Abstract
The Kv1.3 voltage-dependent potassium channel is expressed at high levels in mitral cells of the olfactory bulb (OB). Deletion of the Kv1.3 potassium channel gene (Kv1.3−/−) in mice lowers the threshold for detection of odors, increases the ability to discriminate between odors, and alters the firing pattern of mitral cells. We have now found that loss of Kv1.3 produces a compensatory increase in Na+-activated K+ currents (KNa) in mitral cells. Levels of the KNa channel subunit Slack-B determined by Western blotting are substantially increased in the OB from Kv1.3−/− animals compared with those of wildtype animals. In voltage-clamp recordings of OB slices, elevation of intracellular sodium from 0 to 60 mM increased mean outward currents by 15% in mitral cells from wildtype animals and by 40% in cells from Kv1.3−/− animals. In Kv1.3−/− cells, KNa current could even be detected with 0 mM Na+ internal solutions, provided extracellular Na+ was present, and this current could be abolished by TTX and ZD7288, blockers of Na+ influx through voltage-dependent Na+ channels and H-channels, respectively. The role of enhanced expression of Slack subunits in the increase of KNa current in Kv1.3−/− cells was also confirmed using an RNA interference (RNAi) approach to suppress Slack expression in primary cultures of olfactory neurons. In Kv1.3−/− neurons, treatment with Slack-specific RNAi inhibited approximately 75% of the net outward current, whereas in wildtype cells, the same treatment suppressed only about 25% of the total current. Scrambled and mismatched RNAi oligonucleotides failed to suppress currents. Our findings raise the possibility that the olfactory phenotype of Kv1.3−/− animals results in part from an enhancement of KNa currents.
INTRODUCTION
Kv1.3, a voltage-gated potassium channel of the Shaker subfamily, is expressed at relatively high levels in olfactory bulb (OB) mitral cells, which relay signals from olfactory sensory neurons to central brain olfactory regions (Colley et al. 2004). Deletion of the Kv1.3 gene in mice alters the characteristics of the outward potassium currents and changes the shape of action potentials and the firing pattern of these cells (Fadool et al. 2004). For example, isolated wildtype neurons typically respond to intracellular current pulses with sustained repetitive firing of action potentials, whereas neurons from Kv1.3−/− mice fire a more rapid train of action potentials that progressively decrease in amplitude after the onset of stimulation. Deletion of Kv1.3 also produces changes in signaling pathways and in the number of synaptic glomeruli in the OB and results in a 1,000- to 10,000-fold lower threshold for detection of odors and an increased ability to discriminate between odorants (Biju et al. 2008; Fadool et al. 2004).
Neurons of the olfactory bulb (OBNs) also express K+ channels that are gated by changes in intracellular Na+ levels (KNa channels) (Bhattacharjee et al. 2002). Immunocytochemical labeling indicates that two KNa channel subunits, Slack and Slick (also termed Slo2.2 and Slo2.1, respectively), are expressed at relatively high levels in mitral cells (Bhattacharjee et al. 2002, 2005). KNa channels can be readily detected in single channel recordings of patches from cultured OBNs (Egan et al. 1992b) and in membranes reconstituted from the OB (Egan et al. 1992a). Although the Slack gene can give rise to several different splice variants that are expressed in different cell types, OBNs specifically express high levels of mRNA for one variant, termed Slack-B (Brown et al. 2008). In the absence of the Slack-B subunit, which is required for heteromer formation between Slack and Slack channel subunits (8,9), Slick KNa channel subunits fail to be trafficked efficiently to the plasma membrane (Chen et al. 2009).
Studies with a variety of neurons have indicated that the presence of KNa channels can lead to an adaptation of firing rate in response to maintained stimulation similar to that observed in Kv1.3−/− mitral cells (Bhattacharjee and Kaczmarek 2005; Brown et al. 2008; Descalzo et al. 2005; Foehring et al. 1989; Franceschetti et al. 2003; Kim and McCormick 1998; Sanchez-Vives et al. 2000; Sandler et al. 1998; Schwindt et al. 1989; Wallen et al. 2007). We therefore compared the expression of KNa currents in slices of the OB and in cultured OBNs acquired from wildtype and Kv1.3−/− mice. We found that Slack-dependent KNa currents account for as much as 75% of the tetraethylammonium (TEA)-resistant K+ current in Kv1.3−/− olfactory neurons, compared with only about 25% in wildtype cells. The enhanced expression of KNa channels in Kv1.3-deficient animals may be one component of their extraordinary olfactory phenotype.
METHODS
Plasmids, reagents, and animals
All RNA interference (RNAi) reagents were purchased from Qiagen (Valencia, CA). The nucleotide sequence of RNAi-2757 was CAG GTC ATT GTG GCC ACA ATA, that for RNAi-2581 was CAG GTG GAA TTC TAT GTC AAT, and that for RNAi-2574 was CTG GTC GGG TGT TTA GTA TCA. The control scrambled RNAi negative control sequence was AAT TCT CCG AAC GTG TCA CGT. For investigation of the effects of RNAi reagents on Slack expression in Human Embryonic Kidney 293 cells (HEK cells), we used HEK cells stably transfected with rSlack-enhanced green fluorescent protein (EGFP) (Chen et al. 2009). The refractory Slack mutant (Niwa and Slack 2007) was constructed in the background of rSlack-EGFP (Chen et al. 2009) and the primers used for the mutagenesis were GTG GGT GAT CCA GGT CAT AGT CGC GAC AAT AAG CTT CTT AGAG and CTC TAA GAA GCT TAT TGT CGC GAC TAT GAC CTG GAT CAC CCAC. Cysteine-activated papain, trypsin inhibitor, cytosine arabinoside, and poly-d-lysine hydrobromide (MW 49,300–53,000) were purchased from Sigma-Aldrich (St. Louis, MO). The H-channel inhibitor ZD-7288 and Na+ channel blocker tetrodotoxin (TTX) were from Tocris Bioscience (Ellisville, MO). Dulbecco's modified Eagle medium (DMEM) was from Invitrogen (Carlsbad, CA) and fetal bovine serum (FBS) was from Gemini Bio-Products (West Sacramento, CA).
