple neurons mediate translation-only related responses.
METHODS
Surgeries and experimental setup
These experiments were performed on one adult (∼0.8 kg) female and one male squirrel monkey (Saimiri sciureus). Surgical and animal care procedures followed the Principles of Laboratory Animal Care (National Institutes of Health publication No. 86-23, revised 2002) and were approved by the Northwestern University Animal Care and Use Committee. Surgeries implanting a bolt for holding the head at 15° nose down from the stereotaxic plane, a recording chamber, frontal eye coils in both eyes, and bilateral labyrinthine stimulating electrodes in squirrel monkeys (Chen-Huang and McCrea 1999) were performed under sterile conditions. The chamber was tilted 22° caudally and 5° medially with respect to the stereotaxic coordinates, facilitating an easy access of microelectrodes to the brain stem.
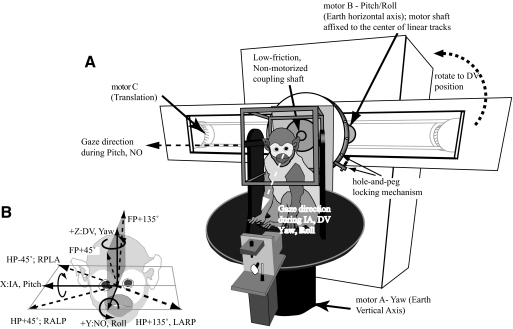
A detailed description of the 3D rotation and translation vestibular stimulator apparatus (Fig. 1), which incorporated two independent rotary motors, one about the earth-vertical axis (motor-A) and the other about an earth-horizontal axis (motor-B), and a belt-driven linear track (motor-C), has been previously published (Chen-Huang and Peterson 2006). This motor-track assembly could be positioned, via a servo controller or a manual locking mechanism (not shown), ±160° from the earth-vertical position, including an earth-horizontal orientation required for translations along the IA axis. A second manual “hole-and-peg” disk allowed the motor-A suprastructure to be locked onto the linear track assembly at 15° intervals. Thus this device allowed the animal to be translated in 3D space by orienting motor-B at various angles while the monkey always maintained an upright posture, by a passive counterrotation of the “hole-and-peg” disk, regardless of the orientation of the linear track. For example, when the linear track was oriented horizontally, IA or NO translations were produced by activating motor-C and by using motor-A to reposition the animal with respect to the linear axis. Similarly, using motor-B to rotate the animal about a horizontal axis, pitch, and roll of the animal, as well as rotations in the planes of the vertical canals, could be produced. When the track was oriented vertically, activation of motor-C produced DV translations. Finally, driving motor-A alone produced yaw rotations. Intermediate orientations on the horizontal (IA–NO), frontal (IA–DV), and sagittal (NO–DV) planes were also examined. In this report, rotation axes were earth-referenced and fixed with respect to the animal that remained upright. Thus horizontal-axis (HA) rotations stimulated vertical semicircular canals (i.e., pitch and roll), whereas vertical-axis (VA) rotations stimulated horizontal canals (i.e., yaw).
Fig. 1.
A: diagram of the 6-degrees-of-freedom vestibular stimulator. Motor-A rotated about the earth-vertical (yaw) axis and motor-B rotated about an earth-horizontal (pitch/roll) axis. The motor shaft of motor-B was affixed to the center of linear tracks. Motor-C was coupled to a belt-pulley system that moved motor-A and the superstructure, including a magnetic field cube, a laser/galvanometers assembly and a primate chamber, along a pair of linear tracks. Motor-A and the superstructure were attached to the linear tracks via a coupling shaft that could be manually locked at 15° intervals, with respect to the long axis of the tracks (“hole-and-peg locking mechanism”). Depending on the gaze direction of the monkey controlled by motor-A, motor-B produced roll or pitch motions and motor-C produced translations along the interaural (IA) or nasooccipital (NO) axis when the track was oriented horizontally. Translations along the dorsoventral (DV) axis were generated by orienting the linear tracks vertically. The yaw motor and suprastructure were manually locked to the tracks so that the animal always remained upright. Yaw, pitch, and roll were produced with the track at either orientation. B: the coordinate frame used in this study. Leftward yaw, right ear down, and pitch nose up rotations were positive rotations. Positive translations were rightward, forward, and upward.
Sinusoidal rotational stimuli were 20°/s at 0.15–2.4 Hz. The translation velocity was kept at 14 cm/s for sinusoidal movements between 0.6 and 3.6 Hz, which produced a translation acceleration of 0.054–0.323 g (g = 980.7 cm/s2). For stimuli at 4.8 Hz, the amplitude was reduced to 10 cm/s (0.308 g), whereas at the lower frequencies of 0.15–0.3 Hz, it was increased to 40 cm/s (0.038–0.077 g). Positive directions used in this report were rightward IA, forward NO, and upward DV. Positive rotations were leftward yaw, nose-up pitch, and right ear down (RED) roll (right hand rule) (Fig. 1B). Static positioning, including roll or pitch tilts, could be achieved as well. Translational and rotational head movements were recorded with a triaxial accelerometer and rate sensor (Memsense, Rapid City, SD). These signals, along with bilateral horizontal and vertical eye positions (Remmel Labs) and x–y target positions (GSI, Bedford, MA), were stored on a computer disk via a data acquisition system (see following text).
Extracellular potentials were recorded with 1.5–3 MΩ Epoxylite-insulated tungsten microelectrodes. A chamber-mounted predrilled circular grid (Crist et al. 1988) (Crist Instrument, Hagerstown, MD) and a motorized microdrive (Narishige International, East Meadow, NY) were used to position and drive the microelectrode to desired recording sites. Extracellular spikes were conventionally amplified, filtered (100–5 kHz), and stored using a 20 kHz, 12-bit acquisition system (DataWave Technologies, Berthound, CO) and, more recently, using the RX8 system (244 kHz, 16-bit sampling rate; Tucker-Davis Technologies, Alachua, FL). Spikes selected by on-line discrimination software were saved to disk, carefully viewed, and “recut” with the Off-line Sorter software (Plexon). Time-stamped spike events, each with a value of 1, were subsequently convolved with a Gaussian kernel (σ = 10 ms) to yield spike density histograms at 1-ms resolution (MacPherson and Aldridge 1979; Richmond et al. 1990).
Neuronal behavior was characterized during the following conditions: 1) eye movement–related responses during 1- to 2-s fixation periods where monkeys made spontaneous saccades; 2) smooth pursuit–related responses when monkeys visually followed a laser spot moving sinusoidally at 20°/s, 0.4–0.6 Hz, along the vertical and horizontal axes; 3) angular VOR (AVOR) suppression responses during 0.6 Hz, 20°/s yaw and pitch as monkeys fixated a visual target moving with the head; 4) AVOR responses during rotations about the yaw, pitch, and roll axes at typically 1.2 Hz, 20°/s, and at the extended frequency of 0.15–2.4 Hz while monkeys fixated an earth-fixed light-emitting diode (LED) target at 40 cm from the eyes; 5) linear VOR (LVOR) responses during translations along the IA, NO, and DV axes at typically 1.2 Hz, 14 cm/s, and additionally 0.15–4.8 Hz while monkeys fixated an earth-fixed LED target at 40 cm from the eyes; and 6) LVOR responses during translations along intermediate directions at 0.3–4.8 Hz.
Electrolytic lesions were used to confirm locations of recordings in the one animal that did not have chronically implanted labyrinthine stimulating electrodes. A constant current of 50 μA was applied via a low impedance (<500 kΩ) tungsten electrode for 20 s near the caudal pole of the left abducens nucleus after the animal was lightly anesthetized (ketamine, 20 mg/kg; acepromazine, 0.5 mg/kg). The animal was then deeply anesthetized with pentobarbital (200 mg/kg) and perfused transcardially with saline and fixative (4.0% formaldehyde, 0.1 M NaPO4, pH 7.4, and 15% picric acid). Brain tissue was freeze-sectioned in the transverse plane of electrode penetrations. Every fourth section, each 40 μm in thickness, was stained with thionine to reveal cell bodies, whereas its adjacent section was stained with Perls' solution, enhanced by 3,3′-diaminobenzidine (Perls/DAB), to reveal the ferric iron caused by rupture of small blood vessels or by the electrolytic lesion-associated electrocoagulation (Machado et al. 2009; Russo and Bruce 2000). Histology was performed by NeuroScience Associates (Knoxville, TN).
Data analysis
TRIAL-BY-TRIAL ANALYSIS.
Neural firing rate, along with records of concomitant stimulus and eye position signals, were binned at 1-ms interval and displayed for visual inspection with the aid of a custom procedure written in IGOR Pro (WaveMetrics). Some records, in which animals' eye positions deviated >3° from expected values or in which saccades occurred (plus 30 ms before and after), were rejected from further analysis. Saccades were identified by their high velocities. Combined neural records from multiple trials that lasted 30 s (36–144 cycles for frequency ≥1.2 Hz) to 3 min (13–54 cycles for 0.15 to 0.6 Hz) were equally divided into three segments (i.e., 9–24 cycles/segment). A bootstrap method was adopted that randomly drew half of the data from each segment (Optican and Richmond 1987) and fit the selected data with Eq. 1. This procedure also included an iterative component that searched for and excluded nonlinear response regions, presumably due to inhibitory saturation, from further analysis. Nonlinear regions were characterized by their large deviations (actual values) from the linear model (expected from fits) that exceeded a preset value (>5 spikes/s) (Chen-Huang et al. 1997). This bootstrap process was repeated five times for each segment (Richmond et al. 1987). Mean coefficients and their SEs (σ) were computed from the final 15 fit results. Note that although coefficients of sinusoid fits were plotted in the polar coordinate (i.e., gain and phase), the neural response (NR) of each segment with respect to time (t, in s) was fit independently, in vectorial form, to sinusoids at the fundamental frequency (fsti) present in stimuli with a least-squares error (LSE) algorithm
| (1) |
where k0 is the background firing rate; Ss/Sc is the component coefficient at temporal 0°/90° with respect to stimulus; Stim is the peak acceleration of translation in g (980 cm/s2) or rotational velocity in deg/s; sensitivity =
in spikes·s−1·g−1 or spikes·s−1·deg−1·s−1; and phase = atan (Sc/Ss) in radians with respect to translation acceleration or rotation velocity.
Coefficients of variation (CVs; i.e., SD over mean interval) and mean interspike intervals were computed from >30 epochs, which were 3–5 s in duration and contained >15 impulses, for each neuron during resting state. The normalized CV (CV*), at mean interval = 15 ms, was interpolated from the linear fits of CV versus mean intervals for all neurons (Chen-Huang et al. 1997).
FREQUENCY-DEPENDENT SPATIOTEMPORAL FIT FOR TRANSLATIONAL RESPONSES.
Responses of VOTS neurons were fitted with a frequency-dependent spatiotemporal model that optimally described each neuron's responses to translations in 3D space at multiple frequencies by a linear summation of responses along three orthogonal directions: IA (X), NO (Y), and DV (Z). Given that SFX(s), SFY(s), and SFZ(s) represent the frequency-dependent response dynamics, or transfer functions, of a vestibular neuron along IA, NO, and DV axes (s = j*ω; ω = 2*π*fsti; fsti in Hz and j is the imaginary unit), the overall neural response, NR(s), to any unitary translation direction in polar coordinates (α-azimuth, β-elevation), is expressed as
| (2) |
where −π ≤ α ≤ π and −π/2 ≤ β ≤ π/2, α and β are in radians, and kx, ky, and kz are axial DC sensitivities. SFX(s), SFY(s), and SFZ(s) are transfer functions along IA (X), NO (Y), and DV (Z) axes that incorporated terms up to two poles and/or two zeroes (appendix), a subset of systems used in previous studies modeling otolith afferents and central vestibular neurons (Angelaki and Dickman 2000; Fernandez and Goldberg 1976c; Schor et al. 1985). Inputs were presumably processed by a cascade of various high-pass/differentiator or low-pass/integrator elements. In addition, a nonminimum phase (NMP) term (appendix), which could be realized as summing an inhibitory high-pass pathway with a parallel excitatory “pure gain” pathway (Schor et al. 1985), was used to model incongruent gain and phase behaviors in some neurons.
