Abstract
Cats actively respond to horizontal perturbations of the supporting surface according to the force constraint strategy. In this strategy, the force responses fall into two groups oriented in either rostral and medial directions or caudal and lateral directions, rather than in strict opposition to the direction of perturbation. When the distance between forelimbs and hindlimbs is decreased, the responses are less constrained and directed more in line with the perturbation. We have recently shown that electromyographic responses from limb muscles of the decerebrate cat resemble those obtained in the intact animal. Our objectives here were to determine whether the decerebrate cat preparation would also exhibit the force constraint strategy and whether that strategy would exhibit the characteristic dependence on limb position on the strategy. Horizontal support surface perturbations were delivered and three-dimensional exerted forces were recorded from all four limbs. Clustered force responses were generated by all four limbs and were found to be statistically indistinguishable between animals decerebrated using two different levels of transection. The directionality of the force responses was preserved throughout successive time epochs during the perturbations. In addition, the clustering of force responses increased with distance between forelimbs and hindlimbs. These results indicate that the force constraint strategy used by terrestrial animals to maintain stability can be generated without the assistance of the cerebral cortices and without prior training. This suggests an important role for the lower brain stem and spinal cord in generating an appropriate strategy to maintain stability.
INTRODUCTION
Radial, horizontal support surface perturbations have been used to examine postural control and stability (Henry et al. 1998; Macpherson 1988a,b). Rather than the expected radially arranged force responses, cats responded with exerted forces that cluster (or appear constrained) into two diagonal XY (horizontal plane) populations extending toward and away from the center of mass of the subject (Macpherson 1988a). This clustering of forces was deemed the “force constraint strategy.” The force constraint strategy is robust in humans and cats, demonstrating its presence in naïve subjects, during support surface rotation, and volitional reaching tasks in humans (Henry et al. 1998; Leonard et al. 2009; Macpherson 1994; Torres-Oviedo et al. 2006). Furthermore, the strategy demonstrates alterations in its constraint characteristics with stance condition, including width of stance in human and distance between forelimbs and hindlimbs in cat (Henry et al. 2001; Torres-Oviedo et al. 2006). In shorter stance conditions, the force constraint dissipates and a more radial force trajectory is achieved. At longer or wider stance conditions, both cats and humans, respectively, demonstrate a more constrained strategy with forces in the rostral/caudal directions, achieving larger magnitudes than those in the medial/lateral dimension.
These constrained force trajectories are associated with directionally selective muscle activation (Henry et al. 1998; Macpherson 1988b). Muscular activity during support surface displacements is quantified using tuning curves, which compare increases or decreases in activity to background activation and graph the resultant against perturbation direction. The tuning curves of both humans and cats illustrate that muscles have a principal direction of activation and are generally active over about 25% of the perturbation space. This selective directional activation is conserved across stance conditions; however, the amplitude of the response does appear to vary in some muscles (Henry et al. 2001; Torres-Oviedo et al. 2006).
There is substantial evidence that the cortex (Adkin et al. 2006; Beloozerova et al. 2003, 2005; Jacobs and Horak 2007; Taube et al. 2006), brain stem (Deliagina et al. 2006, 2008; Mori 1987; Mori et al. 1989; Musienko et al. 2008), and spinal cord (Bosco and Poppele 2001; Lyalka et al. 2005; Stein 2008) all contribute to postural control. Classical literature illustrates that the decerebrate cat generates several postural tasks including the righting reflex (Magnus 1926), suggesting a strong role for brain stem and spinal cord circuits. Furthermore, there is growing evidence that brain stem regions, specifically the pontomedullary reticular formation, relay important information about postural disturbances (Stapley and Drew 2009). However, more recent evidence alludes to a role for the cortex in unpredictable postural disturbances (Adkin et al. 2006; Jacobs and Horak 2007). It is plausible that these structures exist within a hierarchical framework in which each unit is responsible for increasingly complex integration of sensory and environmental information.
We have previously demonstrated that the modified premammillary decerebrate cat exhibits important features of the postural strategy—specifically, appropriately directed muscular activation patterns can be generated in the absence of the cerebral cortices (Honeycutt et al. 2009). Furthermore, although perturbations rarely elicited a muscular response in the intercollicular decerebrate cat, when responses were present they were also directionally appropriate. These results are consistent with reports that the spinalized animal can also produce appropriately directed muscle activation patterns (Macpherson and Fung 1999), indicating that supraspinal structures are not necessary for directionally appropriate muscle activation for horizontal perturbations. Thus we hypothesized that this postural strategy arises from the interaction of limb biomechanics and reflex networks in the spinal cord (Honeycutt et al. 2009; Nichols et al. 1999, 2002).
The generation of directionally appropriate muscle activation patterns does not, however, necessarily indicate that the force constraint strategy will emerge. Muscle activation patterns must not only be of appropriate direction, but their magnitudes must be in appropriate proportions. For example, the spinalized animal, which is capable of producing appropriately tuned muscular responses in some muscles, does not exhibit the force constraint strategy (Macpherson and Fung 1999). These muscle responses, although appropriate directionally, are significantly decreased in amplitude. Moreover, flexor responses are rare, likely resulting from the selective neuromuscular junction degradation seen in chronic spinal animals (Burns et al. 2007; Potluri et al. 2008). Therefore to fully evaluate the presence of a functionally relevant strategy in the absence of cerebral cortices, it becomes important to examine the force responses generated by the decerebrate animal. In addition, since the electromyographic (EMG) responses are not particularly sensitive to stance distance (Henry et al. 2001; Torres-Oviedo et al. 2006), it is important to verify that the force constraint decreases with stance distance if the decerebrate preparation is to be considered a valid model to investigate underlying mechanisms of postural control.