Wildtype (C57BL/6) and Kv1.3−/− mice were used for this study. The properties and generation of the Kv1.3−/− mice, in which a large part of the promoter region and the N-terminal third of the Kv1.3 coding sequence were deleted, have been described previously (Fadool et al. 2004; Koni et al. 2003).
Western blotting
Postnatal day (P) 20 mice were killed by CO2 inhalation in accordance with Florida State University Laboratory Animal Resources and AVMA-approved methods. Olfactory bulbs (OBs) were quickly harvested after decapitation. OBs were immediately homogenized in homogenization buffer (HB, in mM: 320 sucrose, 10 Tris base, 50 KCl, 1 EDTA [pH 7.8]) for 50 strokes with a Kontes tissue grinder (size 20) on ice. HB processed bulbs were used to isolate membrane proteins as previously described (Tucker and Fadool 2002). Membrane proteins (25 μg/lane) were separated on 8–10% acrylamide gels by SDS-PAGE and electrotransferred to nitrocellulose blots as also previously described (Cook and Fadool 2002; Tucker and Fadool 2002).
Nitrocellulose was incubated overnight at 4°C with polyclonal antisera to either Slack B or Slick (1:4,000) and then subunit expression was probed with horseradish peroxidase-conjugated rabbit anti-chicken secondary antibody (1:5,000; Millipore) for 90 min at room temperature (RT). Enhanced chemiluminescence (ECL; Amersham-Pharmacia) exposure on Fugi Rx film (Fisher Scientific, Waltham, MA) was used to visualize labeled proteins. The film autoradiographs were analyzed by quantitative densitometry using a Hewlett-Packard PhotoSmart Scanner (Model 106–816; Hewlett-Packard, San Diego, CA) in conjunction with Quantiscan software (Biosoft, Cambridge, UK). After probing with antibodies against Slack-B or Slick, the blots were stripped and reprobed with monoclonal antiserum against neuron-specific enolase (NSE) at 1:4,500. Line scanning densitometry was performed for channel and NSE probed blots and then pixel quantification was normalized to that of the wildtype condition. Channel expression was then computed as a ratio calculated as the NSE-normalized, Kv1.3−/− signal divided by the NSE-normalized, wildtype (WT) signal (Tucker and Fadool 2002).
Olfactory bulb slice preparation
Coronal slices (350–400 μM) from P7–P14 mice were prepared by conventional methods (Song et al. 2005) and transferred to a recording chamber that was perfused continuously (2 –3 ml/min) with artificial cerebral spinal fluid (ASCF, in mM: 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2 sodium pyruvate, 0.4 ascorbic acid, 3 myo-inositol, 10 glucose, 2.0 CaCl2, and 1 MgCl2, gassed with 95% O2-5% CO2, pH 7.4, at 21–23°C).
Olfactory bulb primary cell culture
OBs were harvested from P2–P4 mice and neuronal primary cultures were prepared using the procedure of Huettner and Baughman (1986) as modified by Egan et al. (1992b) and Fadool et al. (2000). Animals were killed by decapitation according to ICUCC-approved methods. OBs were removed quickly from the cranium and placed into 10 ml serum free DMEM equilibrated previously at 37°C in a 5% CO2 incubator. Olfactory bulbs from four to five animals were incubated whole in a physiological saline solution containing cysteine-activated papain for 1 h at 37°C in the 5% CO2 incubator. The bulbs were then washed in DMEM containing 5% FBS and 5 mg/ml trypsin inhibitor for 10 min to stop the enzymatic activity of the papain. Cells were dissociated by trituration by the use of a graded-size series of fire-polished siliconized Pasteur pipettes. In contrast to earlier studies of cultured neurons of wildtype and Kv1.3−/− animals, the cells were not plated onto a monolayer of astrocytes (Colley et al. 2004; Fadool et al. 2004). Instead the resulting neuron and glia suspension was plated directly onto poly-d-lysine-coated 12-mm glass coverslips and incubated in DMEM supplemented with 2% penicillin/streptomycin and 5% FBS. Cytosine arabinoside (10 μM) was added to the medium for 36 h between days 3 and 5 to stop the overgrowth of dividing cells and to promote better survival of the neurons. Growth medium was changed twice a week. Neurons were used for patch recording 2 to 7 days after plating.