The frequency-dependent spatiotemporal analysis commenced by applying the same transfer functions, where time constants (τ values) were constrained to be equal, across the three canonical axes: i.e., SFX(s) = SFY(s) = SFZ(s). These models were equivalent to fitting NR with the cosine rule, thus referred to as simple models. Complex models fit NR with two (two axes shared the same transfer function with the same time constants, whereas the third axis was different) or three transfer functions independently in which time constants varied freely. In total, 8 simple and 658 complex models were tested. For each model, the normalized residual χ2 was estimated from a least-squares fit weighted by the inverse of SE (appendix, Eq. A4).
Next, the variance accounted for (VAF) and mean square error (MSE) were computed. The initial attempt to find the optimal model based on MSEs was not successful since sorted MSEs were distributed as a multitiered sigmoid curve that could not be differentiated by cluster analysis, indicating many models, complex or simple, shared similar MSEs (see Fig. 4; MSEs are plotted in ascending order). Thus two more tests were included. First, the Akaike information criterion (AIC, Eq. 3a) was used to search for the most parsimonious model that, with fewer fit parameters, was as likely the best model as more complex models (Burnham and Anderson 2002). The Bayesian information criterion was computed for every neuron as well, but will not be reported here since the result was similar to that of AIC. Further, an AIC weight (ωAIC) for each model fit was computed from Eq. 3b that normalized each model's AIC with respect to the sum of all tested models based on the Gaussian distribution (Burnham and Anderson 2004).
| (3a) |
if ΔAICi= AICi − AICmin, then the AIC weight ωAIC is
| (3b) |
Fig. 4.
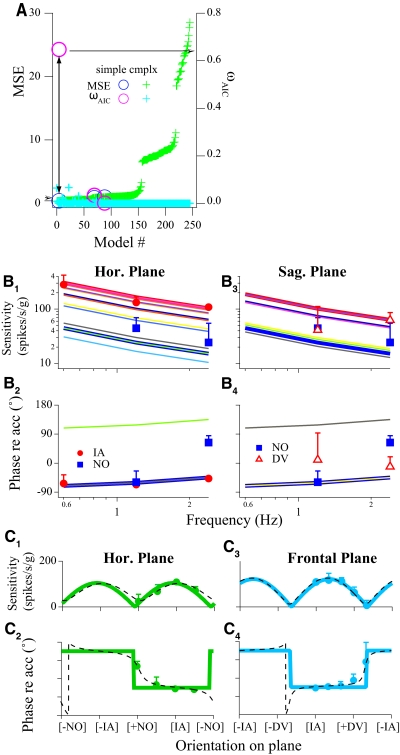
Summary of the modeling results for the VOTS neuron shown in Fig. 3. A: the normalized mean square error (MSE, left ordinate, blue-green) and Akaike information criterion weight (ωAIC; right ordinate, magenta-light blue) obtained from all successfully converged models were computed with the frequency-spatiotemporal fits. Both quantities are plotted according to the ascending order of MSE. Each model is labeled with the same marker style but different colors (e.g., the paired circles indicated by the double arrowheaded line). The simple model with the highest ωAIC (=0.65; magenta circle with arrow) also had a very low MSE (0.35, blue circle with arrow), indicating that this was the most parsimonious model of all models tested. B: comparison of actual and predicted responses from the chosen model indicated in A. Bode plots, i.e., sensitivity and phase vs. frequency, of actual (markers) and predicted (thick lines) responses, are plotted along the IA, NO, and DV axes. Estimated response dynamics in the intermediate orientations, sampled at a 15° interval, are also shown (thin lines). Note that although matching sensitivity and phase curves are graphed, only 2 stacks of phase curves are visible. C: spatial tuning curves of the same VOTS neuron in the horizontal and frontal planes. Actual (markers) response sensitivity and phase vs. translation directions are superimposed on the estimated responses from the global frequency-spatiotemporal model (solid lines). In addition, unrestricted, independent 2-dimensional fits to the horizontal and sagittal plane data are shown in dashed lines. The 2 identical peaks in the sensitivity plots correspond to peak responses in the excitatory and inhibitory directions. Error bars (SEs) in B and C are shown in one direction and may be obscured by symbols.
Further, after sorting on χ2, the model that contained m parameters and produced the lowest χ2 was identified (lowest residual model). F tests were then performed to examine the null hypothesis that models with fewer parameters (h, h < m) fit significantly worse than the lowest residual model (Eq. 4; for details see Chen-Huang and Peterson 2006). A P < 0.05 indicated that (m − h) variables beyond h made a significant contribution to the regression. Otherwise, the null hypothesis that additional (m − h) variables were not needed to improve the goodness of fit was upheld and instead a lower-order model was used
| (4) |
Thus ωAIC represented the probability that a given model was the most parsimonious and robust model among all successful models. The model with the highest ωAIC was considered the unequivocal best model if it also met these criteria: 1) its ωAIC ≥0.1; 2) an F test showed that the most parsimonious model with the highest ωAIC was not significantly different from the lowest residual model (Eq. 4) and its MSE fell within the lowest cluster; and 3) the ratio of ωAIC between the highest model versus its next runner-up model was ≥2.
RECONSTRUCTION OF FREQUENCY-DEPENDENT NEURONAL TUNING TO TRANSLATIONS IN 3D SPACE.
Finally, the expected sensitivity and phase to translations in a complete 3D space, ±180° (azimuth) × ±90° (elevation), were reconstructed from the chosen model at each test frequency with matrices of 1,440 columns and 720 rows. As described in the previous study (Chen-Huang and Peterson 2006), the unitary maximum translation vector Vtmax, with the maximum translation sensitivity (Stmax) and phase (ϕtmax), was searched for and located on the 3D sphere at each frequency. Although these values can be derived by solving a series of complex equations (Angelaki 1993), model simulation was more efficient in obtaining results, e.g., one algorithm was applicable to all models, without sacrificing accuracy. The minimum translation vector Vtmin (with Stmin and ϕtmin) was then searched for in the plane orthogonal to the Vtmax.
For the purpose of comparing our “model-selection first” approach to the conventional method that characterized spatiotemporal properties based on tuning ratios obtained from unrestricted model fits (Angelaki et al. 1992; Bush et al. 1993), we computed tuning ratios, Stmin/Stmax, from the lowest MSE, instead of the chosen model described earlier. These tuning ratios always had values >0. These two approaches produced similar values for complex neurons; however, tuning ratios for simple neurons by our analysis were always zero. Note that tuning ratios in this study were obtained from the 3D maximum–minimum plane. In some cases, 2D tuning ratios from single planes were computed for comparison.
Simple and complex models are readily differentiated in spatial tuning plots. The sensitivity-orientation curves of simple neurons followed the cosine rule with a definite Stmin = 0, or null, in every great-circle plane (Fig. 4C), whereas it was not the case for complex neurons (Fig. 6B; also see Fig. 2 in Chen-Huang and Peterson 2006). In addition, the phase-orientation curves of simple neurons stepped between two values with a 180° interval on any given plane, whereas a gradual phase shift was observed in complex neurons. The two identical peaks in sensitivity plots corresponded to peak responses in the excitatory and inhibitory directions. Since otolith afferents lead acceleration by <30° and that central processing appears to introduce phase lag, not additional phase lead, excitatory responses were defined to be in phase with acceleration (±45 around phase 0°) or velocity (±45 around −90°), i.e., between −135° (lag) and +45° (lead) of acceleration.
Fig. 6.
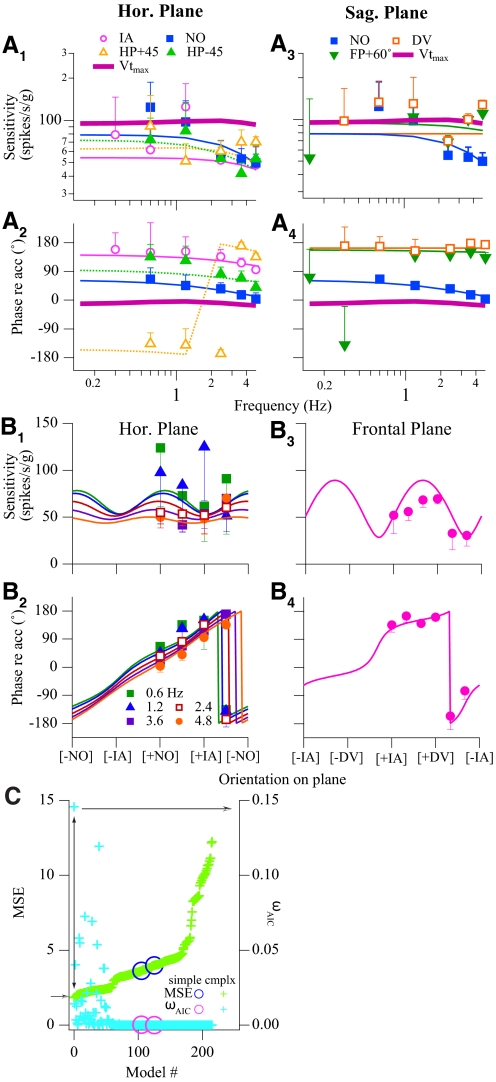
Summary of modeling results for the VOTS neuron shown in Fig. 5. The chosen model had the following parameters: Sx = −54.2, τ1-x = 0.023, kx = 0.21; Sy = 78.7, τ1-y = 0.041, ky = −0.35; Sz = −78.7, kz = 0.10. A: Bode plots, i.e., sensitivity and phase vs. frequency, of actual (markers) and modeled (thin solid or dashed lines) responses along the IA, NO, DV, HP +45°, HP −45°, and FP +60° axes. The estimated responses in maximum translation response vectors (Vtmax) are also shown (thick purple lines). B: spatial tuning property of the same VOTS neuron in the horizontal (0.6–4.8 Hz) and frontal (2.4 Hz) planes. Actual (markers) and modeled (lines) responses are plotted against orientation angle on each plane. The selection of this complex model, which accounted for 90% of variance, was deemed necessary, as shown in C. Normalized MSEs (left ordinate) and ωAIC (right ordinate) obtained from all successfully converged models are plotted according to the ascending order of MSE. The complex model that produced a low MSE and high ωAIC (crosses linked by the double arrowheaded line) was chosen (see text). Error bars (SEs) in A and B are shown in one direction and may be obscured by symbols.
Fig. 2.