Based on our hypothesis that the postural strategy arises from the interaction of limb biomechanics and reflex networks in the spinal cord, we predict that the decerebrate cat will generate the essential features of the force constraint strategy, including constraint adjustments resulting from alteration in stance condition. We propose that the force constraint strategy is not expressed in the spinalized animal because of the paucity of robust muscular responses. We expect that in preparations in which more robust muscular responses are present in a larger set of muscles, as is observed in the decerebrate cat, the force constraint strategy will emerge. If the decerebrate animal is able to generate the force constraint strategy, it will further validate this preparation as a model to evaluate the sensory mechanisms underlying the postural responses and demonstrate that the muscular responses of the decerebrate animal are generating a functionally relevant postural strategy in the absence of the cerebral cortices.
Our objective was to quantify the force constraint strategy of the modified premamillary and intercollicular decerebrate cat, including the dependence on stance width. We indeed found that both preparations of the decerebrate cat exhibit similar responses to the intact animal. Finally, implications of these results on the role of the brain stem and spinal cord in postural control are discussed. These results were previously presented in abstract form (Honeycutt and Nichols 2006).
METHODS
Setup
Force data were obtained from the ten cats whose muscular activation patterns had previously been obtained (Honeycutt et al. 2009) and whose weights ranged from 6.5 to 12 lb. These animals were used in accordance with the issued standards of the National Institutes of Health and the Emory Institutional Animal Care and Use Committee. Animals will be referred to by the date of the experiment. Under isoflurane anesthesia, a tracheotomy was performed and a tube inserted for isoflurane delivery and an intravenous line was inserted in the external jugular vein for hydration and drug delivery. Animals were decerebrated using two techniques: intercollicular and a modified premammillary.
The more traditional intercollicular decerebration consisted of a vertical transection through the superior colliculus (Mori 1987; Sherrington 1898). All brain material rostral to the transection was removed. Since the intercollicular decerebration technique did not produce consistent postural responses (Honeycutt et al. 2009), we used a modification of the premammillary decerebration, a preparation typically used to obtain spontaneous stepping (Grillner and Shik 1973; Whelan 1996). Following the premammillary transection and removal of all brain tissue rostral to the cut, a second, vertical transection was made at the level of the mammillary bodies to prevent spontaneous locomotion. The resulting preparation was considerably more responsive to mechanical perturbations than animals prepared with intercollicular transections.
The animal's head was fixed in a stereotaxic frame and its tail secured through a mechanical clamp at the base of the tail. A sling supported the body of the animal until the animal was removed from anesthesia and weight bearing established. Fixation of the tail instead of the hip gave a large range of motion for the animals' hindquarters and allowed for natural hip placement. The toe pads of all four limbs were glued to squares of tape, which were in turn applied to the surfaces of the force transducers with resolutions of 1/800 N for forces in the X and Y dimensions and 1/400 N for forces in the Z dimension. The force transducers were configured with right-handed coordinate systems, with the X direction pointing rightward. The natural turnout of the foot was used for each animal. The large, central pad of the foot was not secured, thus allowing natural movement of the foot during perturbations. Intact animal kinematics determined the natural stance placement of the toe approximately 1 cm behind the greater trochanter in the sagittal plane. Depending on animal size this placement resulted in a 28- to 32-cm distance between the center of the forelimb and hindlimb transducers. The center of right and left forelimb and hindlimb transducers were 7 cm apart. In three experiments (6/8, 1/22, 9/7), forces were evaluated at two additional stance conditions that altered the interlimb spacing (distance between forelimbs and hindlimbs). Both hindlimbs were moved 5 cm forward or 4 cm backward from the natural interlimb spacing and perturbations were completed from the new stance condition.
Perturbations
After the decerebration and positioning were completed, the animals were gradually removed from anesthesia to limit sudden increases in blood pressure. Data were taken under both the anesthetized and active (after complete removal of anesthesia) conditions. Muscle tone and activity were monitored in the anesthetized condition to ensure that the animal was not responsive. Active data were quantified only when the animal had achieved active muscular responses (visualized through EMG recordings) in three trials consisting of the full complement of 16 directions. This typically occurred within 30 min after removal of the anesthetic. The support surface was translated in 16 different, horizontal directions using two motors: rotational and linear. The rotational motor would position a linear motor that performed the perturbation so that the length inputs would have the same characteristics in all directions. In all but two experiments, the support surface was perturbed 4 cm over 400 ms, with an acceleration of 0.5 (m/s2). In the 6/8 experiment the platform was moved 8 cm over 400 ms and in the 11/7 experiment the platform was moved 4 cm over 150 ms. The perturbation parameters were chosen based on previous studies in intact cats (Macpherson 1988a,b) for comparison purposes. Unique to the decerebrate cat, the animal's head and tail were in a fixed position. Therefore the limbs were perturbed, held at the extended position for 1,000 ms, and then returned 4 cm over 400 ms to the initial position. In the 10/8 experiment the hold was 500 ms and in the 11/7 and 2/23 experiments the hold was 3,000 ms.