Whole cell patch-clamp recording
Whole cell recordings (Hamill et al. 1981) were made from mitral cells in slices of OB, cultured OB, and transfected HEK cells using an EPC-7 amplifier (HEKA Elektronik, Lambrecht, Germany) for voltage-clamp recordings. All of the experiments were performed at RT (21–22°C). Electrodes for whole cell recordings had a resistance of 3–4 MΩ when filled with an intracellular solution containing the following (in mM): 60 NaCl, 80 KCl, 5 EGTA, and 10 HEPES. When no [Na]i was added, 60 mM NMDG (N-methyl-d-glucamine) was used as the substitute. Cell capacitance and series resistances were electronically compensated using the compensation controls of the EPC-7 patch clamp, which were also used to estimate cell capacitance. The series resistance for whole cell recordings from mitral cells was 8–12 MΩ and compensated at 30–50%. Under these conditions, at the most positive command potentials (> +50 mV) errors in potential applied at the cell are expected to be within approximately 4–8% of the command potential. No corrections were made for liquid-junction potentials. Recordings were rejected if the holding current at −90 mV was >50 pA (cultured cells) or 100 pA (cells in slices) or if the series resistance was >15 MΩ. Data were filtered at 2 kHz, sampled at 10 kHz, and analyzed using Clampfit v. 9.0 software (Molecular Devices, Sunnyvale, CA). To eliminate potential contributions of altered sodium transients to the measurements of the amplitude of KNa currents, current–voltage (I–V) relations were always plotted for current measured at the end of command pulses, when sodium levels are likely to have reached a steady state. Statistical data were analyzed with the Student's t-test except where noted. All data are presented as means ± SE.
RNAi transfection
One day after the seeding of the primary culture of neurons, 1.5 μl RNAi (20 μM) was mixed with 10 μl of HiPerfect and DMEM without serum and the volume was adjusted to 100 μl. Within 10 min after mixing at RT, the complex was added to the cells in 35 mm culture dishes, which were then shaken slowly and incubated at 37°C in a 5% CO2 incubator for 3 h. The medium was then changed to normal DMEM/FBS culture medium with antibiotics. Whole cell patch-clamp recordings were carried out ≥3 days after RNAi transfection.
To test the effects of RNAi on wildtype and refractory Slack-B channels expressed in HEK293 cells, the cells were transfected with rSlack-B or the refractory mutant rSlack-B using Superfect in accordance with the manufacturer's protocol (Qiagen). One day later, 1.5 μl RNAi (20 μM) was mixed with 10 μl of HiPerfect plus DMEM without serum and the volume was adjusted to 100 μl. Subsequent incubation steps were as for the primary neuronal cultures and recordings were carried out 1–3 days after transfection.
RESULTS
Expression of Slack-B is enhanced in OBs of Kv1.3−/− mice
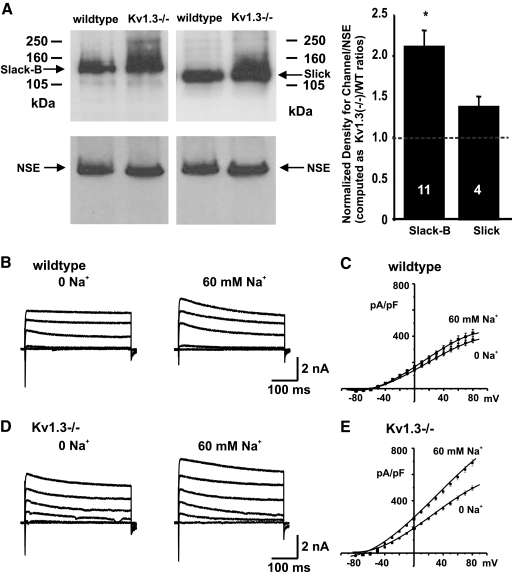
Immunoblotting was used to examine relative levels of the Slack-B and Slick KNa channel subunits in OB membrane preparations from wildtype and Kv1.3−/− mice (Fig. 1A). A previously characterized antibody directed against the Slack-B-specific N-terminal domain was used to detect Slack subunits (Bhattacharjee et al. 2002; Brown et al. 2008). To detect Slick subunits, an antibody that recognizes an epitope in the cytoplasmic C-terminal domain of Slick that has no similarity with Slack was used. The properties, specificity, and distribution of immunoreactivity for this antibody have also been described previously (Bhattacharjee et al. 2005; Chen et al. 2009). Quantitative densitometry indicated that the level of Slack-B was increased over twofold in the OB of Kv1.3−/− mice over that in wildtype mice (Fig. 1A, P < 0.0025, n = 11, Student's t-test with arc-sine transformation). The increase in pixel density found for Slick immunoreactivity did not reach statistical significance (Fig. 1A). No changes were detected on Western blots in levels of neuron-specific enolase (NEN), a control protein not expected to change with the presence or absence of Kv1.3 (Fig. 1A).
Fig. 1.
Slack levels and KNa currents are enhanced in the olfactory bulb (OB) of Kv1.3−/− mice. A: immunoblots of OB membrane fractions from P20 wildtype and Kv1.3−/− mice were probed with antibodies against Slack-B or Slick (top left) and then stripped and reprobed with monoclonal antiserum against neuron-specific enolase (NSE) at 1:4,500 (bottom left). Arrows denote the expected Mr for each protein. Bar graph on the right shows the mean (±SE) pixel immunodensity of labeled Slack-B and Slick proteins. Line scanning densitometry was performed for channel and NSE probed blots and then pixel quantification was normalized to that of the wt condition. Channel expression was then computed as a ratio calculated as the NSE-normalized, Kv1.3−/− signal divided by the NSE-normalized, wildtype (WT) signal. Sample sizes are indicated on the bar graph. * Significantly different, arc-sine transformation for percentage data, Student's t-test, alpha <0.0025. B: representative whole cell currents in mitral cells in slices of OB acquired from WT mice, recorded with either 0 or 60 mM Na+ in the patch pipette and with 1 mM tetraethylammonium (TEA) in the extracellular solution. Currents were evoked by stepping from a holding potential of −80 mV to test potentials of −80, −50, 10, 40, and 70 mV for 400 ms. C: grouped current–voltage (I–V) relations with 0 or 60 mM Na+ in the pipette (n = 19, 16, respectively). D and E: as for B and C but for Kv1.3−/− mice (n = 21, 30, respectively).