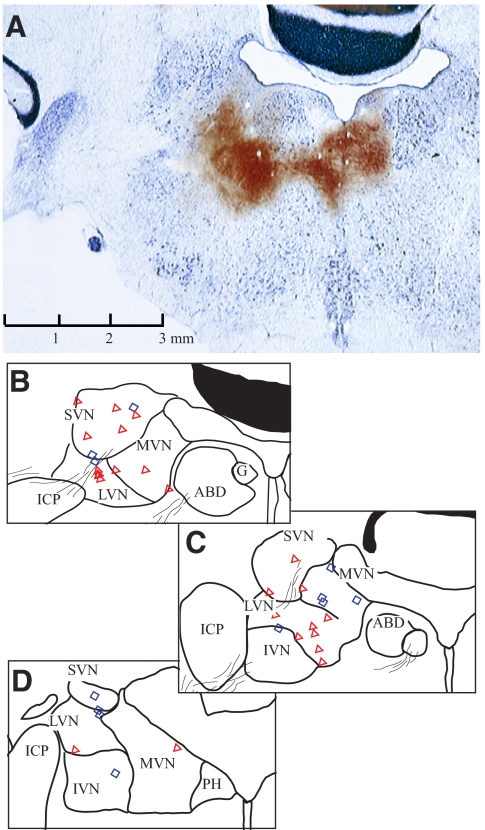
Anatomical locations of recorded vestibular neurons from one squirrel monkey. Cerebellum and brain stem were sectioned in the coronal plane of electrode penetration, tilting 22° dorsocaudally and 15° laterally with respect to the stereotaxic plane. The brain was sliced at 40 μm intervals. Every fourth section was stained with Perls/3,3′-diaminobenzidene (Perls/DAB, brown), whereas its next adjacent section was stained with thionine (blue). A: confirmation of recording sites with electrolytic lesions (see methods). Currents were injected at 2 sites, 2 mm apart, near the left abducens nucleus. The only one section where electrolytic lesions were revealed with Perls/DAB stain was superimposed on its adjacent section with thionine stain. B–D: line drawings, with reconstructed neurons' locations, of sections corresponding to A (B) and the following 2 sections at 0.48 (C) and 1.28 (D) mm caudal to A. Neurons located within 0.48 mm anterior/posterior to A–B are shown on B (symbols). Neurons at 0.5 to <1 mm caudal to A are superimposed on C, whereas those at 1–2.5 mm caudal are shown in D. Neurons are marked as simple (red triangle) or complex (blue diamond) types. Calibration in A applies to all sections. ABD, abducens nucleus; SVN/MVN/LVN/IVN, superior/medial/lateral/inferior vestibular nucleus; ICP, inferior cerebellar peduncle; G, genu of facial nerve; PH, nucleus prepositus hypoglossi.
Simple and complex neurons exhibited a distinctive frequency-related sensitivity/phase relationship (Bode plots). The slope of sensitivity versus frequency curves was constant and the phase-frequency curves were fixed in simple neurons, regardless of stimulation directions (Fig. 4B), since simple responses had the same transfer functions in all directions. As such, response dynamics along the optimal Stmax direction (Vtmax) was identical to other directions. In contrast, the response dynamics of complex neurons demonstrated a clear interaction between frequency and translation directions (Fig. 6A). The family of sensitivity curves did not show a constant trend across frequencies, nor did phase curves remain fixed with respect to frequency because the direction of translations changed. Furthermore, Vtmax was not invariant across frequencies (Fig. 9, arrow) and response dynamics along the Vtmax was different from responses along other directions (Fig. 6A, thick lines).
Fig. 9.
Projections of frequency-related Vtmax for complex neurons onto the horizontal (A), sagittal (B), and frontal (C) planes. Symbols indicate neuron's dynamics, whereas colors/line styles code frequency-related migration of Vtmax. Vectorial length and polar angle denote the projected component magnitude and direction onto each plane. Gray thin lines in A illustrate great circles (meridians) through ±IA pole sliced at 15° interval. The projected circles of latitude (dashed lines) correspond to vectorial angular deviation of 30° (radius = 0.87) or 60° (r = 0.5) away from a given plane. The neuron tagged by an arrowhead was the same neuron shown in Figs. 5 and 6. The map was tilted forward 15° from the stereotaxic plane, as how the animal's head was held.
3D ROTATIONAL RESPONSES.
The trial-by-trial analysis of rotational responses was handled the same way as translational responses in trial-by-trial analysis. However, rotational responses about all three orthogonal axes—yaw, pitch, and roll—and occasionally two additional left anterior–right posterior (LARP) and right anterior–left posterior (RALP) axes, for neurons in this report were available only at 1.2 Hz (some lower-frequency tests were available but only along one axis). Initial fitting results indicated that the weighted LSE function could not differentiate complex versus simple models based on this data set. Thus simple models were used in fitting rotational neural responses (NRr, in spikes·s−1·deg−1·s−1)
| (5) |
where αr and βr express azimuth and elevation angles of rotation axes and SFr(sr) represents a first-order system with one pole term (τr) and a fractional phase shift component (λr; −0.5 ≤ λr ≤ +0.5) (see appendix, Eq. A1) with respect to angular velocity. The DC sensitivity along each rotational axis krx, kry, and krz; the time constant (τr); and the fractional term (λr) were solved by a weighted LSE fitting procedure. The unitary rotational maximum vector Vrmax and its related sensitivity (Srmax) and phase (ϕrmax) at 1.2 Hz were directly derived as
RESULTS
A total of 289 neurons were recorded from the superior/medial/lateral/inferior vestibular nuclei (SVN/MVN/LVN/IVN) of two squirrel monkeys. Most neurons were located within 3 mm posterior and 2.5 mm lateral to the left abducens nucleus (Fig. 2). The location of recordings was confirmed in one animal in which electrolytic lesions were made at the conclusion of experiments (Fig. 2A). Stimulation of vestibular afferents was performed in the other animal. Short-latency field potentials (N1 ≅ 0. 6–0.8 ms), similar to those shown in Fig. 3A, were always evoked from the ipsilateral ear in those penetrations where translation-related neurons were recorded.
Fig. 3.
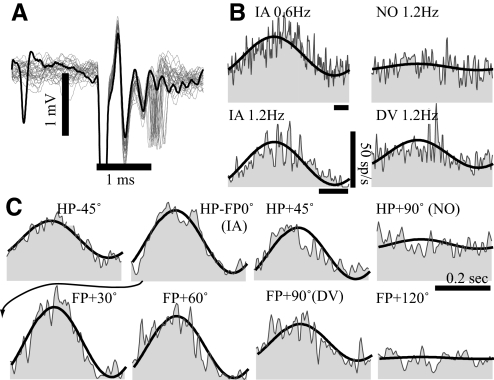
A typical simple vestibular-only translation sensitive (VOTS) neuron. A: action potentials (traces) evoked by 0.1 ms, 150 μA of cathodal electrical pulses delivered via a pair of chronically implanted labyrinthine electrodes in the ipsilateral ear. Occasional failure (thick trace) due to the refractory inhibition left the field potential intact. The latency for field potentials and action potentials was 0.7 and 0.9–1.3 ms, respectively. B and C: averaged responses of the same VOTS neuron to 0.6–1.2 Hz, 53–106 cm/s2 translations along the interaural (IA), nasooccipital (NO), and dorsoventral (DV) axes (B) and 2.4 Hz, 210 cm/s2 sinusoidal translations on the horizontal plane (HP) and frontal plane (FP) (C). Neural responses (shaded), aligned in time to the start of positively directed acceleration, are superimposed with the model fits (solid lines) chosen from the frequency dependent spatiotemporal fit. See Fig. 1 for the chosen coordinate frame. Time calibration bars in B and C denote 0.2 s. The arrow indicates the placement of the IA response in the frontal plane series.
Trial-by-trial analysis (see methods) was performed in all 289 neurons and results were tabulated. However, only data from 67 neurons, including 20 eye movement–related neurons, were deemed sufficient for the frequency-dependent spatiotemporal analysis (see methods), which required ≥10 translational experiments conducted with various frequency and movement directions. A statistical analysis with reduced fit parameters is under development to examine the remaining data set (unpublished data). Results from the 47 vestibular-only translation sensitive (VOTS) neurons are included in this report. Nine neurons were tested in the one monkey that had chronically implanted bilateral labyrinthine simulating electrodes. Five neurons responded to labyrinthine simulation at monosynaptic (0.8–1.2 ms) and one at disynaptic (1.3–1.8 ms) latency. Among the neurons receiving monosynaptic inputs from the ipsilateral labyrinths, four were simple VOTS neurons not sensitive to rotations and one had a complex translation response and responded to vertical axis (VA) rotation.
Neurons with simple tuning properties
Figure 3 illustrates this approach on a VOTS neuron that was classified as exhibiting simple tuning properties. This neuron appeared to receive monosynaptic inputs from vestibular afferents since ipsilateral labyrinthine stimulation at fourfold threshold current (150–200 μA) evoked spikes with 0.8–1.2 ms latency (Fig. 3A). Otolith afferents were the most likely source of the monosynaptic drive since this neuron, along with three other monosynaptic neurons, responded only to translations, not to rotations. Common features of these four electrically identified neurons were strong excitatory responses to contraversive IA motions and a phase lag, −8 to −59°, with respect to the contraversive linear acceleration.
Responses to translations were tested under 12 conditions, including intermediate orientations on the horizontal plane (HP) and frontal plane (FP). Neural responses were temporally aligned with respect to stimulus acceleration (not shown). Averaged responses to 14 cm/s translations at 0.6–2.4 Hz are shown in Fig. 3, B and C, superimposed with the predicted responses (thick lines) from the best model chosen by criteria described in methods. Weighted mean square errors (MSEs, left ordinate, symbols in blue-green) and normalized Akaike criteria (ωAIC, right ordinate, symbols in magenta-light blue) from model fits are plotted in Fig. 4A. Better-fit models had higher ωAIC values and lower MSEs that clustered near the left axis since models were plotted in ascending order of their MSEs. The highest ωAIC (0.65) was obtained from a simple model with a very low MSE (circles in magenta and blue paired by the arrowheaded line). In addition, an F test showed that this simple model, which accounted for 98% of weighted variance, was statistically indistinguishable from the lowest χ2 model (P = 0.58).
Estimated responses from this chosen model, incorporating a pole (t = 2.7 s) and a zero (t = 0.64 s), are shown in Fig. 3, B and C (thick lines), overlaying the averaged single-cycle neural responses. The predicted (lines) and actual responses (symbols) versus frequency (Bode plot) are plotted in Fig. 4B. The actual (symbols) and predicted (lines) responses to 2.4 Hz translations in the HP and FP are plotted in Fig. 4C. These results showed that a simple cosine model was sufficient to model the responses of this VOTS neuron.
An important property of neurons exhibiting simple tuning is that their response dynamics is independent of movement orientations. As shown in Fig. 4B, the slope of frequency-dependent sensitivity reduction (−9.4 dB/decade) for this VOTS neuron was constant across multiple translation directions, including the direction that was expected to produce the maximum sensitivity (Vtmax: α = 8°, β = 30°). Similarly, the relationship between response phase and frequency was fixed regardless of translation directions, except for shifting by 180° when the stimulus changed from an excitatory to an inhibitory direction. At any given frequency, the sensitivity versus orientation curves followed the cosine rule where sensitivity null was located 90° from the Vtmax (Fig. 4, C1 and C3). Response phases remained constant for orientations within ±90° of Vtmax and changed by 180° during translations in the opposite direction (Fig. 4, C2 and C4).
The quantity Stmin/Stmax (sensitivity tuning ratio), or rather its 2D projection, has been used extensively in previous studies as the indicator of spatiotemporal tuning behavior. Based on fits to data obtained from 2.4 Hz, single-plane experiments, the 2D tuning ratios were 0.23 and 0.16 for the HP and FP, respectively (Fig. 4C, black dotted lines). A single 3D tuning ratio from the lowest MSE model (see reconstruction of frequency-dependent neuronal tuning to translations in 3d space in methods) for this neuron was 0.06, 0.09, and 0.15 at 0.6, 1.2, and 2.4 Hz, at which experiments were conducted. These 3D ratios, although varied across frequencies, were within the range of simple neurons, Stmin/Stmax <0.3, in our previous 3D study. However, the 2D ratios exceeded the 0.1–0.15 values designated as simple neurons reported by other labs. This example illustrates the plane dependence of 2D ratios and the inexact relationship between 2D and 3D tuning ratios. Differences between 2D and 3D tuning ratios were previously reported (Chen-Huang and Peterson 2006).
