Abstract
The human neocerebellum has been hypothesized to contribute to many high-level cognitive processes including attention, language, and working memory. Support for these nonmotor hypotheses comes from evidence demonstrating structural and functional connectivity between the lateral cerebellum and cortical association areas as well as a lack of somatotopy in lobules VI and VII, a hallmark of motor representations in other areas of the cerebellum and cerebral cortex. We set out to test whether somatotopy exists in these lobules by using functional magnetic resonance imaging to measure cerebellar activity while participants produced simple or complex movements, using either fingers or toes. We observed a previously undiscovered somatotopic organization in neocerebellar lobules VI and VIIA that was most prominent when participants executed complex movements. In contrast, activation in the anterior lobe showed a similar somatotopic organization for both simple and complex movements. While the anterior somatotopic representation responded selectively during ipsilateral movements, the new cerebellar map responded during both ipsi- and contralateral movements. The presence of a bilateral, task-dependent somatotopic map in the neocerebellum emphasizes an important role for this region in the control of skilled actions.
INTRODUCTION
The presence of topographic maps provides important constraints on hypotheses concerning the functional role of many neural regions. Indeed the characterization of somatotopic maps has led to important advances in our understanding of both function and connectivity of various cortical and subcortical areas (Thivierge and Marcus 2007).
Within the cerebellum, multiple somatotopic maps have been identified. Stimulation and cellular recording studies in animals (e.g., Snider and Stowell 1944; see review in Manni and Petrosini 2004) as well as functional neuroimaging studies in humans (Grodd et al. 2001; Habas et al. 2004a,b; Nitschke et al. 1996; Rijntjes et al. 1999; Thickbroom et al. 2003) have revealed two body representations in the cerebellum, one in the anterior lobe (lobules I–V) and a second in the inferior aspect of the posterior lobe (chiefly lobules VIIB and VIII). These maps reinforce functional hypotheses concerning the role of the cerebellum in the planning and control of movements.
To date, no clear somatotopy has been identified for the phylogenetically younger, superior posterior lobe of the cerebellum, a region we will refer to as neocerebellum in this paper. Indeed the lack of somatotopy, along with evidence of connections between this region (via the dentate nucleus) and association areas of the cerebral cortex (Dum et al. 2002; Kelly and Strick 2003; Middleton and Strick 2001), has led to a de-emphasized role of the neocerebellum in sensorimotor control (e.g., Desmond et al. 2005; Ravizza et al. 2006; Schmahmann and Caplan 2006; Stoodley and Schmahmann 2009). Rather, functional hypotheses have focused on cognitive operations such as attention shifting (Courchesne and Allen 1997), lexical retrieval (Desmond et al. 1998), and the maintenance of information in working memory (Fiez et al. 1996; reviewed in Strick et al. 2009).
Previous fMRI studies of cerebellar somatotopy have generally involved simple, single-joint movements (Grodd et al. 2001; Rijntjes et al. 1999; Thickbroom et al. 2003). However, evidence for engagement of lobules VI and VIIA of the neocerebellum during motor tasks has been observed, typically when movements are complex, requiring greater sensorimotor coordination than simple one-dimensional joint actions (Diedrichsen et al. 2005; Greger et al. 2004; Miles et al. 2006). In the present study, we asked whether the form and extent of somatotopic representation in the cerebellum varies with movement complexity.
Consistent with previous studies, we define complexity here in terms of the degrees of freedom required to execute an action (Ingram 2008; Todorov and Grahramani 2004; Weiss and Flanders 2004). Movements with high degrees of freedom, for example, those that require some effectors to flex while other effectors extend, are considered complex. Movements with low degrees of freedom, for example, the simultaneous flexion or extension across a set of digits, are considered simple. In the cerebral cortex, complex finger movements engage a broad bilateral network of areas on the precentral gyrus (Cramer et al. 1999; Hanakawa et al. 2005) including regions that are not activated during the production of simple finger movements (Verstynen et al. 2005). We hypothesized that the coordination demands associated with complex movements would produce more activation in neocerebellar regions (Diedrichsen et al. 2005; Greger et al. 2004). Assuming this prediction was confirmed, our principle goal in this study was to determine if this activation would be somatotopically organized.