KNa currents are enhanced in mitral cells of Kv1.3−/− mice
To determine whether the increased levels of Slack-B immunoreactivity in the OBs of Kv1.3−/− animals were associated with an increased level of KNa current, we carried out whole cell recordings of mitral cells in OB slices, using an intracellular pipette solution that contained either 0 or 60 mM Na+, a concentration close to that reported for 50% activation of KNa channels in different systems (Bhattacharjee and Kaczmarek 2005; Bhattacharjee et al. 2003). To minimize the contribution of other K+ channel subunits to the outward K+ current, we added the K+ channel blocker TEA (1 mM) to the external solution. Slack and Slick channels are insensitive to this level of TEA (Bhattacharjee et al. 2003; Joiner et al. 1998; Yang et al. 2006). For mitral cells from both wildtype and Kv1.3−/− animals, the level of outward current was greater when the internal solution contained 60 mM Na+ rather than zero Na+, consistent with the presence of KNa currents across both genotypes (Fig. 1, B and D). Examination of the grouped current density–voltage (I–V) relations for the two genotypes, however, clearly showed that elevation of Na+ to 60 mM produced a much greater increase in current magnitude in mitral neurons from Kv1.3−/− mice than that from wildtype (Fig. 1, C and E). In neurons from Kv1.3−/− mice, increasing Na+ from 0 to 60 mM produced an approximately 40% increase in the mean outward current density (from 419 ± 17 pA/pF [n = 21] to 580 ± 22 pA/pF [n = 30], measured at +60 mV, P < 0.001). In contrast, elevation of Na+ to 60 mM in neurons from wildtype animals produced only an approximately 14% increase in outward current density (from 323 ± 20 pA/pF [n = 19] to 376 ± 27 pA/pF [n = 16]).
KNa channels are activated by Na+ influx through NaV channels and H-channels
In cells transfected with the Slack-B gene, it is possible to record macroscopic currents in the whole cell mode even when the pipette solution contains no added Na+ ions (Bhattacharjee et al. 2003; Joiner et al. 1998). In contrast, removal of Na+ from the cytoplasmic face of excised inside-out patches containing Slack-B channels eliminates channel activity (Bhattacharjee et al. 2003). This suggests that, in whole cell recordings, Slack channels may be activated by Na+ influx through the plasma membrane, even with nominally Na+-free internal solutions. To test this possibility for mitral cells in slices of OB, neurons were exposed to TTX (1 μM), a specific blocker of voltage-dependent Na+ channels, and to ZD7288 (20 μM), a specific blocker of Ih channels, which are Na+-permeable nonselective cation channels. In initial whole cell experiments with mitral cells from Kv1.3−/− mice, application of either TTX or ZD7288 alone produced a variable 15–20% reduction in outward current at +60 mV(n = 6, TTX; n = 4, ZD7288) when a 0 Na+ internal solution was used. To quantify further the contribution of Na+ influx to activation of KNa currents, the effect of co-application of both channel blockers was tested using intracellular pipettes containing either 0 or 60 mM Na+ ions.
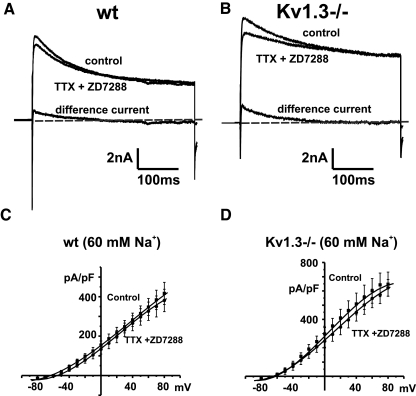
Co-application of the channel blockers produced only a minimal change in net outward current when KNa currents were already activated by high intracellular Na+ (60 mM) in the pipette solution (Fig. 2, A and B). No statistically significant difference was found in the mean I–V relations measured at the end of a 400 ms command pulse for either genotype before and after co-application of TTX and ZD7288 (Fig. 2, C and D; n = 6, wildtype; n = 6, Kv1.3−/−).
Fig. 2.
Effects of tetrodotoxin (TTX) and ZD7288 on outward currents in mitral cells recorded in a slice preparation with 60 mM internal Na+ ions. A: whole cell currents evoked by stepping from −80 to +30 mV for 400 ms before (control) and after application of TTX (1 μM) and ZD7288 (20 μM) to block voltage-dependent Na+ current and H-current in wild type (WT) mitral cells. Also shown is the difference current obtained by subtraction of the current after application of TTX and ZD7288 from the control current (difference current). B: as for A, but for Kv1.3−/− mice. Holding current in A and B is represented by a dashed gray line. C: grouped current–voltage (I–V) relations for mitral cells recorded using 60 mM Na+ in the pipette before (control) and after application of TTX and ZD7288 (TTX + ZD7288) (n = 6) for wt (C) and Kv1.3−/− (D) mice.