Neurons with complex tuning properties
Spatial and frequency-related responses of complex neurons could not be adequately modeled with the simple cosine law. An exemplary complex neuron is shown in Fig. 5, where experiments were performed over 35 different combinations of stimulus frequencies and directions. Important features differentiating complex from simple neurons are 1) frequency-related response dynamics changed with orientations and the Vtmax changed with frequency, 2) response phases shifted as a function of spatial orientations, and 3) the modulation of sensitivity in most planes did not have a clear null. These results are summarized in Fig. 6, where neural responses are plotted with respect to frequency (Fig. 6A) and spatial orientation (Fig. 6B). In contrast to the Bode plots in Fig. 4B, this neuron's sensitivity as a function of frequency was modulated by the orientation of translations (Fig. 6, A1 and A3) and the sensitivity–orientation curves did not have a null on most planes (Fig. 6, B1 and B3). The gradual shift in phases was observed in most planes (Fig. 6, A2 and A4 and B2 and B4).
Fig. 5.
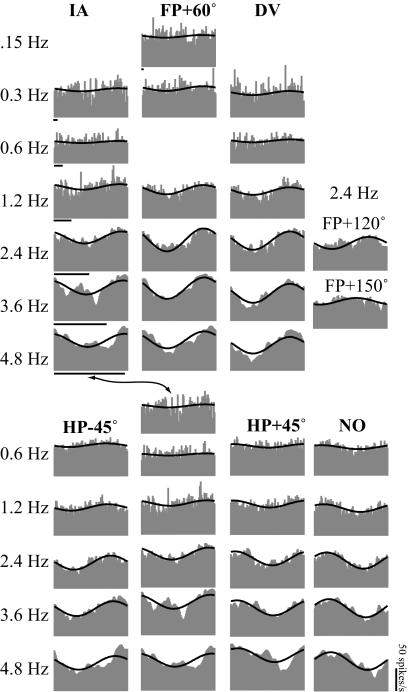
Averaged responses of a complex VOTS neuron to 0.15–4.8 Hz, 38–300 cm/s2 sinusoidal translations along IA, NO, and DV axes and along intermediate orientations on the horizontal plane (HP) and frontal plane (FP). Neural responses (shaded), aligned with the acceleration in time, are superimposed with the model fits (solid lines) chosen from the frequency-dependent spatiotemporal fit. Time calibration bars denote 0.2 s. The firing rate calibration bar applies to all graphs. See Fig. 1 for the chosen coordinate frame. Note that IA responses are repeated and presented in both plane series (arrow).
The frequency-dependent spatiotemporal analysis confirmed that complex models were necessary to fit the responses of this neuron. As shown in Fig. 6C, fits with higher ωAIC (magenta-light blue) and lower MSE (blue-green) were obtained from complex models. The best model (solid lines in Figs. 5 and 6, A and B) with the highest ωAIC accounted for 90% of the weighted variance. Furthermore, F tests rejected both simple models whose fits converged successfully (paired circles in magenta and blue in Fig. 6C). As predicted by spatiotemporal convergence, this neuron's Stmax and ϕtmax (thick purple lines in Fig. 6A) at test frequencies manifested response properties different from those observed at other orientations. Although this neuron's Vtmax shifted with frequency (see Fig. 9 for more details), its maximum responses were nearly in phase with acceleration and flat across frequencies. Finally, its 3D tuning ratios exhibited considerable variation, 0.28–0.55, for experiments in 0.15–4.8 Hz.
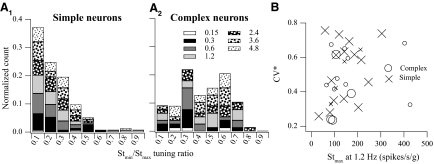
Response dynamics
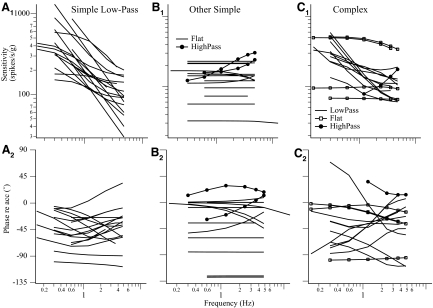
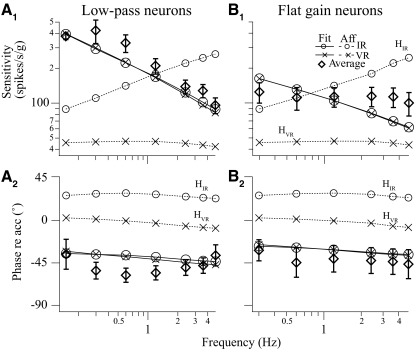
Of 47 neurons where the comprehensive frequency-dependent spatiotemporal analysis was performed, simple cosine tuning models were sufficient for 32 neurons, whereas complex models were necessary for 15 neurons. The chosen models accounted for 67–98% (median = 89%) of weighted variance. The percentage of neurons, 32%, exhibiting complex tuning properties was comparable to that of our previously published results. As demonstrated earlier, the response dynamics of simple neurons was described by a single transfer function regardless of translation directions. The slope of sensitivity versus frequency at Vtmax in the range of frequencies tested in most neurons was <20 dB/decade (Fig. 7), i.e., one pole or zero, although a higher-order system might be needed to model their overall behavior.
Fig. 7.
Response dynamics of the 47 VOTS neurons along the Vtmax estimated from their respective chosen model based on the frequency-dependent spatiotemporal analysis. The Stmax and ϕtmax of low-pass VOTS simple neurons are plotted in A, whereas those of the flat-gain (line) and high-pass (circles) simple neurons are plotted in B. Likewise, the predicted Stmax and ϕtmax of complex neurons that exhibited low-pass, high-pass, or flat dynamics are plotted in C. Responses were reconstructed at the frequency at which neural recordings were made. Neurons with an averaged sensitivity slope ≤4 or ≥ −4 dB/decade were classified as flat dynamics, >4 dB/decade were high-pass, and < −4 dB/decade were low-pass.
The predicted frequency-dependent responses of VOTS neurons, calculated at Vtmax from the chosen models, were grouped into flat, low-pass, or high-pass types if the slope of sensitivity versus frequency was within 0 ± 4, < −4, or >4 dB/decade. Seventeen simple neurons exhibited low-pass, 13 neurons flat, and 2 neurons high-pass responses (Fig. 7, A and B). Of 15 complex neurons, 4 neurons exhibited flat, 10 low-pass, and one high-pass response (Fig. 7C). Results of ANOVA (simple/complex model × dynamics, two-way, random model) on Stmax across frequencies, excluding the high-pass neurons and 0.15 Hz experiments due to insufficient sample size, showed significance only in the interaction effect, i.e., the sensitivity of simple flat neurons was lower than that of all other types. In contrast, results of ANOVA on ϕtmax showed a significant effect due to dynamics, i.e., low-pass neurons lagged flat neurons, and a significant effect due to spatiotemporal tuning, i.e., simple neurons lagged more than complex neurons, as well as an interaction effect (P < 0.05). In general, low-pass simple neurons tended to lag more than neurons exhibiting complex, flat dynamics (Fig. 7).
In short, complex and simple tuning neurons shared similar dynamic characteristics, where neurons with low-pass responses were more common than those with flat responses, and high-pass neurons were the least numerous. The response dynamics of VOTS neurons with respect to those of published vestibular afferents will be compared in the discussion.
Spatial tuning properties
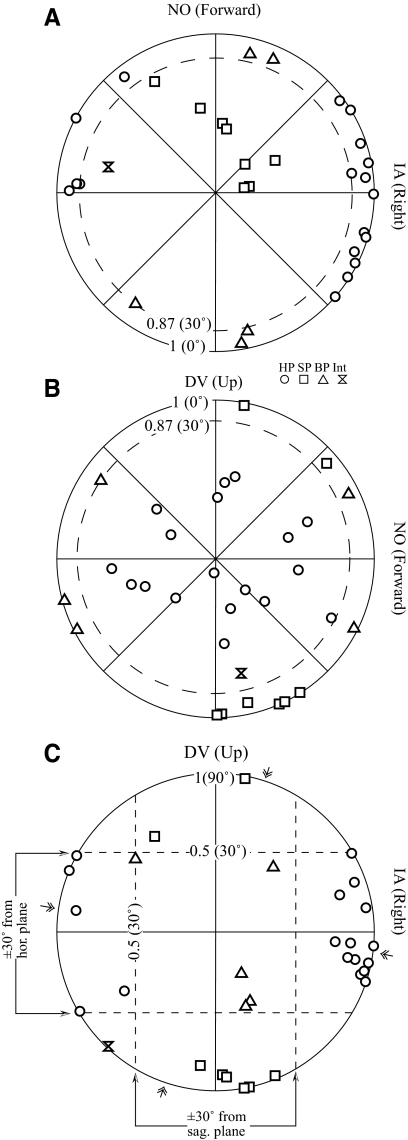
Since the polarization vectors of most otolith afferents lie near the plane of utriculus or sacculus, which are nearly coplanar with the horizontal and sagittal planes (see discussion), we next examined the spatial distribution of predicted Vtmax by plotting their projections onto the horizontal (Fig. 8A), sagittal (Fig. 8B), and frontal (Fig. 8C) planes. Vectors that lay within 30° of the horizontal plane or sagittal plane would have projection lengths >0.87 on these planes (dashed circle, Fig. 8, A and B) and fall within the ±30° latitude parallels on the frontal plane (dashed lines, Fig. 8C). Of 32 simple neurons, 18 had vectors near the horizontal plane (circles) and 8 had vectors close to the sagittal plane (squares). An additional 5 neurons were within 30° of the horizontal and sagittal planes (both-plane or BP neurons; triangles) and thus cannot be unambiguously assigned to either the horizontal plane or the sagittal plane. Finally, one neuron had a vector that was not close to either the horizontal or sagittal plane (intermediate plane). Thus the Vtmax of all but one simple neuron was closely aligned with the horizontal plane and/or the sagittal plane.
Fig. 8.
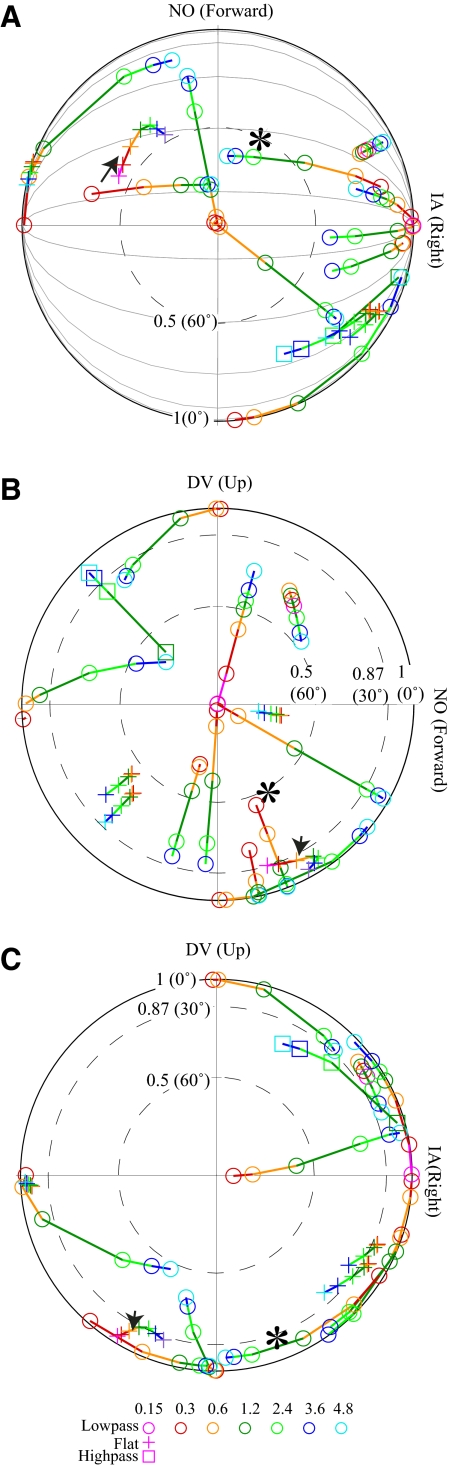
Projections of Vtmax for simple VOTS neurons onto the horizontal (HP, A), sagittal (SP, B), and frontal (FP, C) planes. Vectorial length and polar angle denote the projected component magnitude and direction onto each plane. Simple neurons were classified as HP or SP type if the angular deviation of Vtmax from the horizontal or sagittal plane, respectively, was <30°. Thus the length of projected vectors onto these planes was ≥0.87 (dashed circles, A and B) and fell within the ±30° latitude parallels on the FP (dashed lines, C). Both-plane (BP) neurons lay near both planes, whereas one intermediate (Int) neuron was near neither. Symbols indicate neuron's planar preference. The map was tilted forward 15° from the stereotaxic plane, as how the animal's head was held. Double arrows on the perimeter of polar plot C denote the mean polarization vectors of otolith afferents recorded from the superior and inferior vestibular nerves (Fernandez and Goldberg 1976b).