Force data analysis and quantification
Force data from the horizontal plane (X: medial–laterial, Y: rostral–caudal) were analyzed for the purposes of this study. Trials that included stepping behavior or in which the foot was lifted from the force transducer were excluded from analysis. Force data were demeaned and low passed at 30 Hz. The change in force per ms was calculated by finding the area under the force curve for 100-ms time intervals and dividing by 100 ms. The initial time period evaluated was 50–150 ms. This time interval was chosen because it avoids the inertial properties associated with the motors during the first 50 ms and it corresponds to the automatic postural response reported in intact animals (Macpherson 1988a,b). Additional time intervals were analyzed to evaluate the extended force responses past the typical automatic postural response time period. To visualize and further quantify the force constraint, the average change in force for all perturbation directions was graphed from origin.
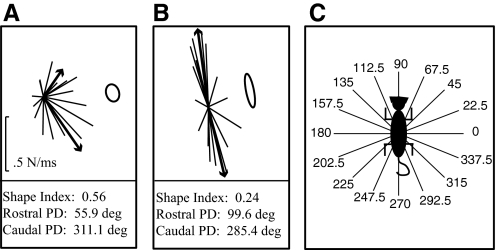
The constraint or clustering of the force responses was further quantified using principal direction and stiffness ellipses. Principal directions were calculated by first separating the two populations based on their squared Euclidean distance from one another using the MATLAB function kmeans. The principal direction of each population was then calculated by averaging the remaining force vectors and determining the direction of the resultant vector. Stiffness ellipses were derived by dividing the 16 force vectors by the distance traveled in the x- and y-directions appropriately. The shape index of the ellipse was calculated by dividing the minor axis by the major axis. A shape index close to 1 represents a circle or nonconstrained force responses, whereas a shape index close to 0 represents a line or highly constrained force responses. Figure 1 shows example principal direction and shape index quantifications along with the perturbation directions.
Fig. 1.
Figure demonstrates the key variables used for quantifying the force responses: principal direction (PD), shape index, and perturbation direction. Each X and Y force curve is integrated for 100-ms time windows and divided by 100 to get the average change in force/ms. The resultant XY vector is then graphed originating at zero. The black lines represent the force traces for the left forelimb (LF) and right hindlimb (RH) from the 6/8 experiment. The heavier and arrowed black lines represent the 2 PD vectors calculated through the cluster analysis. The stiffness ellipses calculated for these force traces are shown to the right. The shape index, used to quantify force constraint, is a ratio of the minor axis to the major axis, where 1 represents a circle and 0 represents a line. The PDs and shape indexes evaluated for these 2 force strategies are presented below the figure.
Shape indices and principal directions were statistically compared during different time epochs, interlimb spacing, and decerebration techniques. Shape indices and principal directions were calculated for each full set of 16 directions under each condition. Three trials (full sets of 16 directions) were quantified for each animal and statistics computed. There was one exception, which was the 9/7 long stance condition where statistics were computed on only two trials. The variance was determined to be equivalent in all cases using an F-test. Therefore a two-tailed t-test for independent variables of equal variance was used to determine whether statistical differences were present in the shape indices and principal directions under each condition. Degrees of freedom for statistical significance were adjusted appropriately based on the number of observations in each muscle; t-tests were performed at a 0.95 confidence interval or P value of 0.05. Results were compared qualitatively between the intercollicular and modified premammillary preparations and between the modified premammillary decerebrate preparation and the intact animal.
RESULTS
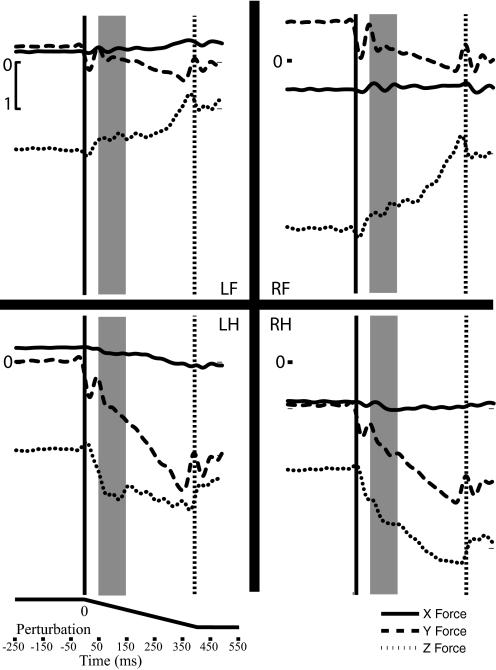
Raw force traces demonstrate that the decerebrate cat generally produced greater maximum force in the rostral/caudal direction than that in the medial/lateral direction. Figure 2 shows representative raw, filtered force traces from all four limbs in a premammillary decerebrated animal (9/11 experiment). Forces are shown nondemeaned to demonstrate the active force generated by the animal during standing. These preparations, in particular the modified premammillary, generated strong muscular activity in between perturbations, often resisting their constraints at the head and tail. They required no support of their abdominal region for proper back alignment. On average, animals generated around 20–25% of their weight on the force transducers. In Fig. 2, the solid vertical line indicates perturbation initiation, whereas the dashed line indicates perturbation termination. The force responses of Fig. 2 illustrate the increase of force through the dynamic phase of the perturbation, which finds a maximum that is maintained during the steady-state hold phase. X forces (medial/lateral) in all animals generally found a maximum around 0.5 N, whereas Y forces (rostral/caudal) typically could reach 1.5 N. Decerebrated animals occasionally produced Z forces as large as 10 N, although more typically the maximum Z force responses were between 2 and 2.5 N during the perturbation. Finally, Fig. 2 portrays a shaded region that represents the initial analysis time period (50–150 ms) used to calculate the average change in force. The average change in force is graphed originating at zero for further analysis of the force constraint and its principal directions.