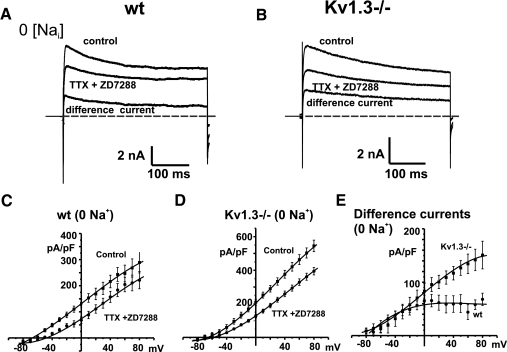
In contrast, co-application of these channel blockers to neurons recorded with a zero Na+ internal solution, a condition under which Na+ influx contributes to activation of KNa currents, produced a reduction in outward currents in mitral cells from both wildtype and Kv1.3−/− animals (Fig. 3, A and B). An examination of the grouped data I–V curves showed that the reduction in outward current was greater for mitral cells from Kv1.3−/− mice (Fig. 3, C and D; n = 7, wildtype; n = 7, Kv1.3−/−). The difference currents obtained by subtracting the traces obtained after application of TTX and ZD7288 from those before addition of these agents (Fig. 3, A, B, and E) were also calculated. These difference currents recorded with zero internal Na+ represent KNa currents activated by Na+ influx. At a command potential of +60 mV, the difference currents recorded for neurons from Kv1.3−/− mice (134 ± 18 pA/pF, n = 7) were significantly greater than those measured in neurons from wildtype mice (44 ± 14 pA/pF, n = 7, P < 0.01).
Fig. 3.
Effects of TTX and ZD7288 on outward currents in mitral cells recorded in a slice preparation with 0 mM internal Na+ ions. A: whole cell currents evoked by stepping from −80 to +30 mV for 400 ms before and after application of TTX (1 μM) and ZD7288 (20 μM) to block voltage-dependent Na+ current and H-current in wild type (WT) mitral cells. Also shown is the difference current obtained by subtraction of the current after application of TTX and ZD7288 from the control current. B: as for A, but for Kv1.3−/− neurons. Holding current in A and B is represented by a dashed gray line. C: grouped I–V relations for mitral cells from wildtype mice with zero Na+ in the pipette before and after application of TTX and ZD7288 (n = 7). D: as for C, but for mitral cells from Kv1.3−/− mice (n = 8). E: grouped I–V relations for the current density of the difference currents for wt and Kv1.3−/− mitral cells recorded with zero internal Na+ ions.
Enhanced KNa current in Kv1.3−/− mice results from increased Slack gene expression
To determine whether the basal KNa currents in mitral neurons of wildtype animals and the increase in these currents in Kv1.3−/− mice can be directly attributed to changes in gene expression of Slack channel subunits, we used an RNA interference (RNAi) approach to down-regulate expression of Slack subunits. For this purpose, a 21-mer RNAi oligonucleotide (called RNAi-2757) that is an exact match to a sequence corresponding to part of transmembrane segment S2 in the mouse Slack gene was designed.
To test the functional efficacy of RNAi-2757, we first examined its effect on voltage-activated whole cell currents in HEK cells transfected with the Slack-B gene. Currents were recorded from Slack-B expressing cells, ≥24 h after transfection with RNAi-2757. We found that treatment with RNAi-2757 led to a >80% elimination of Slack-B currents relative to cells not treated with this RNAi (Fig. 4, A and B; reduction from 143 ± 32 to 27 ± 5 pA/PF at +60 mV [n = 7, 6, respectively]). As a control for the specificity of action of this RNAi reagent, a refractory mutant of the Slack-B gene was generated. Specifically three point mutations (T1969A 1971C and C1974G) were made within the target region for RNAi-2757. These three nucleotide substitutions do not alter the amino acid sequence in this region but destroy the match between RNAi-2757 and its target in the Slack gene. The expression of this refractory Slack-B construct in HEK cells was not found to be affected by treatment with RNAi-2757 (Fig. 4, C and D; change from 56 ± 8 to 52 ± 8 pA/PF at +60 mV; n = 12, 15, respectively), indicating that RNAi-2757 acts specifically on the wildtype Slack-B gene to reduce expression.
Fig. 4.
Slack-specific RNA interference (RNAi) eliminates currents in Slack-B-expressing Human Embryonic Kidney 293 (HEK) cells. A: recordings of whole cell outward current evoked by stepping from a holding potential of −80 mV to test potentials of −80, −50, 10, 40, and 70 mV for 400 ms in control Slack-B-transfected HEK cells (left) and in those transfected with RNAi-2757 1 day after transfection with the Slack-B gene (right). Recordings were carried out 2 days after transfection with RNAi-2757. B: grouped I–V relations for Slack-B-transfected HEK cells in the absence or presence of RNAi-2757 (n = 7, 7). C: lack of effect of RNAi-2757 transfection on a refractory Slack-B mutant expressed in HEK cells. The refractory mutant gene has the same amino acid sequence as wt Slack-B but has 3 mismatched nucleotides in the target sequence for RNAi-2757. Voltage paradigm as in A. D: grouped I–V relations for refractory Slack-B mutant-transfected HEK in the absence or presence of RNAi-2757 (n = 12, 15, respectively).
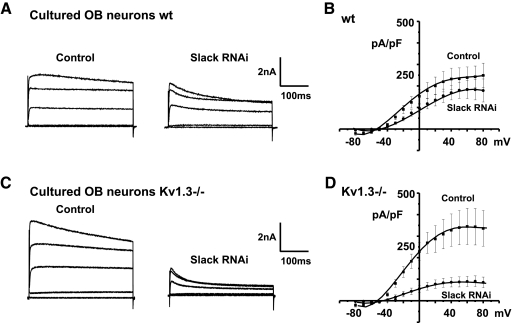
To examine the effects of down-regulation of Slack expression on native neuronal KNa currents, primary cultures of isolated olfactory neurons that have been used previously to characterize K+ currents in wildtype and Kv1.3−/− neurons were used (Fadool et al. 2004). Mitral cells in such cultures can readily be distinguished from other cell types by their size and pyramidal morphology. Currents in the isolated neurons were recorded using pipettes containing 60 mM Na+ ions. Treatment with RNAi-2757 reduced the net outward current in mitral cells prepared from both wildtype and Kv1.3−/− mice. Consistent with the results obtained using OB brain slices, the suppression of outward current was significantly greater for neurons from Kv1.3−/− mice than that from wildtype mice. In the wildtype cultured cells, RNAi-2757 inhibited about 23% of the current (Fig. 5, A and B; n = 5). In contrast, in the Kv1.3−/− cultured cells, RNAi-2757 inhibited approximately 75% of the outward current (Fig. 5, C and D; from 345 ± 84 to 89 ± 24 pA/pF at +60 mV; n = 7, 7, respectively).