The distribution of Vtmax in simple neurons on the horizontal plane (Vtmax–HP), sagittal plane (Vtmax–SP), and frontal plane (Vtmax–FP) was not uniform. The Vtmax–HP of most horizontal neurons (circles) clustered ±45 within rightward (contraversive; n = 13) or leftward IA (ipsiversive; n = 6). Thus 73% simple-tuning horizontal plane VOTS neurons preferred contraversive translations, corresponding to the polarization vectors of the medial part of ipsilateral utriculus. The mean Vtmax–FP tilted 1 ± 5° ventromedially from the horizontal plane and 8 ± 5° dorsolaterally from the sagittal plane for HP and SP neurons, respectively, similar to that of otolith afferents (arrows in Fig. 8C). No difference was observed in the resting discharge (65 ± 16, 57 ± 19, 59 ± 21 spikes/s, P > 0.6, Kruskal–Wallis test) among SP, HP, and BP neurons. The mean Stmax of SP and HP neurons at 2.4 Hz (145 ± 28 vs. 155 ± 15 spikes·s−1·g−1) were similar, whereas BP neurons had a lower sensitivity (107 ± 19 spikes·s−1·g−1). On average, SP neurons lagged acceleration (−51 ± 12°) more than HP (−36 ± 9°) and BP (−25 ± 13°) neurons. These differences were not statistically significant (P > 0.2, Kruskal–Wallis test).
Since the orientation-dependent response phase varied across frequencies in complex neurons (e.g., Fig. 6), it was expected from the linear summation of spatiotemporal responses that their maximum response direction Vtmax would change with frequency. This frequency-dependent Vtmax was manifested by changes of component magnitude (MHP, MSP, MFP) and direction (δHP, δSP, δFP) in the projections onto canonical planes. For example, the neuron marked with an arrowhead (Fig. 9), which was described in Figs. 5 and 6, had moderate changes in its Vtmax–HP and Vtmax–SP. In contrast, Vtmax–HP of the neuron marked with * changed significantly in component magnitude and direction (MHP = 0.86–0.36, δHP = 13.2–82.5°, 0.3–4.8 Hz), whereas its Vtmax–SP maintained a constant δSP (−69°) in spite of the big change in MSP (0.55–0.99). In other words, the frequency-dependent Vtmax followed a path in which the projection on HP traversed in arc, whereas that on SP traveled radially on the polar coordinate. This migration pattern can be easily visualized as following the spherical surface of a great circle connecting the ±IA axis (gray lines in Fig. 9A) and was restricted mostly to the frontal plane (Fig. 9C).
With this new insight, we calculated changes in the component magnitude and direction of the frequency-dependent Vtmax–HP, Vtmax–SP, and Vtmax–FP and estimated the frequency-dependent migration “path” followed by each complex neuron. An overall centering pole was identified if the maximum frequency-dependent change in direction was ≤15° and the change in component magnitude was >0.1 on the orthogonal plane. For example, the frequency-dependent Vtmax was determined to travel along the ±IA–pole meridian if the change in δSP was <15° and in MSP was >0.1 (see neuron tagged with *).
The frequency-dependent Vtmax path of each complex neuron was successfully mapped onto one of the meridians centering IA, DV, or NO, except for one neuron (arrowhead) whose projected Vtmax changed >15° in all canonical planes. As shown in Fig. 9, three neurons traversed in DV–pole meridians (Fig. 9A), 10 neurons, including the one marked with *, in IA–pole meridians (Fig. 9B), and one neuron in NO–pole meridians (Fig. 9C). The IA group of neurons not only outnumbered other groups combined, its predominant preference toward IA at low frequency was especially striking. This observation, where the Vtmax of some complex neurons transitioned from the DV axis at higher frequency toward the horizontal plane, especially the IA axis, at lower frequency revealed a novel spatio-filtering mechanism in processing otolith-related signals.
3D tuning ratio and coefficient of variation
In this study, the spatiotemporal tuning properties of VOTS neurons were determined by testing the null hypothesis that complex spatiotemporal models were significantly superior to simple models. Next, we examined whether this approach produced similar results to previous reports in which neurons were classified based on their tuning ratios, computed from unconstrained fits (see reconstruction of frequency-dependent neuronal tuning to translations in 3d space in methods), and how simple and complex neurons exhibited differential population distribution of tuning ratios across frequencies. As shown in Fig. 10A, the distribution of 3D tuning ratios, normalized with respect to the total population count in each class, was different between simple and complex neurons. The 3D tuning ratio rarely exceeded 0.3 at any given translation frequency for simple neurons. The 3D tuning ratios in complex neurons had higher values and a wider dispersion. This result that a significant fraction of complex neurons (∼40%) had 3D tuning ratios ≤0.3 was not surprising since complex neurons might exhibit simple tuning property in a narrow frequency range and thus tend to have broadly scattered tuning ratios.
Fig. 10.
A: distribution histogram of the 3D tuning ratio, Stmin/Stmax, in simple (A1) and complex (A2) neurons at various frequencies (see legend). The ordinate lists count fractions normalized with respect to the total number of cases in each class. B: distribution of normalized coefficient of variation (CV*), to t = 15 ms, with respect to the maximum translation sensitivity (Stmax) at 1.2 Hz. Simple (cross) and complex (circle) VOTS neurons that exhibited significant differential responses during 0.3 Hz tilts and translation are emphasized with larger symbols.
The mean CV* (0.50 ± 0.18 vs. 0.45 ± 0.16), mean interspike intervals (25 ± 4 vs. 18 ± 2 ms), or background discharge rate (60 ± 6 vs. 58 ± 11 spikes/s) was not significantly different between simple and complex neurons. Further, similar to the observation in vestibular afferents, CV* was not correlated with Stmax in VOTS neurons (Fig. 10B).
Rotational responses
Rotational responses about the yaw, pitch, and roll axes (see Fig. 1 and 3d rotational responses in methods) were recorded in all VOTS neurons (n = 47) at 1.2 Hz and other additional frequencies (0.15, 0.3, or 0.6 Hz). Twenty-one neurons showed significant responses at 1.2 Hz (R2 ≥ 0.5, sensitivity ≥2SE) to vertical axis (VA) (yaw, n = 6) or horizontal axis (HA) (pitch/roll and/or LARP/RALP, n = 15) rotations. The mean maximum rotational sensitivity (Srmax, 0.9 ± 0.2 vs. 0.9 ± 0.1 spikes·s−1·deg−1·s−1) and phase (ϕrmax, −5 ± 9 vs. 32 ± 12° with respect to angular velocity) at maximum rotation direction (Vrmax) were similar between HA- and VA-sensitive VOTS neurons. Three HA neurons also responded weakly to VA rotations, but none of the VA neurons responded significantly to HA rotations.
Two thirds of complex neurons (10/15) and one third of simple neurons (11/32) responded to rotations. Further, although a roughly equal fraction from either class of neurons, 4/32 versus 2/15, responded strongly to VA rotations, complex neurons were significantly more likely to respond to HA rotations (8/15) than simple neurons (7/32, P < 0.05, two-tailed Fisher's exact test). There was no difference in rotational sensitivity or phase between simple or complex neurons that responded to rotations.
The rotational vectors on the pitch–roll plane (Vrmax–HP) of 15 HA rotational VOTS neurons are shown in Fig. 11A. There was a significant preponderance of HA neurons tuning near the left anterior/right posterior canal (LAC–RPC) plane (n = 12) versus the left posterior/right anterior canal (LPC–RAC) plane (n = 3; P = 0.003). The Vtmax–HP of the same 15 neurons at 1.2 Hz is plotted in Fig. 11B. Note that rotational and translational vectors are graphed for the ease of comparing the effects of gravitation-inertia forces, such that pitch-up and left ear-down are aligned with forward NO and rightward IA, respectively.
Fig. 11.
Projections of unitary maximum rotational (Vrmax–HP, A) and translational (Vtmax–HP, B) response vectors during 1.2 Hz 20°/s rotation or 14 cm/s translation onto the horizontal plane. Only neurons that responded significantly to 1.2 Hz HA rotations are shown. The polar graph for Vrmax–HP was remapped so that the direction of gravitation-inertial force (GIF) during rotation and translation was aligned, i.e., pitch-nose up rotation to forward translation and right ear down (RED) rotation to leftward translation. Rotation vectors corresponding to left anterior/posterior canal (LAC/LPC) and right anterior/posterior canal (RAC/RPC) are also shown in A. C: comparison of the orientation of Vtmax–HP and Vrmax–HP in HA rotation sensitive VOTS neurons (symbols). The line of unity denotes congruent spatial responses of VOTS neurons to HA rotations and GIF. Distance between tick marks is 90°. The polar angle for each orientation was chosen to minimize angular differences between paired Vtmax–HP and Vrmax–HP. D: distribution of the angular alignment differences between Vrmax–HP and Vtmax–HP in selected HA rotation VOTS neurons whose Vtmax at 1.2 Hz was not predominantly along the DV axis, i.e., the magnitude of vector length on the horizontal plane >0.6 (dashed line in B).
As a population, there was no correlation between Vtmax and Vrmax on the horizontal plane at 1.2 Hz for VOTS neurons sensitive to HA rotations (P > 0.1, nonparametric circular correlation; Fisher and Lee 1982; Fig. 11C). For further examination, the angular alignment between Vtmax and Vrmax on the horizontal plane at 1.2 Hz, defined as δ(t − r)HP = δtHP − δrHP by angle subtraction, showed two noticeable features : 1) the Vtmax of many vertical canal VOTS neurons (n = 7; 3 simple and 4 STC neurons) pointed out of the horizontal plane and was predominantly (component magnitude >0.6; dashed line in Fig. 11B) aligned with the DV, such that LAC neurons (n = 4) responded to downward DV, whereas RPC neurons (n = 2) responded to upward DV. This congruent pattern of alignment between Vtmax and Vrmax suggests that these neurons play a role in mediating compensatory rotation/translation motor functions. Oppositely directed Vtmax and Vrmax were also observed in one LPC neuron (up pitch–downward DV). 2) There appeared a pattern in the alignment of Vrmax–HP and Vtmax–HP in the remaining 8 vertical canal neurons (4 complex and 4 simple) (Fig. 11D). The Vtmax–HP of simple neurons (squares) were in the opposite orientation from Vrmax–HP, whereas a closer alignment was seen in complex neurons (circles, Fig. 11D). The low probability (P = 0.07, two-tailed Fisher's exact test) in this small number of sample neurons revealed an important tuning pattern differentiating simple versus complex VOTS neurons. The presence versus the lack of a synergistic alignment between Vtmax–HP and Vrmax–HP, as observed in complex versus simple neurons, indicates differential otolith–canal interaction mechanisms at brain stem.
Similarly, a synergistic alignment between Vtmax and Vrmax was observed in all but two horizontal canal VOTS neurons. Four neurons responded maximally to rightward IA and rightward rotation, whereas two neurons responded to leftward yaw and rightward IA. No difference was observed between simple and complex neurons.