Fig. 2.
The raw force traces (low passed at 30 Hz) for all 4 limbs during a perturbation direction of 90° from the 9/11 experiment are depicted. Three-dimensional force data from the right forelimb (RF), left forelimb (LF), right hindlimb (RH), and left hindlimb (LH) are depicted during a forward (90°) horizontal perturbation. Perturbation initiation and termination are denoted with a solid and dashed line, respectively. Forces were additionally demeaned (not depicted) prior to quantification to get an accurate reading of the change in force. Forces are quantified over 100-ms time windows. The first time period evaluated is marked by a gray box and begins 50 ms after perturbation initiation, to avoid the inertial properties of the initial perturbation. For quantification of these responses, each force curve is integrated individually for all 16 directions. This animal weighed about 10 lb.
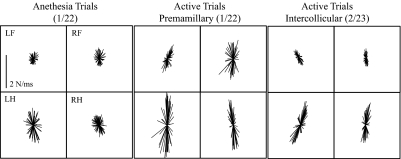
Animals decerebrated at the premammillary level more consistently produce muscular responses to perturbation (8 of 9 animals, or 88.9%) than intercollicular decerebrated animals (3 of 28 animals, or 11.7%); however, the force responses associated with these two preparations were remarkably similar. One of the premammillary animals that did generate forces had additional interventions and therefore is not reported here. The other 25 intercollicular decerebrate animals tested did not generate muscular responses. These preparations were used for other purposes and are not reported here. Figure 3 shows representative active force responses from two animals decerebrated at the premammillary (center) and intercollicular (right) level. Force responses of an anesthetized premammillary animal are also depicted (left). The force responses obtained under anesthesia were mostly radial in nature, although a slight tendency for clustering appears to be present. In comparison, the active responses of both the premammillary and intercollicular animals are larger in amplitude and more constrained with larger rostral–caudal forces than medial–lateral forces. The rostral–caudal forces were additionally offset from the Y-axis and demonstrated a 2–13° inward turn toward the center of the animal. The asymmetry in principal directions observed between left and right limbs was most likely due to the fact that the animal was supported by a clamp applied to the tail. The clamp provided an additional route for force transmission and could accommodate small differences in weight support or tone in the two limbs.
Fig. 3.
Representative horizontal plane force projections from the modified premammillary (active and anesthetized) and intercollicular decerebrate animal. Depicts the typical XY force projections of the modified premammillary decerebrate cat (1/22 experiment) under anesthesia and after the animal was removed from isoflurane (active). Force projections were evaluated during a window of 50–150 ms after perturbation initiation and graphed originating from zero. The anesthesia trials are less constrained and smaller in amplitude than the modified premammillary animals. The modified premammillary animals demonstrate a force constraint or clustering where the forces in the rostral–caudal dimension are larger than those in the medial–lateral dimension.
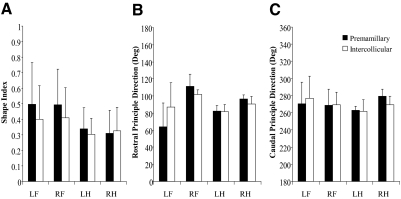
Animals decerebrated at the premammillary and intercollicular decerebration levels produced statistically equivalent shape indices in all four limbs. Figure 4 illustrates the quantification of the force constraint of all four limbs' force responses attained in animals decerebrated at the premammillary (black) and intercollicular (white) levels. The amplitudes of the intercollicular preparations' force responses are slightly smaller in appearance and slightly more constrained (except in the RH). However, none of the differences, shape index (A), or principal direction (B: rostral, C: caudal), is statistically significant.
Fig. 4.
Principal direction (PD) and shape index quantification for the modified premammillary and intercollicular decerebrate cat. PD and shape index were quantified for all 4 limbs (LH, left forelimb; RF, right forelimb; LH, left hindlimb; RH, right hindlimb) in all animals that generated active muscular responses. The black columns represent the mean and SD calculated from the 7 modified premammillary decerebrated animals, whereas the white columns represent the mean and SD from the 3 intercollicular decerebrated animals. All PDs and shape index quantifications were found to be statistically equivalent between the 2 types of preparations.
Although animals decerebrated at the premammillary and intercollicular levels produce constrained forces in all four limbs, the hindlimbs show more constrained shape indices and less variable principal directions (Fig. 4) than those of the forelimbs. The average hindlimb shape index values range from 0.30 to 0.34, whereas the average forelimb shape indices range from 0.40 to 0.50. The force constraint in the hindlimbs was also more consistent with SDs <0.15, whereas forelimbs showed higher variability, ranging from 0.19 to 0.27. This same trend in variability was present in principal directions. Hindlimb principal directions present small SDs <14°, whereas forelimb principal directions were more variable, generally showing SDs >20°. In addition to the consistent nature of the hindlimb responses, the principal directions were almost mirror images of one another. The left hindlimb generated principal directions of 82 and −96 (276), whereas the right hindlimb produced principal directions of 96 and −80 (or 260). This result generally demonstrates symmetry between the hindlimb force responses, despite the small imbalances accommodated by the tail clamp.