Fig. 5.
Slack-specific RNAi eliminates a component of outward current in cultured primary OB mitral cells. A and C: representative whole cell currents under control (control) or RNAi-2757 transfected conditions (Slack RNAi) using neurons from wildtype (wt) or Kv1.3−/− mice. Currents were recorded with an internal solution containing 60 mM Na+ ion and evoked by stepping from −80 mV to test potentials of −80, −50, 10, 40, and 70 mV for 400 ms. B and D: grouped I–V relations in the absence or presence of RNAi-2757 (n = 7, 9 and 5, 7, respectively).
As a control for the effects of RNAi-2757 in cultured mitral cells of wildtype and Kv1.3−/− mice, the effects of a scrambled RNAi oligonucleotide on outward current magnitude was examined. No significant effect on outward current density was observed with the scrambled RNAi under conditions identical to those used for RNAi-2757 (Control, 336 ± 83 pA/pF [n = 7]; RNAi-2757, 254 ± 34 pA/pF [n = 7] at +80 mV). In addition we assayed the effects of two other 21-mer RNAi oligonucleotides, with sequences that differ from a perfect match with the mouse Slack target sequence by either one (RNAi-2581) or two (RNAi-2574) nucleotides. When tested in cultured mitral cells prepared from Kv1.3−/− mice and using 60 mM Na intracellular solution, a mismatch of only one nucleotide (RNAi-2581) resulted in a degree of suppression of outward current magnitude that was comparable to that observed with the perfect match RNAi-2757 (Control, 336 ± 83 pA/pF [n = 7]; RNAi-2581, 76 ± 40 pA/pF [n = 6] at +60 mV). In contrast, the mismatched RNAi-2574, which differs from the Slack sequence in two positions, produced a much smaller reduction of outward current that did not reach statistical significance (Control, 336 ± 83 pA/pF [n = 7]; RNAi-2574, 172 ± 39 pA/pF [n = 6] at +60 mV). These collective findings indicate that the enhancement of KNa current that occurs in mitral cells of Kv1.3-deficient mice specifically requires expression of the Slack potassium channel subunit.
DISCUSSION
Imaging studies have demonstrated that large changes in intracellular Na+ occur during normal physiological signaling, particularly in restricted compartments such as dendrites (Rose 2002; Rose and Ransom 1997), where intracellular Na+ levels can rise to as high as 45–100 mM during repetitive stimulation (Rose and Konnerth 2001). Such Na+ transients trigger the activation of KNa channels, which have been reported in a very wide range of neurons, as well as in cardiac cells (Bhattacharjee and Kaczmarek 2005; Dryer 1994; Kameyama et al. 1984). In many neurons, the presence of KNa currents has been shown to result in slow Na+-dependent afterhyperpolarizations (sAHPs) that produce adaptation of firing rate during a maintained stimulus (Descalzo et al. 2005; Foehring et al. 1989; Franceschetti et al. 2003; Kim and McCormick 1998; Kubota and Saito 1991; Sanchez-Vives et al. 2000; Sandler et al. 1998; Schwindt et al. 1989; Wallen et al. 2007). The activation of KNa channels during repetitive firing has also been shown to regulate rates of bursting (Franceschetti et al. 2003) and to increase the timing accuracy of action potentials during high rates of stimulation (Yang et al. 2007). Particularly high levels of KNa channels have been found in olfactory mitral cells and granule cells using both electrophysiological and immunostaining approaches (Bhattacharjee et al. 2002, 2005; Egan et al. 1992a,b).
Our present studies have confirmed the presence of KNa currents and channel subunits in olfactory neurons and have provided direct evidence that the native KNa current results from the expression of the Slack channel subunit. In addition, we have shown that deletion of Kv1.3, the major voltage-dependent K+ channel subunit in mitral cells (Fadool et al. 2004), results in an very marked up-regulation of Slack channel subunits and of KNa current. Using these neurons, we have demonstrated that KNa currents can be activated by Na+-influx through voltage-dependent Na+ channels and nonselective cation H-channels even when recordings are made using Na+-free internal solutions. Recent findings by others have also provided evidence that Slack is required for expression of KNa current in mitral cells and cortical pyramidal cells (Budelli et al. 2009).
The degree of suppression of the KNa current in Kv1.3−/− neurons by anti-Slack RNAi in isolated mitral cells is generally consistent with the estimates of the contribution of KNa currents to total outward currents in mitral cells recorded with internal 60 mM Na+ in slices. Specifically, in neurons in OB slices, we found that increasing the internal Na+ from 0 to 60 mM produced an approximately 40% increase in outward current, whereas blocking Na+ influx with TTX and ZD7288 with zero internal Na+ produced an almost 40% reduction of the basal current. This provides an estimate that KNa currents account for just under 60% of the total outward current recorded under these conditions (1 mM external TEA and 60 mM internal Na+). The use of RNAi to suppress Slack expression in the isolated Kv1.3−/− neurons produced a reduction of nearly 75% of the outward current recorded under the same conditions.