Rotational versus translational responses at lower frequency
Responses to low-frequency (0.15–0.3 Hz) dynamic tilts and translations were compared in 32 of the reported VOTS neurons. Pairs of tilt versus translation (e.g., roll vs. IA, pitch vs. NO, or LARP vs. HP −45°, etc) were tested, regardless of whether the neuron was sensitive to higher-frequency rotations. The paired movements were chosen based on each neuron's translational sensitivity observed during 1.2 or 2.4 Hz experiments. In other words, although the selected translational orientation might not be the absolute “maximum,” as determined by the off-line calculation, it was always larger than other tested directions.
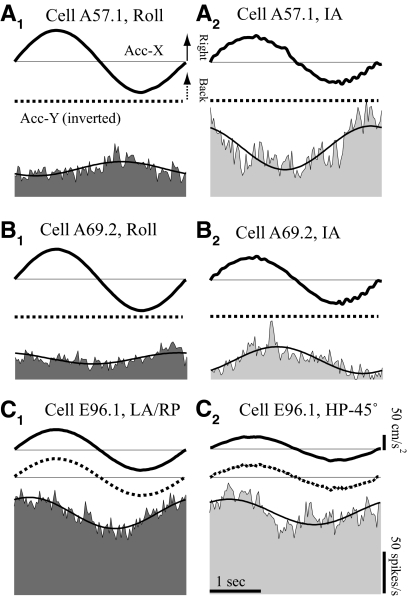
The comparison of responses to 0.3 Hz tilt/translation is illustrated for three neurons in Fig. 12. The first, simple neuron (Fig. 12A) did not respond to HA rotations at 1.2 Hz, whereas the other two neurons (Fig. 12, B and C) modulated during HA rotations and exhibited complex tuning properties. During 0.3 Hz roll, the sensitivity of the simple neuron in Fig. 12A to the gravitation-inertial force (GIF) was significantly reduced relative to that during IA translation. Similarly, the complex neuron in Fig. 12B exhibited significantly different responses to the IA translation and roll rotation. In contrast, the difference on the second complex neuron (Fig. 12C) was not significant.
Fig. 12.
Exemplary VOTS neurons in which diverse GIF-related responses during 0.3 Hz HA rotations and HP translations were observed. The GIFs produced during 0.3 Hz HA rotations and horizontal plane translations are similar in profile. Responses to 0.3 Hz, 10°/s roll (A, B) or left-anterior–right-posterior (LA–RP, C) rotations and to 0.3 Hz 75 cm/s2 IA (A, B) or HP −45° (C) translations are shown for a simple (A) and 2 complex (B, C) VOTS neurons. The peak effective GIF in the horizontal plane, i.e., the vector sum of accelerations along the IA (Acc-X, thick solid lines) and NO (Acc-Y, thick dashed lines) axes recorded by a head-mounted triaxial accelerometer, in roll/LA–RP was 0.092 and 0.076 g in translations. Average neural responses (shaded) are superimposed with sinusoidal fits. For graphing purposes, Y-axis (NO) acceleration records are inverted so that neural responses are aligned with rightward IA and backward NO. Calibration bars in C apply to all graphs.
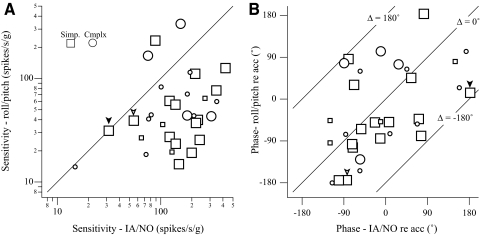
Response sensitivity and phase with respect to linear acceleration due to changes in the gravitation force during HA rotations were compared with those obtained during the same frequency translations in 32 neurons (Fig. 13). Vectorial differences between paired utriculus-related responses to 0.3 Hz IA/NO translations and to roll/pitch were computed for each neuron. In most cases, neural sensitivity during 0.3 Hz roll/pitch was lower than that during translation (Fig. 13A). The phase was widely distributed, although roll/pitch responses tended to lag translation responses by about 90° (Fig. 13B). Next, a paired t-test was performed on the magnitude of the vectorial differences between dynamic tilts and translation responses for each neuron. Note that the vectorial differences for neurons with similar sensitivity during tilts and translations (marked with inverted arrowheads in Fig. 13A) might reach significance if the phase differences were large (Fig. 13B). This tilt–translation difference was significant in 15/19 simple neurons (P < 0.05; Fig. 13). In contrast, only 4 of 13 complex neurons exhibited significantly different responses to tilt and translation. Thus as a population, complex neurons were significantly different from simple neurons in their responses to 0.3 Hz tilt versus translation (P = 0.01, two-tailed Fisher's exact test).
Fig. 13.
A: paired comparison of response sensitivity (A) and phase (B) tested during 0.3 Hz, 0.076 g, horizontal plane translations vs. 0.3 Hz 10°/s HA rotations that produced similar GIFs (0.092 g). Responses were computed as raw modulation (spikes/s) with respect to effective GIFs (g) evoked during translations and rotations. Thus the sensitivity and phase during rotations are expressed with respect to linear acceleration, not the customary angular velocity. The line of unity in A denotes equality in their rotation vs. translation responses. The lines in B demarcate phase differences of −180, 0, and +180°. Phase values are expressed with respect to the positive, not necessarily the excitatory, stimulus direction, i.e., rightward/forward translations. Neurons responding to paired 0.3 Hz dynamic tilts and translations with significant difference (paired t-test) are noted with larger markers. The inverted arrowheads highlight 2 neurons where the sensitivities to tilts and translations are similar yet phase differences are very large.
Although complex neurons were more likely than simple neurons to respond to HA rotations (see preceding text), HA–rotation VOTS neurons were no more likely to respond differently to 0.3 Hz tilts and translations, as confirmed by the nonparametric analysis (contingency table, IGOR Pro statistics package; Zar 1998) of the two categories, spatiotemporal tuning type (χ2 = 5.36, P < 0.05) and HA rotation (χ2 = 0.26, P = 0.6), on tilt versus translation responses. In short, only the spatiotemporal tuning type, not rotational responses or other parameters tested, was significantly correlated with differential tilt versus translation responses.
DISCUSSION
Results of this study raise the possibility that complex and simple VOTS neurons constitute distinctive vestibular pathways where complex neurons are well suited to drive tilt-related responses, whereas simple neurons mediate translation-related responses. Complex VOTS neurons exhibited frequency-related path migration that, in most cases, appeared to follow along IA–pole meridians that produced IA-bound low-frequency gain enhancement. Furthermore, complex VOTS neurons were more sensitive to mid-frequency HA rotations and more likely to respond similarly to changes in GIF during low-frequency dynamic tilts and linear acceleration during translations. In contrast, the maximum translation vectors of simple neurons were frequency independent and distributed near the horizontal and sagittal planes, similar to those of otolith maculae. Last, responses of simple VOTS neurons were less sensitive to HA rotations and GIF during low-frequency dynamic tilts.
Validity of the 3D global frequency spatiotemporal modeling
As stated in the introduction, our approach, a global statistics-based analysis, assumed that the spatiotemporal tuning properties of VOTS neurons could be modeled by linearly summing frequency-related (temporal) responses in 3D space. This single equation (Eq. 2) imposes constraints on frequency-dependent spatiotemporal tuning properties absent from our previous piecewise, frequency-blind analysis (Chen-Huang and Peterson 2006). Although nonlinear summation of spatial and temporal responses in vestibular neurons has been reported (Musallam and Tomlinson 2002), our results show that linear frequency-dependent spatiotemporal models provide sufficient goodness of fit.
Another important feature of our analysis was that the necessity of complex over simple models for fitting VOTS neural responses was examined statistically. This method independently confirmed previous results that 3D tuning ratios exhibited a bimodal distribution with two peaks representing simple and complex neurons (Chen-Huang and Peterson 2006). The roughly 40% of ambiguity, where the tuning ratios of simple and complex neurons overlapped (Fig. 10), was not unexpected since 3D tuning ratios in complex neurons varied widely with frequency. Our results suggest that 3D tuning ratios predict best in the frequency range of 2.4–3.6 Hz. At lower frequency, comprehensive frequency-dependent analysis was clearly superior.
Last, since all neurons, simple or complex, were optimally modeled with fewer than 10 total parameters, the frequency-dependent spatiotemporal analysis may be further streamlined. It appeared that higher-order (>2) transfer functions were rarely needed to model neurons with complex tuning. Thus a data set of neural recordings at five directions, including the three canonical axes, tested over two to three wide-range frequencies, appears sufficient for a limited frequency-dependent spatiotemporal analysis.
Comparisons of VOTS neurons to otolith afferents
Fernandez and Goldberg (1976a) estimated that the polarization vectors of roughly 77% otolith afferents fell within 30° of the (left) utriculus and sacculus (i.e., ∼10 and ∼15° clockwise from the horizontal and sagittal planes, respectively). Since the mean polarization directions of our VOTS simple neurons appeared closer to these two planes than did afferents, it is reasonable to assume that most simple neurons lying within ±30° of the horizontal plane or the sagittal plane receive predominantly superior (utriculus) or inferior (sacculus) vestibular afferent inputs (Fernandez and Goldberg 1976b). The exception was the five BP neurons that may receive inputs from either sacculus and/or utriculus. In short, the polarization vectors of simple VOTS neurons closely resembled those of vestibular afferents in squirrel monkeys.
In contrast, response dynamics of VOTS neurons differed from otolith afferents in many aspects. As shown in Fig. 14, the mean response phase of simple neurons at Vtmax lagged that of regular and irregular afferents by about 30 and 60°, respectively (cf. Fernandez and Goldberg 1976c; Goldberg et al. 1990). The frequency-dependent sensitivity enhancement, prominent in irregular afferents, was uncommon in our data set (Fig. 7). The signal processing interposed between afferents, regularly or irregularly firing type, and flat-gain or low-pass simple VOTS neurons probably included transfer functions performing “fractional leaky integration” (Angelaki 1993; Schor et al. 1985). Responses of both low-pass and flat neurons were fit equally well with an interposing fractional leaky integrator [ks*(1 + τps)h, −1 ≤ h < 0], using irregular or regular afferents as inputs (VAF >90%). Thus simple VOTS neurons with low-pass or flat-gain properties could be readily modeled as receiving irregular or regular afferent inputs and the transfer functions shared common features.
Fig. 14.
Comparisons of average responses of simple VOTS neurons to 2 classes of otolith afferents across frequency. The frequency-dependent sensitivity and phase of low-pass neurons (A) and flat-gain neurons (B) are adequately modeled with a fractional leaky integrator, in the form of ks*(1 + τps)h, −1 < h < 0, with very regular (HVR, h ≅ −0.3) or irregular (HIR, h ≅ −0.7) afferent inputs at test frequencies. Afferent responses were obtained from previously published data in squirrel monkeys (Goldberg et al. 1990).
Since irregular and regular vestibular afferents differed significantly in response phase and gain (Fernandez and Goldberg 1976c; Goldberg et al. 1990), the complex spatiotemporal tuning properties observed on central vestibular neurons probably result from summing spatially distributed irregular and regular afferent inputs (Angelaki 1993). This is an attractive hypothesis, but it should be noted that we have yet to find any nonrotational complex VOTS neuron receiving direct otolith afferent inputs. Thus we cannot rule out the possibility that indirect, polysynaptic pathways may play an obligatory role in generating the responses observed in complex VOTS neurons.
These dynamically diverse signals, transmitted by either regular/irregular afferents or direct/indirect pathways, appeared to originate from different otolith endorgans. The Vtmax of simple neurons tended to align closely with the polarization vectors of afferents (see preceding text), whereas complex neurons deviated from both planes or migrated from one to the other. The distinctive spatial tuning patterns between simple and complex VOTS neurons suggest that the spatiotemporal convergence was strongly correlated to the convergence of cross-macula inputs. This hypothesis is further supported by the similarity between the percentage of complex neurons (34% of all VOTS neurons) in this study and that of convergent vestibular neurons (36% of all otolith-related neurons), identified with electrical stimulation of saccular and utricular nerves in decerebrate cats, in a previous study (Kushiro et al. 2000).