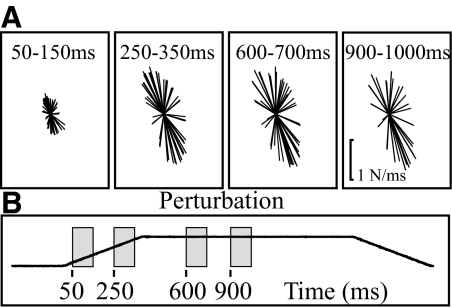
Time epoch affects the magnitude of the force responses but had little effect on shape index and principal direction. Figure 5 depicts the representative right hindlimb force responses from one experiment taken at four different time epochs. The first two time periods are during the beginning and end of the dynamic or perturbation phase (50–150, 250–350 ms), whereas the second two time periods are during the steady-state or hold period (600–700, 900–1000 ms). The data from three experiments were further quantified (shape index and principal direction) and are reported in Table 1. The first two panels of Fig. 5A show that the force responses increase in amplitude through the dynamic phase, reaching a maximum that is maintained through the steady-state hold period. These amplitude shifts are also seen in the raw force traces depicted in Fig. 1. The second time period of the hold phase does demonstrate a slight adaptation with forces slightly smaller during this phase than the first time period of the hold.
Fig. 5.
Horizontal force trajectories during different time epochs. Representative force traces of the right hindlimb (9/7) during 4 time periods (50–150, 250–350, 600–700, and 900–1,000 ms) are depicted. All forces were graphed on the same scale across experiments and conditions. The force constraint or shape index appears to be maintained through the time periods. However, the amplitude is affected by time. Shape index and principal directions were further quantified for this and 2 additional experiments and are presented in Table 1.
Table 1.
Principal direction (PD) and shape for four different time epochs in three representative experiments
| Time, ms | Shape Index | Rostral PD | Caudal PD |
|---|---|---|---|
| 9/11/2007 | |||
| 50–150 | 0.36 (0.07) | 92.4 (2.8) | 279.6 (9.1) |
| 250–350 | 0.37 (0.33) | 92.4 (1.5) | 277.2 (8.3) |
| 600–700 | 0.33 (0.08) | 89.6 (2.0) | 274.1 (13.3) |
| 900–1,000 | 0.34 (0.08) | 89.4 (1.9) | 274.7 (11.6) |
| 10/8/2007 | |||
| 50–150 | 0.28 (0.02) | 91.5 (2.9) | 267.1 (1.8) |
| 250–350 | 0.35 (0.03) | 90.2 (0.6) | 271.1 (1.3) |
| 600–700 | 0.29 (0.03) | 93.1 (1.3) | 269.1 (3.7) |
| 900–1,000 | 0.28 (0.04) | 90.2 (1.3) | 270.6 (1.6) |
| 9/7/2007 | |||
| 50–150 | 0.37 (0.03) | 101.8 (4.2) | 250.3 (3.8) |
| 250–350 | 0.32 (0.01) | 103.1 (1.3) | 251.0 (2.4) |
| 600–700 | 0.36 (0.01) | 95.9 (2.2) | 248.9 (3.1) |
| 900–1,000 | 0.37 (0.01) | 92.8 (0.6) | 248.9 (3.1) |
Mean and SD values of the shape index and principal direction of the right hindlimb were evaluated over four time windows (50–150, 250–350, 600–700, and 900–1,000 ms) in three experiments (9/11, 10/8, and 9/7).
Whereas the amplitude of the force responses is altered by time epoch, the shape index and principal direction are affected minimally across all time epochs; t-tests confirmed that principal directions are statistically equivalent in all three experiments. Furthermore, the shape indices for all time epochs are found to be statistically equivalent to the first time epoch except the second time epoch of the dynamic phase in the 10/8 and 9/7 experiments. Although these two were shown to be statistically different, neither represents a difference >11% from the initial time epoch.
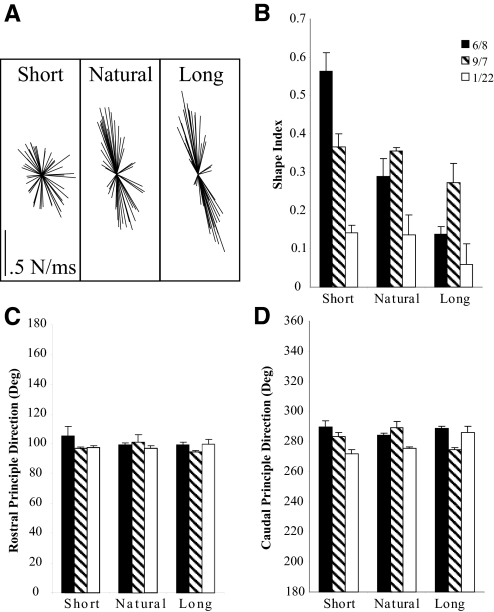
The premammillary decerebrate cat shows more constrained forces as quantified by shape index as stance distance is increased. Figure 6A shows the force responses of the right hindlimb for the 6/8 experiment under all three stance conditions: short, natural, and long. The short force trajectories appear more radial, whereas the natural and long conditions show increasing force constraint. Figure 6, B–D depicts the quantification across experiments of the shape index and principal directions for each of the three stance conditions. The shape index values were the most affected by stance condition (Fig. 6A). Whereas the 6/8 experiment showed the most dramatic change in shape index from 0.56 at the shorter stance to 0.14 at the longer stance, all of the experiments show decreasing shape index values. Shape index values from each experiment were statistically different from short to long stance and shape indices from natural to long were highly significantly different (6/8) or showed a trend toward significance (9/7, 1/22). However, only the 6/8 experiment demonstrated highly significant differences between the short and natural conditions. All of the rostral principal directions were found to be statistically indistinguishable in each experiment. The caudal principal directions showed some significant differences across conditions but the percentage difference was not >9%, indicating the shifts were only slight.