METHODS
Participants
Thirteen healthy, right-handed individuals (18–29 yr, 7 female) participated in the experiment. One individual was excluded from the analyses due to excessive head movement. All experimental procedures were approved by the University of California Berkeley Committee for the Protection of Human Subjects, and informed consent was obtained prior to the experiment.
Tasks
Participants performed simple and complex movements with either their fingers or toes. Simple actions required simultaneous flexion and extension of many digits, while complex actions involved sequences of individual digit flexion and extension. These two movement types are sufficiently different in planning and control to evoke differential responses in cortical motor areas (Verstynen et al. 2005). Participants were instructed to rhythmically flex and extend all four fingers (thumb excluded) in the simple finger condition or all five toes in the simple toe condition. We allowed participants to move all five toes because withholding movement of just the big toe was difficult.
The complex finger condition required sequential digit movements that were either medial to lateral (index to pinky) or lateral to medial (pinky to index). During the complex toe condition, participants produced sequential movements of the toes, attempting to flex and extend the big toe, followed by flexion and extension of the second, third, and fourth toes; and finally abduction of the fifth toe (or the same sequence in reverse). Although participants varied in their ability to produce the toe sequences, they were all capable of producing three distinct toe gestures (based on visual observations of the experimenter), thus remaining a complex movement by requiring additional degrees of freedom (e.g., Todorov and Ghahramani 2004). Finger and toe movements were tested in separate functional magnetic resonance imaging (fMRI) runs.
Movements were produced at a rate of ∼2 Hz. Participants were instructed to restrict finger and toe movements to flexion-extension about the metacarpophalangeal and metatarsalphalangeal joints, respectively. All participants were visually monitored and guided during an initial supervised training period to ensure performance matched the instructions with care taken to minimize movements about the wrist and ankle.
Each trial began with an instruction period in which a visual cue was presented on the screen. The cue indicated the type of movement (simple or complex) and the effector to be used for that trial. The instruction screen remained visible for 2 s during which time the participants were to prepare the response pattern. Immediately after the instruction period, the cue turned green indicating the start of the movement period. Participants produced the target pattern in a continuous manner for 8 s (∼16 movements per trial given the target 2 Hz rate). The word “stop” was presented in the center of the screen to indicate the end of the trial and the beginning of a 12 s rest period.
The instructional cue consisted of a text display that indicated the limb (i.e., “left finger” or “right toe”) and pattern for the upcoming trial. Participants were presented with four asterisks (* * * *) for the simple conditions and one of two numeric sequences (1 2 3 4 or 4 3 2 1) for the complex conditions.
During the scanning session, participants completed eight scanning runs, four for finger movements and four for toe movements. Each scanning run consisted of 24 trials. Half of these trials involved simple movements (6 with the right hand or foot, 6 with the left), and half complex movements (6 with the right, 6 with the left). Thus over the course of the scanning session, participants completed 24 trials for each of the eight conditions [side (left/right) × effector (fingers/toes) × movement type (simple/complex)]. Finger/toe order was counterbalanced across subjects, and within a run, the trial order was fully randomized.
fMRI acquisition
Imaging data were obtained with a Varian Unity Inova 4 Tesla scanner (Varian, Palo Alto, CA). Head movements were attenuated by a custom-fitted mouthpiece formed to the dental impressions of each participant. While high field strength MR systems can result in reduced signal near sinus cavities, a particular problem for the lateral regions of the cerebellum, we recently reported that this Varian 4T system provides adequate cerebellar coverage. In fact, the signal obtained with a general purpose whole-head coil was similar to that obtained with a surface coil placed directly over the cerebellum (Spencer et al. 2007). Given this, we opted to use the whole-head coil in the current study.
Stimuli were back-projected onto a screen that participants viewed through a mirror mounted inside the head coil. Oblique single-shot Echo Planar Imaging (EPI) acquisitions were angled ∼70° from horizontal, selected to maximize coverage of the cerebellum and motor cortex. Data sets were acquired as a series of 264 volumes (18 slices, TR 2, TE 28 ms, 3 mm slice thickness, 0.5 mm slice gap, 3 mm in plane resolution), each preceded by 10 dummy scans. A total of 8 functional data sets of 528 s were acquired for each participant. Anatomical images were collected using a three-dimensional (3D) MPFLASH sequence at the end of the experiment.