Response dynamics in VOTS neurons has been reported in anesthetized or decerebrate animals (Bush et al. 1993; Schor 1974; Schor and Miller 1982) and alert animals (Angelaki and Dickman 2000; Xerri et al. 1987). Similar to the study in alert cats, in which translations along only the dorsoventral axis were tested (Xerri et al. 1987), high-pass neurons were far less numerous than low-pass and flat-gain VOTS neurons and responses leading acceleration were uncommon in this report, in contrast to other studies (Angelaki and Dickman 2000; Bush et al. 1993; Schor 1974; Schor and Miller 1982). This difference may be attributed to species used, wakefulness, or differential responses from various areas of vestibular nuclei (cf. Schor 1974).
Compensatory LVOR versus orienting ocular responses
Translational head movements evoke predominantly compensatory LVOR that is modulated by gaze direction and viewing distances, appropriate for stabilizing visual images on the retina (Paige et al. 1996; Telford et al. 1997). Compensatory LVOR exhibits high-pass dynamics (with respect to head velocity), is not dependent on head orientation with respect to gravity (Paige and Tomko 1991), and follows Listing's Law (Angelaki et al. 2003; Walker et al. 2004). In addition, translations on the horizontal plane evoke a second type, though at a much lower sensitivity in normal animals, of eye movements that exhibits low-pass dynamics and is not sensitive to behavioral context (Angelaki and Hess 1996; Telford et al. 1998). Existing evidence suggests that the evoked torsional/vertical eye movements to IA/NO translations are part of orienting ocular responses most prominent during changes of head orientation with respect to gravity, i.e., tilts (Kushiro et al. 2002; Merfeld et al. 2001). Since epithelial hair cells on otolith maculae are equally excited by inertial- or gravitation-driven shearing forces, the diminished orienting ocular responses to head translations on the horizontal plane and compensatory LVOR to tilts suggest that the animals' ability to distinguish translation versus tilt is an active neural process.
Models addressing the issue of how central vestibular systems differentiate tilt versus translation based on the ambiguous otolith inputs focus on the importance of additional canals inputs and the different operating frequency range by these two functions (Angelaki et al. 2000, 2002; Green and Angelaki 2004; Mayne 1974; Merfeld and Zupan 2002; Paige and Tomko 1991). Incorporating the canal–otolith interaction hypothesis, Green and Angelaki (2004) provided a theoretical framework for brain stem neural mechanisms discriminating ambiguous otolith inputs and predicted responses of neurons within the network that implements the head orientation–dependent coupling between canal and otolith sensory inputs. They hypothesized that gravitation-related signals, carried by “tilt-only neurons,” are produced by the canal–otolith cross product via a neural network that performs a mathematical integration, such as the velocity storage integrator. Subsequently, inertial-driven, pure translation signals are isolated by subtracting tilt-only signals from the undifferentiated otolith afferent-like signals. Recent reports suggest that independent tilt and translation-related signals are present in vestibular nuclei and cerebellar uvula-nodulus (Angelaki et al. 2004; Yakusheva et al. 2008; Zhou et al. 2006).
Simple VOTS neurons in this study appear to carry inertia-driven translation signals and exhibit responses similar to the model prediction (Green and Angelaki 2004). Response vectors of simple neurons are independent of frequency and distributed near the horizontal and sagittal planes. Their sensitivity to GIF was significantly attenuated during low-frequency (0.15–0.3 Hz) dynamic tilts compared with translations at the same frequency. Unique spatial alignment patterns between mid-frequency Vrmax and Vtmax support the notion that translational and rotational signals carried by most simple neurons are appropriate for driving angular VOR (AVOR) and LVOR premotor neural networks where AVOR- and LVOR-related responses coexpress in the eye movement–related VOR neurons (Chen-Huang and McCrea 1999; Green et al. 2001). On the other hand, the observation that Vrmax and Vtmax on the horizontal plane (Fig. 11) in the few simple neurons sensitive to HA rotations and HP translations were oppositely directed was intriguing, possibly reflecting the residual effects of a mechanism that “cancels” gravitational inputs.
Although responses of complex neurons do not fit in the catalog of vestibular neurons predicted by the previous theoretical model (Green and Angelaki 2004), our results suggest that they play an important role in mediating orienting responses. The mid-frequency Vrmax and Vtmax in the horizontal plane were closely aligned in HA rotation sensitive complex neurons. In other words, during dynamic tilts, the rotational and gravity-driven inputs in complex neurons appeared synergistic. Further, the Vtmax of most complex neurons were closely aligned with the DV axis at higher frequency and became aligned with the IA axis during low-frequency translations. Last, we showed that responses of most complex neurons during 0.15 or 0.3 Hz roll/pitch were not significantly different from those during the same frequency translations. Thus in contrast to simple neurons, most complex neurons did not appear to distinguish between tilts versus translations. However, their responses did not simply mimic that of otolith afferents, which are excited by gravitational and inertial forces alike (Fernandez and Goldberg 1976a). Rather, the frequency-dependent tuning of Vtmax and the pattern of convergent vertical canal inputs in complex neurons underscore an intricate neural network in mediating gravity-dependent motor functions. Although their projection targets are unknown, their signals appear appropriate for driving vertical/oblique and, to a much smaller extent, horizontal, extraocular motoneurons (Graf et al. 1993), both during mid- to high-frequency vertical translations and HA rotations and during low-frequency translations in the horizontal plane, dynamic, and possibly static tilts.
If complex neurons are indeed an integral part of the neural mechanism that mediates gravity-related responses, two important features can be readily appreciated. First, the frequency-dependent spatial tuning of complex neurons provides a unique spatiotemporal filtering mechanism, independent of and complementary to the canal-driven responses. Second, since complex neurons appear to receive cross-macular inputs, sacculus inputs probably play a role as well. Canal-independent ocular orienting responses, with a gain of 0.1 to 0.6, have been demonstrated during static tilt (Diamond and Markham 1983; Haslwanter et al. 1992; Hess and Angelaki 1999; Maruta et al. 2001, 2008), low-frequency translations on the horizontal plane (Angelaki 1998; Paige and Tomko 1991), and constant centrifugation. Although the counterrolling eye movements are mostly a linear function of the acceleration along IA and NO axes (Graybiel and Woellner 1959), GIF along the vertical axis, presumably transmitted via sacculus or, to a much lesser degree, the anterior pars medialis of utriculus (Fernandez and Goldberg 1976a; Fernandez et al. 1972), was suggested to play a role (Maruta et al. 2008; Moore et al. 2001). The contribution of sacculus versus utriculus to torsional eye movements recorded in human subjects on centrifuges was estimated roughly at a 1:3 ratio (De Graaf et al. 1996). Although fewer in the number of relay neurons and not as strong in their synaptic drive, torsional eye movements could be elicited via polysynaptic pathways from both utriculus and sacculus (Curthoys 1987; Fluur and Mellstrom 1971; Isu et al. 2000; Sasaki et al. 1991; Uchino et al. 1996).
Although it is not clear whether utricular and saccular inputs arrive at the motor nuclei independently or via a shared pathway, the convergence of cross-macular and vertical canal inputs as manifested in complex neurons appears appropriate for producing the ocular orienting responses. The unique spatiotemporal tuning properties of complex neurons to translations, coupled with their preponderance to receiving vertical canal inputs, make them a suitable pathway for driving the vertical/oblique premotor network during vertical translations and dynamic tilts. Thus the behavior of complex neurons provides an important insight into how central otolith signals may be processed with regard to compensatory and orienting responses that have not been predicted by previous theoretical models.
GRANTS
This research was supported by National Eye Institute Grant EY-16444 to C. Chen-Huang.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. George Alheid at Northwestern University, Department of Physiology, for assisting the perfusion of an experimental monkey and providing guidance in preparing the brain tissue.
APPENDIX
The transfer functions, SFX(s), SFY(s), and SFZ(s), along IA (X), NO (Y), and DV (Z) axes incorporated terms up to two poles and two zeroes in one of the following forms
1) 1/(τ1s + 1)
2) (τ3s + 1)
3) 1/(τ1s + 1)(τ2s + 1)
4) (τ3s + 1)/(τ1s + 1)
5) (τ3s + 1)/(τ1s + 1)(τ2s + 1)
6) (τ3s + 1)(τ4s + 1)/(τ1s + 1)(τ2s + 1)
7) s*(τ3s + 1)/(τ1s + 1)(τ2s + 1)
8) 1
Although combinations of these eight transfer functions successfully fit responses of most neurons, nonminimum phase (NMP) systems were needed in some other neurons (Angelaki and Dickman 2000; Schor et al. 1985). The NMP system was used in modeling responses where incongruent sensitivity and phase behavior with respect to frequency were observed. The NMP version of SFx/y/z(s) was expressed as
| (A1) |
The time constant (τ) in the phase shift element was set to 100 s. The fractional exponent −0.5 ≤ λx/y/z ≤ 0.5 provided an additional phase shift within ±90° to that of SF(s). The model selection-related rule also applied to λ, such that λx = λy = λz in simple tuning models and unequal λ values for complex models. All neurons were first modeled with the conventional minimum phase systems, without the phase shift element. NMP systems were used when large uncertainty (SE) associated with fit coefficients was produced by conventional system or that NMP systems greatly improved fits (ΔVAF >10%).