Fig. 6.
Shape index of the force responses is most affected by changes in stance condition. Depiction of the right hindlimb force trajectories for 3 different interlimb spacing conditions (6/8 experiment). Both hindlimbs were moved 3 cm forward (short) and backward (long) from natural stance and perturbations reapplied and forces reevaluated. Whereas the 6/8 experiment shows the most dramatic change in shape index from 0.56 at the shorter stance to 0.14 at the longer stance, all of the experiments show decreasing shape index values. Principal directions are less affected by changes in interlimb spacing; however, some statistical significance did occur, although they did not represent changes >9% difference.
DISCUSSION
Summary
Our objective was to quantify the force constraint strategy of the modified premamillary and intercollicular decerebrate cat, including the dependence on stance width. Exerted force responses obtained from active trials were larger and more constrained than the responses obtained while the animal was under anesthesia. We found that the active force responses of both the premammillary and intercollicular decerebrate preparations were constrained and statistically indistinguishable in shape index and principal direction. Time epoch had little effect on the force constraint shape index and direction, whereas stance condition showed differences in shape index but not principal direction. These data show that the muscle activation patterns seen in these animals (Honeycutt et al. 2009) are of appropriate strength to generate functionally relevant force responses. These observations further demonstrate the appropriateness of the decerebrate cat model in evaluating the neural and sensory mechanisms underlying postural control.
Comparison with the intact animal
Both the modified premammillary and intercollicular decerebrate animals produced constrained force responses in all four limbs (i.e., the force constraint strategy) that closely resemble those reported in the intact animal. The decerebrate animal, like the intact animal, showed a stronger force constraint (lower shape index) in the hindlimbs than that in the forelimbs. Furthermore, the decerebrate animal displayed the characteristic dependence on stance condition, producing less constrained responses as interlimb spacing was decreased.
The only distinction from the intact animal noted was smaller variability in principal direction. The decerebrate cat consistently produces an inward rotation of the force constraint varying from 2 to 13° off the rostral/caudal axis. Conversely, the intact animal generates more variable inward rotations ranging from small rotations of 5–10° (control data: Macpherson and Fung 1999; prior experience: Ting and Macpherson 2004) to large rotations of almost 45° (Macpherson 1988a, 1994). The cause for the increased variability in the intact animal is not apparent. It is possible that the constraints brought by our methodological design, including fixation of the head and tail, limit the directionality of the force constraint. Indeed, our method affords more rigorous control of the kinematic conditions such as toe placement 1 cm behind the greater trochanter, sagittal and frontal plane paw spacing, hip height, and foot turnout. The intact animal has more flexibility to choose and adjust its limb configuration. There are also likely biomechanical discrepancies between the animals such as weight, height, and morphological composition that certainly influence the force constraint rotation. Regardless, the inward rotation demonstrated in the decerebrate cat is well within the range of observed principal directions in the intact animal.
Central mechanisms
The data presented here suggest that the force constraint strategy used by terrestrial animals to maintain stability when faced with balance challenges can be generated without the assistance of the cerebral cortices. The data presented herein indicate that the muscular responses generated by the decerebrate cat are of appropriate direction and proportional strength to one another to generate the force constraint strategy. The knowledge that the decerebrate cat can generate the force constraint strategy suggests a critical role for the brain stem and spinal cord in postural control; however, the contribution of each of these structures remains uncertain. Two potential hypotheses regarding the role of these structures are 1) the brain stem provides critical processing necessary for generation of the postural strategy or 2) the brain stem is playing a permissive or scaling role allowing for adaptation to changing circumstances, whereas the spinal cord mediates the directional attributes of muscle activation and provides the necessary integration for production of the response.
Evidence in support of the first hypothesis is that of data demonstrating that the spinalized animal is not able to generate the force constraint strategy. This could indicate that this “postural strategy” requires processing from higher neural structures. Nonetheless, the spinalized cat can generate elements of the postural response, specifically directionally appropriate muscle activation curves in the anterior biceps femoris, sartorius, gluteus medius, rectus femoris, and vastus medialis muscles (Macpherson and Fung 1999). Apart from the magnitude of these responses and the latency at which they occur, these muscle activation patterns have directional tuning similar to that of the activation patterns in the decerebrate and intact animals.
The different temporal patterns and smaller size of the spinalized cat response likely result from transmission through an injured spinal cord. Chronic spinalization results in increased inhibitory neurotransmitters (Edgerton et al. 2001) and flexor neuromuscular degradation (Burns et al. 2007; Potluri et al. 2008), which likely contribute to the small, delayed muscular responses and a lack of flexor activation, respectively. Furthermore, these animals are afflicted with clasp-knife inhibition where strong force generation in muscle, which would occur during the early stages of the postural response, is quickly followed by an abrupt decline of force in those muscles (Bonasera et al. 1994; Nichols and Cope 2001).
Therefore we suspect that the failure of the spinalized animal to generate the force constraint strategy is attributable to the lack of prolonged, strong muscle activation in a broad set of muscles. In contrast, the decerebrate animal, with an intact and uninjured cord, readily generates robust muscle activation patterns in a wide variety of muscles and subsequently generates the force constraint strategy. In light of this, we propose that the inability of the spinalized animal to generate the force constraint strategy is due to the detrimental impact of the injury, which limits appropriate muscle recruitment.