We found that the KNa current that is activated by Na+ influx through TTX and ZD7288-sensitive channels in neurons in slices of the OB has a transient and a sustained component. The most likely explanation for the observed transient component in the difference current is that it reflects transient activation of KNa by a peak of sodium entry at the onset of depolarization (Bhattacharjee et al. 2005). Such transients are also likely to be smaller in neurons maintained in culture. Our measurements of KNa current magnitude were therefore made at the end of the pulses, when sodium levels are likely to have reached a steady state. In contrast, the slow activation of Slack-B channels in HEK cells reflects the previously reported slow activation of this Slack isoform in cells in which there is little or no sodium current during activation (Bhattacharjee et al. 2003; Joiner et al. 1998). We cannot, however, eliminate the possibility that Slack/Slick isoforms and/or putative ancillary subunits also differ between these cell preparations.
Potassium channels are made up of a tetramer of pore-forming α-subunits, which may associate with ancillary proteins such as β-subunits or other proteins. Mitral cells of the OB express two different pore-forming KNa channel α-subunits, Slack-B and Slick (Bhattacharjee et al. 2002, 2005). Although RNAi against the Slack channel subunit produced a major reduction in the native KNa current, our experiments do not allow us to determine whether this major current represents homomeric Slack channels or heteromeric Slack/Slick channels. In particular, it has been demonstrated that the presence of specific cytoplasmic N-terminal domain of Slack-B is required for heteromer formation with Slick subunits and for the efficient trafficking of these heteromeric channels to the plasma membrane (Chen et al. 2009). Thus the suppression of Slack-B expression would be expected to produce a parallel reduction in heteromeric Slack/Slick channels in the plasma membrane.
Deletion of the Kv1.3−/− gene in mice reduces their threshold for the detection of odors by a factor of 1,000–10,000, increases their ability to discriminate between odors, and reduces the time required for these animals to find the location of a hidden odor (Fadool et al. 2004). Some of these changes, such as the increased discrimination between odors, can be mimicked by acute suppression of Kv1.3 current (Marks et al. 2009). Other changes, such as the reduced threshold for odor detection, however, require longer-term modifications of the function of olfactory neurons. For example, Kv1.3−/− mice have olfactory glomeruli that are smaller and more numerous than those of wildtype mice and, at the molecular level, the expression of a number of channels, receptors, and signaling molecules has been found to be altered within the olfactory bulb (Biju et al. 2008; Fadool et al. 2004). Thus it is unlikely that the alterations in olfactory behaviors can be attributed to a single cellular change. Nevertheless, it is very likely that the enhancement of KNa current in mitral cells may contribute to the alterations of their firing pattern that were previously described. In particular, increased expression of Slack is consistent with the finding that, in response to maintained depolarization, neurons from Kv1.3−/− mice fire trains of action potentials that adapt with a progressive decrease in amplitude, whereas wildtype neurons respond with sustained repetitive firing that contains clusters of action potentials interspersed with periods of inhibition (Balu et al. 2004; Fadool et al. 2004). Agents that block the delayed rectifier alone do not produce this type of adaptation (Balu et al. 2004). As stated earlier, however, such adaptation is a feature of many neurons in which KNa currents have been characterized (Descalzo et al. 2005; Foehring et al. 1989; Franceschetti et al. 2003; Kim and McCormick 1998; Kubota and Saito 1991; Sanchez-Vives et al. 2000; Sandler et al. 1998; Schwindt et al. 1989; Wallen et al. 2007) and a similar change in adaptation has been observed in computer simulations of increased Slack currents in model neurons (Brown et al. 2008). Furthermore, if as has been observed in auditory neurons (Yang et al. 2007), elevations in KNa current increase the temporal accuracy with which mitral cells lock their action potentials to synaptic inputs and to incoming sensory stimuli, the cerebral cortical neurons that receive inputs from these mitral cells may more readily detect patterns of synchronous activity that correspond to specific odors.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC-01919 and DC-003381.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank J. T. Zorn and P. G. Barry for participation in experiments related to these studies.
Present address of P. Das: Department of Physiology and Pharmacology, University of Toledo College of Medicine, Toledo, OH 43606.