The fitting procedure, written in IGOR Pro (WaveMetrics), sought to minimize the squared error between the actual (aNRi) and the expected neural response (eNRi)
| (A2a) |
where Sc and Ss are from Eq. 1
| (A2b) |
In the case of NMP systems
| (A2c) |
where i is the index of each array and j is the imaginary unit
| (A3) |
Taking the trial-by-trial noise into consideration, the squared error was weighted by the inverse of SE (σ) associated with Ss and Sc from Eq. 1 to give rise to normalized residual χ2
| (A4) |
REFERENCES
- Angelaki DE. Spatio-temporal convergence (STC) in otolith neurons. Biol Cybern 67: 83–96, 1992 [DOI] [PubMed] [Google Scholar]
- Angelaki DE. Spatial and temporal coding in single neurons. Biol Cybern 69: 147–154, 1993 [DOI] [PubMed] [Google Scholar]
- Angelaki DE. Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. III. Responses to translation. J Neurophysiol 80: 680–695, 1998 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Bush GA, Perachio AA. A model for the characterization of the spatial properties in vestibular neurons. Biol Cybern 66: 231–240, 1992 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol 84: 2113–2132, 2000 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Hess BJ. Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. II. Inertial detection of angular velocity. J Neurophysiol 75: 2425–2440, 1996 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Merfeld DM, Hess BJ. Low-frequency otolith and semicircular canal interactions after canal inactivation. Exp Brain Res 132: 539–549, 2000 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Newlands SD, Dickman JD. Inactivation of semicircular canals causes adaptive increases in otolith-driven tilt responses. J Neurophysiol 87: 1635–1640, 2002 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature 430: 560–564, 2004 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Zhou HH, Wei M. Foveal versus full-field visual stabilization strategies for translational and rotational head movements. J Neurosci 23: 1104–1108, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information–Theoretic Approach New York: Springer, 2002, p. xxvi, 488 [Google Scholar]
- Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 33: 261–304, 2004 [Google Scholar]
- Bush GA, Perachio AA, Angelaki DE. Encoding of head acceleration in vestibular neurons. I. Spatiotemporal response properties to linear acceleration. J Neurophysiol 69: 2039–2055, 1993 [DOI] [PubMed] [Google Scholar]
- Chacron MJ, Maler L, Bastian J. Feedback and feedforward control of frequency tuning to naturalistic stimuli. J Neurosci 25: 5521–5532, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YS, Shum DK, Lai CH. Neuronal response sensitivity to bidirectional off-vertical axis rotations: a dimension of imbalance in the bilateral vestibular nuclei of cats after unilateral labyrinthectomy. Neuroscience 94: 831–843, 1999 [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, McCrea RA. Effects of viewing distance on the responses of vestibular neurons to combined angular and linear vestibular stimulation. J Neurophysiol 81: 2538–2557, 1999 [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, McCrea RA, Goldberg JM. Contributions of regularly and irregularly discharging vestibular-nerve inputs to the discharge of central vestibular neurons in the alert squirrel monkey. Exp Brain Res 114: 405–422, 1997 [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, Peterson BW. Three dimensional spatial-temporal convergence of otolith related signals in vestibular only neurons in squirrel monkeys. Exp Brain Res 168: 410–426, 2006 [DOI] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122, 1988 [DOI] [PubMed] [Google Scholar]
- Curthoys IS. Eye movements produced by utricular and saccular stimulation. Aviat Space Environ Med 58: A192–A197, 1987 [PubMed] [Google Scholar]
- De Graaf B, Bos JE, Groen E. Saccular impact on ocular torsion. Brain Res Bull 40: 321–330, 1996 [DOI] [PubMed] [Google Scholar]
- Diamond SG, Markham CH. Ocular counterrolling as an indicator of vestibular otolith function. Neurology 33: 1460–1469, 1983 [DOI] [PubMed] [Google Scholar]
- Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol 88: 3518–3533, 2002 [DOI] [PubMed] [Google Scholar]
- Ezure K, Graf W. A quantitative analysis of the spatial organization of the vestibulo-ocular reflexes in lateral- and frontal-eyed animals. I. Orientation of semicircular canals and extraocular muscles. Neuroscience 12: 85–93, 1984 [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol 39: 970–984, 1976a [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force–response relations. J Neurophysiol 39: 985–995, 1976b [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol 39: 996–1008, 1976c [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM, Abend WK. Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurophysiol 35: 978–987, 1972 [DOI] [PubMed] [Google Scholar]
- Fluur E, Mellstrom A. The otolith organs and their influence on oculomotor movements. Exp Neurol 30: 139–147, 1971 [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP. On reaching. Annu Rev Neurosci 9: 147–170, 1986 [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol 63: 781–790, 1990 [DOI] [PubMed] [Google Scholar]
- Graf W, Baker J, Peterson BW. Sensorimotor transformation in the cat's vestibuloocular reflex system. I. Neuronal signals coding spatial coordination of compensatory eye movements. J Neurophysiol 70: 2425–2441, 1993 [DOI] [PubMed] [Google Scholar]
- Graf W, Simpson JI, Leonard CS. Spatial organization of visual messages of the rabbit's cerebellar flocculus. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol 60: 2091–2121, 1988 [DOI] [PubMed] [Google Scholar]
- Graybiel A, Woellner RC. A new and objective method for measuring ocular torsion. Am J Ophthalmol 47: 349–352, 1959 [DOI] [PubMed] [Google Scholar]
- Green AM, Angelaki DE. An integrative neural network for detecting inertial motion and head orientation. J Neurophysiol 92: 905–925, 2004 [DOI] [PubMed] [Google Scholar]
- Green AM, Dickman JD, Angelaki DE. Differential coding of sensorimotor signals on eye-movement-sensitive neurons during rotation and translation (Abstract). Ann NY Acad Sci 942: 468, 2001 [DOI] [PubMed] [Google Scholar]
- Haslwanter T, Straumann D, Hess BJ, Henn V. Does counterrolling violate Listing's law? Ann NY Acad Sci 656: 931–932, 1992 [DOI] [PubMed] [Google Scholar]
- Hess BJ, Angelaki DE. Oculomotor control of primary eye position discriminates between translation and tilt. J Neurophysiol 81: 394–398, 1999 [DOI] [PubMed] [Google Scholar]
- Isu N, Graf W, Sato H, Kushiro K, Zakir M, Imagawa M, Uchino Y. Sacculo-ocular reflex connectivity in cats. Exp Brain Res 131: 262–268, 2000 [DOI] [PubMed] [Google Scholar]
- Johnson MT, Coltz JD, Hagen MC, Ebner TJ. Visuomotor processing as reflected in the directional discharge of premotor and primary motor cortex neurons. J Neurophysiol 81: 875–894, 1999 [DOI] [PubMed] [Google Scholar]
- Kushiro K, Dai M, Kunin M, Yakushin SB, Cohen B, Raphan T. Compensatory and orienting eye movements induced by off-vertical axis rotation (OVAR) in monkeys. J Neurophysiol 88: 2445–2462, 2002 [DOI] [PubMed] [Google Scholar]
- Kushiro K, Zakir M, Sato H, Ono S, Ogawa Y, Meng H, Zhang X, Uchino Y. Saccular and utricular inputs to single vestibular neurons in cats. Exp Brain Res 131: 406–415, 2000 [DOI] [PubMed] [Google Scholar]
- Levi R, Varona P, Arshavsky YI, Rabinovich MI, Selverston AI. The role of sensory network dynamics in generating a motor program. J Neurosci 25: 9807–9815, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado AG, Baker KB, Schuster D, Butler RS, Rezai A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res 1280: 107–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson JM, Aldridge JW. A quantitative method of computer analysis of spike train data collected from behaving animals. Brain Res 175: 183–187, 1979 [DOI] [PubMed] [Google Scholar]
- Maruta J, Raphan T, Simpson JI, Cohen B. Vertical (Z-axis) acceleration alters the ocular response to linear acceleration in the rabbit. Exp Brain Res 185: 87–99, 2008 [DOI] [PubMed] [Google Scholar]
- Maruta J, Simpson JI, Raphan T, Cohen B. Orienting otolith-ocular reflexes in the rabbit during static and dynamic tilts and off-vertical axis rotation. Vision Res 41: 3255–3270, 2001 [DOI] [PubMed] [Google Scholar]
- Masino T, Knudsen EI. Horizontal and vertical components of head movement are controlled by distinct neural circuits in the barn owl. Nature 345: 434–437, 1990 [DOI] [PubMed] [Google Scholar]
- Mayne R. A systems concept of the vestibular organs. In: Vestibular System, Part 2: Psychophysics, Applied Aspects, and General Interpretations, edited by Kornhuber HH. Berlin: Springer-Verlag, 1974, p. 493–580 [Google Scholar]
- Merfeld DM, Zupan LH. Neural processing of gravitoinertial cues in humans. III. Modeling tilt and translation responses. J Neurophysiol 87: 819–833, 2002 [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Zupan LH, Gifford CA. Neural processing of gravito-inertial cues in humans. II. Influence of the semicircular canals during eccentric rotation. J Neurophysiol 85: 1648–1660, 2001 [DOI] [PubMed] [Google Scholar]
- Moore ST, Clement G, Raphan T, Cohen B. Ocular counterrolling induced by centrifugation during orbital space flight. Exp Brain Res 137: 323–335, 2001 [DOI] [PubMed] [Google Scholar]
- Musallam S, Tomlinson RD. Asymmetric integration recorded from vestibular-only cells in response to position transients. J Neurophysiol 88: 2104–2113, 2002 [DOI] [PubMed] [Google Scholar]
- Optican LM, Richmond BJ. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. III. Information theoretic analysis. J Neurophysiol 57: 162–178, 1987 [DOI] [PubMed] [Google Scholar]
- Paige GD, Barnes GR, Telford L, Seidman SH. Influence of sensorimotor context on the linear vestibulo-ocular reflex. Ann NY Acad Sci 781: 322–331, 1996 [DOI] [PubMed] [Google Scholar]
- Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol 65: 1170–1182, 1991 [DOI] [PubMed] [Google Scholar]
- Richmond BJ, Optican LM, Podell M, Spitzer H. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. I. Response characteristics. J Neurophysiol 57: 132–146, 1987 [DOI] [PubMed] [Google Scholar]
- Richmond BJ, Optican LM, Spitzer H. Temporal encoding of two-dimensional patterns by single units in primate primary visual cortex. I. Stimulus–response relations. J Neurophysiol 64: 351–369, 1990 [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Supplementary eye field: representation of saccades and relationship between neural response fields and elicited eye movements. J Neurophysiol 84: 2605–2621, 2000 [DOI] [PubMed] [Google Scholar]
- Sasaki M, Hiranuma K, Isu N, Uchino Y. Is there a three neuron arc in the cat utriculo-trochlear pathway? Exp Brain Res 86: 421–425, 1991 [DOI] [PubMed] [Google Scholar]
- Schor RH. Responses of cat vestibular neurons to sinusoidal roll tilt. Exp Brain Res 20: 347–362, 1974 [DOI] [PubMed] [Google Scholar]
- Schor RH, Angelaki DE. The algebra of neural response vectors. Ann NY Acad Sci 656: 190–204, 1992 [DOI] [PubMed] [Google Scholar]
- Schor RH, Miller AD. Relationship of cat vestibular neurons to otolith-spinal reflexes. Exp Brain Res 47: 137–144, 1982 [DOI] [PubMed] [Google Scholar]
- Schor RH, Miller AD, Timerick SJ, Tomko DL. Responses to head tilt in cat central vestibular neurons. II. Frequency dependence of neural response vectors. J Neurophysiol 53: 1444–1452, 1985 [DOI] [PubMed] [Google Scholar]
- Simpson JI, Leonard CS, Soodak RE. The accessory optic system of rabbit. II. Spatial organization of direction selectivity. J Neurophysiol 60: 2055–2072, 1988 [DOI] [PubMed] [Google Scholar]
- Soechting JF, Flanders M. Moving in three-dimensional space: frames of reference, vectors, and coordinate systems. Annu Rev Neurosci 15: 167–191, 1992 [DOI] [PubMed] [Google Scholar]
- Telford L, Seidman SH, Paige GD. Dynamics of squirrel monkey linear vestibuloocular reflex and interactions with fixation distance. J Neurophysiol 78: 1775–1790, 1997 [DOI] [PubMed] [Google Scholar]
- Telford L, Seidman SH, Paige GD. Canal-otolith interactions in the squirrel monkey vestibulo-ocular reflex and the influence of fixation distance. Exp Brain Res 118: 115–125, 1998 [DOI] [PubMed] [Google Scholar]
- Uchino Y, Sasaki M, Sato H, Imagawa M, Suwa H, Isu N. Utriculoocular reflex arc of the cat. J Neurophysiol 76: 1896–1903, 1996 [DOI] [PubMed] [Google Scholar]
- Walker MF, Shelhamer M, Zee DS. Eye-position dependence of torsional velocity during interaural translation, horizontal pursuit, and yaw-axis rotation in humans. Vision Res 44: 613–620, 2004 [DOI] [PubMed] [Google Scholar]
- Wylie DR, Bischof WF, Frost BJ. Common reference frame for neural coding of translational and rotational optic flow. Nature 392: 278–282, 1998 [DOI] [PubMed] [Google Scholar]
- Xerri C, Barthelemy J, Harlay F, Borel L, Lacour M. Neuronal coding of linear motion in the vestibular nuclei of the alert cat. I. Response characteristics to vertical otolith stimulation. Exp Brain Res 65: 569–581, 1987 [DOI] [PubMed] [Google Scholar]
- Yakusheva T, Blazquez PM, Angelaki DE. Frequency-selective coding of translation and tilt in macaque cerebellar nodulus and uvula. J Neurosci 28: 9997–10009, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushin SB, Raphan T, Cohen B. Spatial properties of central vestibular neurons. J Neurophysiol 95: 464–478, 2006 [DOI] [PubMed] [Google Scholar]
- Zhou W, Tang BF, Newlands SD, King WM. Responses of monkey vestibular-only neurons to translation and angular rotation. J Neurophysiol 96: 2915–2930, 2006 [DOI] [PubMed] [Google Scholar]