Although we cannot completely refute the first hypothesis, there is accumulating evidence for a supportive or scaling role for the brain stem. Recent recordings from the reticular formation of the chronically implanted cat during postural disturbances have shown that cells respond strongly to foot drop perturbations (Stapley and Drew 2009). However, since these cells reacted similarly to all perturbations regardless of which foot was dropped, the authors suggested that the reticular formation contributes to the overall postural response, but likely does not specify the details of the response necessary for compensation. The authors conjectured instead that the descending command might modulate the excitability of spinal neurons and more generally contribute to the postural response. This complements our results, demonstrating that more robust muscle responses are generated when the brain stem is left intact as in our decerebrate model. All of these results together point to the second hypothesis indicating that the brain stem provides support and/or scaling to the spinal cord, which houses the circuitry to encode the details of the postural response, especially the directional tuning of muscle responses. This pattern, which does not require training, appears to be intrinsic to the spinal cord and therefore constitutes a default strategy for postural control. The relevant brain stem areas could then modify the magnitudes of the responses when the default spinal pattern is inappropriate and would lead to instability.
The evidence presented here suggests the neural organizational structure of postural control parallels that of locomotion, where the spinal cord generates the primary rhythmic activation patterns and the brain stem supports and shapes the expression of those activation patterns. Rhythmic muscle activation is readily generated by the isolated spinal cord (Edgerton et al. 2001), indicating that supraspinal structures are not required for the expression of locomotion. Evidence also exists that mechanisms intrinsic to the spinal cord are responsible for adjusting patterns of muscular activation in response to changes in external loading (Timoszyk et al. 2002). The brain stem has been shown to influence the expression of these spinally mediated patterns through a hierarchical organization—upper brain stem regions, subthalmic nucleus, stimulate lower brain stem regions, mesencephalic locomotor region—leading to activation of locomotor circuits within the spinal cord (Grillner and Shik 1973; Mori et al. 1989).
Evidence for a similar hierarchical framework in postural control (including regional differences in brain stem control) comes from reports demonstrating that lower brain stem regions, specifically the ventral and dorsal tegmental fields (VTF and DTF), can excite or inhibit postural tone, respectively (Mori 1987). These authors additionally used horseradish peroxidase to determine which regions were being stimulated through passing fibers. They determined that the most effective VTF stimulation sites also stimulated upper brain stem regions such as the hypothalamus and subthalamic nucleus, whereas the most effective DTF stimulation sites corresponded with the diencephalon and the dorsal posterior and lateral hypothalamus. Although these authors could not precisely determine the destination of the indirectly stimulated upper brain stem fibers, their report does indicate that increasing the excitability of these upper brain stem regions increases the effectiveness of the lower brain stem regions.
Our data from two levels of brain stem transection reinforce this notion of upper brain stem support of lower brain stem regions. Intercollicular and premammillary decerebrated animals produce statistically indistinguishable force results, implying that the upper brain stem is not required for appropriate force responses. However, intercollicular animals rarely (4 of 28 animals) were able to elicit muscular responses to support surface perturbation. Finally, the close association of the postural tone regions (VTF and DTF) to the expression of locomotion further indicates that these two systems are likely similarly controlled. These observations strongly imply that regions rostral to the superior colliculus control the expression of the lower brain stem (regions caudal to the superior colliculus), which in turn support the generation of the force constraint strategy in the spinal cord.
Origins of the force constraint
If indeed the spinal cord houses the primary circuitry required to generate the force constraint strategy, this suggests that the strategy itself is not created out of supraspinal processing. Instead, it becomes more likely that the strategy arises out of the mechanical organization of the limb musculature. By fixing the head and the tail of the cat, we were able to observe the forces without the inertial influences of body movement. The inertial influences of the limbs, as observed during anesthesia trials, are mostly radial in nature and do not demonstrate a strong force constraint. This implies that the exerted forces generated by muscular activation are primarily responsible for the clustering of force responses in the decerebrate animal. Generally, the nonsagittal actions of the hindlimb musculature are not as strong as the actions in parasagittal planes. Experimental evidence comes from intramuscular stimulation data in decerebrate cat (Honeycutt 2009). Three-dimensional exerted force measurements of 17 hindlimb muscles were evaluated. Data demonstrated that most muscles had small nonsagittal actions. Furthermore, when interlimb spacing was altered to shorter distances between the hindlimbs and forelimbs, the nonsagittal actions resulting from intramuscular stimulation became stronger, corresponding to similar adjustments in the force constraint strategy during postural perturbations. This mechanical basis of the strategy is further substantiated by empirical model results demonstrating that the feasible force set—the calculated maximum force that the limb musculature can exert in all directions—is constrained in a similar fashion to the force constraint strategy (McKay et al. 2007). These considerations suggest that the arrangement of muscles and force-transmitting structures in the limb and short-latency spinal pathways underlie the force constraint strategy and its variation in different orientations of the limb.
GRANTS
This work was supported by National Institutes of Health Grants NS-20855 and HD-32571.
ACKNOWLEDGMENTS
We thank V. Stahl, Dr. Jinger Gottschall, Dr. Kyla T. Ross, and Dr. Clotilde Huyghues-Despointes for assistance in data collection and surgical expertise; B. Goolsby for creation of the software necessary for data collection; R. Kiser and G. Schmitt for construction of major equipment; and Dr. Eric Perreault for assistance with the stiffness ellipse analysis.