REFERENCES
- Balu R, Larimer P, Strowbridge BW. Phasic stimuli evoke precisely timed spikes in intermittently discharging mitral cells. J Neurophysiol 92: 743–753, 2004 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the Slack potassium channel in the rat central nervous system. J Comp Neurol 454: 241–254, 2002 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci 23: 11681–11691, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+. Trends Neurosci 28: 422–428, 2005 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, von Hehn CA, Mei X, Kaczmarek LK. Localization of the Na+-activated K+ channel Slick in the rat central nervous system. J Comp Neurol 484: 80–92, 2005 [DOI] [PubMed] [Google Scholar]
- Biju KC, Marks DR, Mast TG, Fadool DA. Deletion of voltage-gated channel affects glomerular refinement and odorant receptor expression in the mouse olfactory system. J Comp Neurol 506: 161–179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Spilianakis CG, Flavell RA, von Hehn CA, Bhattacharjee A, Kaczmarek LK. Amino-termini isoforms of the Slack K+ channel, regulated by alternative promoters, differentially modulate rhythmic firing and adaptation. J Physiol 586: 5161–5179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budelli G, Hage TA, Wei A, Rojas P, Ivy Jong YJ, O'Malley K, Salkoff L. Na(+)-activated K(+) channels express a large delayed outward current in neurons during normal physiology. Nat Neurosci 12: 745–750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kronengold J, Yang Y, Gazula V, Brown MR, Ma L, Ferreira G, Yan Y, Bhattacharjee A, Sigworth F, Salkoff L, Kaczmarek LK. The N-terminal domain of Slack determines the formation and trafficking of Slick/Slack heteromeric sodium-activated potassium channels. J Neurosci 29: 5654–5665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley B, Tucker K, Fadool DA. Comparison of modulation of Kv1.3 channel by two receptor tyrosine kinases in olfactory bulb neurons of rodents. Receptors Channels 10: 25–36, 2004 [PMC free article] [PubMed] [Google Scholar]
- Cook KK, Fadool DA. Two adaptor proteins differentially modulate the phosphorylation and biophysics of Kv1.3 ion channel by SRC kinase. J Biol Chem 277: 13268–13280, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descalzo VF, Nowak LG, Brumberg JC, McCormick DA, Sanchez-Vives MV. Slow adaptation in fast-spiking neurons of visual cortex. J Neurophysiol 93: 1111–1118, 2005 [DOI] [PubMed] [Google Scholar]
- Dryer SE. Na(+)-activated K+ channels: a new family of large-conductance ion channels. Trends Neurosci 17: 155–160, 1994 [DOI] [PubMed] [Google Scholar]
- Egan TM, Dagan D, Kupper J, Levitan IB. Na(+)-activated K+ channels are widely distributed in rat CNS and in Xenopus oocytes. Brain Res 584: 319–321, 1992a [DOI] [PubMed] [Google Scholar]
- Egan TM, Dagan D, Kupper J, Levitan IB. Properties and rundown of sodium-activated potassium channels in rat olfactory bulb neurons. J Neurosci 12: 1964–1976, 1992b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool D, Tucker K, Phillips JJ, Simmen JA. Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1.3. J Neurophysiol 83: 2332–2348, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron 41: 389–404, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring RC, Schwindt PC, Crill WE. Norepinephrine selectively reduces slow Ca2+- and Na+-mediated K+ currents in cat neocortical neurons. J Neurophysiol 61: 245–256, 1989 [DOI] [PubMed] [Google Scholar]
- Franceschetti S, Lavazza T, Curia G, Aracri P, Panzica F, Sancini G, Avanzini G, Magistretti J. Na+-activated K+ current contributes to postexcitatory hyperpolarization in neocortical intrinsically bursting neurons. J Neurophysiol 89: 2101–2111, 2003 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- Huettner J, Baughman R. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci 6: 3044–3060, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Tang MD, Wang LY, Dworetzky SI, Boissard CG, Gan L, Gribkoff VK, Kaczmarek LK. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat Neurosci 1: 462–469, 1998 [DOI] [PubMed] [Google Scholar]
- Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature 309: 354–356, 1984 [DOI] [PubMed] [Google Scholar]
- Kim U, McCormick DA. Functional and ionic properties of a slow afterhyperpolarization in ferret perigeniculate neurons in vitro. J Neurophysiol 80: 1222–1235, 1998 [DOI] [PubMed] [Google Scholar]
- Koni PA, Khanna R, Chang MC, Tang MD, Kaczmarek LK, Schlichter LC, Flavella RA. Compensatory anion currents in Kv1.3 channel-deficient thymocytes. J Biol Chem 278: 39443–39451, 2003 [DOI] [PubMed] [Google Scholar]
- Kubota M, Saito N. Sodium- and calcium-dependent conductances of neurones in the zebra finch hyperstriatum ventrale pars caudale in vitro. J Physiol 440: 131–142, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci 29: 6734–6751, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Slack F. Ins and outs of RNA interference analysis. In: Evaluating Techniques in Biochemical Research, edited by Zuk D. Cambridge, MA: Cell Press, 2007, p. 34–36 [Google Scholar]
- Rose CR. Na+ signals at central synapses. Neuroscientist 8: 532–539, 2002 [DOI] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. J Neurosci 21: 4207–4214, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Regulation of intracellular sodium in cultured rat hippocampal neurones. J Physiol 499: 573–587, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci 20: 4286–4299, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler VM, Puil E, Schwarz DW. Intrinsic response properties of bursting neurons in the nucleus principalis trigemini of the gerbil. Neuroscience 83: 891–904, 1998 [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Crill WE. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. J Neurophysiol 61: 233–244, 1989 [DOI] [PubMed] [Google Scholar]
- Song P, Yang Y, Barnes-Davies M, Bhattacharjee A, Hamann M, Forsythe ID, Oliver DL, Kaczmarek LK. Acoustic environment determines phosphorylation state of the Kv3.1 potassium channel in auditory neurons. Nat Neurosci 8: 1335–1342, 2005 [DOI] [PubMed] [Google Scholar]
- Tucker K, Fadool DA. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J Physiol 542: 413–429, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen P, Robertson B, Cangiano L, Low P, Bhattacharjee A, Kaczmarek LK, Grillner S. Sodium-dependent potassium channels of a Slack-like subtype contribute to the slow afterhyperpolarization in lamprey spinal neurons. J Physiol 585: 75–90, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Desai R, Kaczmarek LK. Slack and Slick K(Na) channels regulate the accuracy of timing of auditory neurons. J Neurosci 27: 2617–2627, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Gribkoff VK, Pan J, Damagnez V, Dworetzky SI, Boissard CG, Bhattacharjee A, Yan Y, Sigworth FJ, Kaczmarek LK. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology 51: 896–906, 2006 [DOI] [PubMed] [Google Scholar]