REFERENCES
References
- Adkin AL, Quant S, Maki BE, McIlroy WE. Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp Brain Res 172: 85–93, 2006 [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during postural corrections. J Neurophysiol 93: 1831–1844, 2005 [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA, Orlovsky GN, Popova LB, Deliagina TG. Activity of different classes of neurons of the motor cortex during postural corrections. J Neurosci 23: 7844–7853, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasera SJ, Pratt CA, Price CMJI, Cope TC, Nichols TR. Stance training preserves intermuscular reflexes and muscle properties in chronic spinal cats. Soc Neurosci Abstr 20: 572, 1994 [Google Scholar]
- Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev 81: 539–568, 2001 [DOI] [PubMed] [Google Scholar]
- Burns AS, Jawaid S, Zhong H, Yoshihara H, Bhagat S, Murray M, Roy RR, Tessler A, Son YJ. Paralysis elicited by spinal cord injury evokes selective disassembly of neuromuscular synapses with and without terminal sprouting in ankle flexors of the adult rat. J Comp Neurol 500: 116–133, 2007 [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev 57: 212–221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology (Bethesda) 21: 216–225, 2006 [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol 533: 15–22, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Shik ML. On the descending control of the lumbosacral spinal cord from the “mesencephalic locomotor region.” Acta Physiol Scand 87: 320–333, 1973 [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. EMG responses to maintain stance during multidirectional surface translations. J Neurophysiol 80: 1939–1950, 1998 [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. Effect of stance width on multidirectional postural responses. J Neurophysiol 85: 559–570, 2001 [DOI] [PubMed] [Google Scholar]
- Honeycutt CF. Mechanisms Underlying Muscle Recruitment in Response to Postural Perturbations (PhD dissertation) Atlanta, GA: Georgia Institute of Technology/Emory University, 2009 [Google Scholar]
- Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol 101: 2751–2761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Nichols TR. Force responses of the postural strategy in the decerebrate cat. Soc Neurosci Abstr 452.5, 2006 [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm 114: 1339–1348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JA, Brown RH, Stapley PJ. Reaching to multiple targets when standing: the spatial organization of feedforward postural adjustments. J Neurophysiol 101: 2120–2133, 2009 [DOI] [PubMed] [Google Scholar]
- Lyalka VF, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol 94: 3677–3690, 2005 [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol 60: 204–217, 1988a [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. II. Electromyographic activity. J Neurophysiol 60: 218–231, 1988b [DOI] [PubMed] [Google Scholar]
- Macpherson JM. The force constraint strategy for stance is independent of prior experience. Exp Brain Res 101: 397–405, 1994 [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Fung J. Weight support and balance during perturbed stance in the chronic spinal cat. J Neurophysiol 82: 3066–3081, 1999 [DOI] [PubMed] [Google Scholar]
- Magnus R. Physiology of posture. Lancet 11: 531–581, 1926 [Google Scholar]
- McKay JL, Burkholder TJ, Ting LH. Biomechanical capabilities influence postural control strategies in the cat hindlimb. J Biomech 40: 2254–2260, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol 28: 161–195, 1987 [DOI] [PubMed] [Google Scholar]
- Mori S, Sakamoto T, Ohta Y, Takakusaki K, Matsuyama K. Site-specific postural and locomotor changes evoked in awake, freely moving intact cats by stimulating the brainstem. Brain Res 505: 66–74, 1989 [DOI] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Lyalka VF, Orlovsky GN, Deliagina TG. Postural performance in decerebrated rabbit. Behav Brain Res 190: 124–134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR, Cope TC. The organization of distributed proprioceptive feedback in the chronic spinal cat. In: Methods and Frontiers in Neurobiology, edited by Cope TC. Boca Raton, FL: CRC Press, 2001, p. 305–326 [Google Scholar]
- Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Exerc Sport Sci Rev 27: 255–284, 1999 [PubMed] [Google Scholar]
- Nichols TR, Wilmink RJH, Burkholder TJ. The multidimensional and temporal regulation of limb mechanics by spinal circuits. In: Progress in Motor Control: Structure–Function Relations in Voluntary Movements, edited by Wrisberg CA, Latash ML.Champaign, IL: Human Kinetics, 2002, p. 179–194 [Google Scholar]
- Potluri S, Himes TB, Hyun J, Tessler A, Son YJ. Selective vulnerability of neuromuscular junctions in ankle flexors to the paralysis elicited by spinal cord injury. Soc Neurosci Abstr 572.13, 2008 [Google Scholar]
- Sherrington CS. Decerebrate rigidity and reflex co-ordination of movements. J Physiol 22: 319–332, 1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol 101: 1334–1350, 2009 [DOI] [PubMed] [Google Scholar]
- Stein PS. Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Rev 57: 118–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube W, Schubert M, Gruber M, Beck S, Faist M, Gollhofer A. Direct corticospinal pathways contribute to neuromuscular control of perturbed stance. J Appl Physiol 101: 420–429, 2006 [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, De Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J Neurophysiol 88: 3108–3117, 2002 [DOI] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. Ratio of shear to load ground-reaction force may underlie the directional tuning of the automatic postural response to rotation and translation. J Neurophysiol 92: 808–823, 2004 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96: 1530–1546, 2006 [DOI] [PubMed] [Google Scholar]
- Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol 49: 481–515, 1996 [DOI] [PubMed] [Google Scholar]