Data processing
The imaging data were reconstructed using an Inverse Fast Fourier Transform with custom-written, in-house software. Functional data sets were realigned and head motion was estimated and corrected using SPM2 (http:www.fil.ion.ucl.ac.uk/spm/software/spm2/). The functional and structural data were manually coregistered and normalized using the spatially unbiased infra-tentorial (SUIT) atlas (Diedrichsen 2006). Statistical analysis was performed using a general linear model (GLM) in SPM2 (Kiebel 1999) as well custom scripts written in MATLAB (www.mathworks.com). Block regressors convolved with the canonical hemodynamic response function were created for each of the four movement types (left and right, simple and complex) and fit to each individual's data. Group analyses were performed separately for the finger and toe conditions using a random effects model.
To restrict analysis to the individual sections of the cerebellum, a region of interest (ROI) approach was used. Two anatomical regions were defined within the left and right cerebellar hemispheres, corresponding to the anterior lobes (lobules III, IV, and V) and neocerebellar regions (lobules VI, VIIA) using the probabilistic atlas of the cerebellum (Diedrichsen et al. 2009; see Fig. 5 in that paper for a visualization of the anatomical masks used in the present analyses). Within these regions we quantified the extent of activity across different task conditions. To identify significantly active voxels, we determined the false-discovery rate (FDR) of activity within the ROI and set an appropriate threshold to maintain an adjusted FDR of 0.05 (adjusted t-threshold range = 1.73–4.44) (Genovese et al. 2002). In this manner, we addressed the problem of multiple comparisons by anticipating the expected number of type-I errors. We then used a repeated-measures ANOVA to evaluate the effects of movement type and effector.
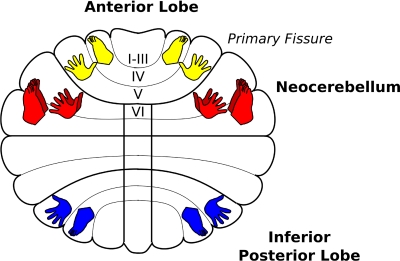
Fig. 5.
A schematic of the cerebellar homunculi including those within the anterior lobe in yellow, the inferior posterior lobe in blue (adapted from Grodd et al. 2004) as well as the new neocerebellar representation in red.
To determine whether there was a significant spatial shift in activation between hand and foot movements, we followed methods that have been described previously (Verstynen et al. 2005). Briefly, we first identified the center-of-mass (COM) of task-related activity within each ROI
where Ai is the task-related t-value of the ith voxel in an ROI of V voxels, and Xi is the x, y, z coordinate position of that voxel. This returns the coordinate positions of the center of highest activity density for a given condition. For each movement type (i.e., simple or complex and left or right), we subtracted the COM for foot movements from the COM for hand movements. The x, y, and z components of the spatial shift in peak activation between hand and foot movements was submitted to a MANOVA. The intercept term, reflecting the sum of the eigenvalues of the dot-product of the within- and between-group sums of squares, provides a test for whether the direction of the spatial shift in activity was consistent across subjects.
RESULTS
Functional organization of cerebellar areas
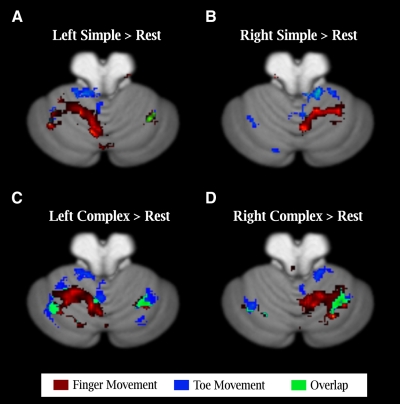
We computed activations as simple contrasts (t-test) of the movement conditions against an implicit baseline, considering the simple and complex conditions separately. Consistent with previous reports, the data from the simple movement condition revealed a somatotopic map in the ipsilateral anterior lobe (Grodd et al. 2001; Habas et al. 2004a,b; Rijntjes et al. 1999; Thickbroom et al. 2003). Finger-related activity was centered near the primary fissure and toe-related activity was shifted in the anterior and medial direction (Fig. 1, A and B). This same pattern (distribution and activation magnitude) was also evident during complex movements.
Fig. 1.
Differential activation in the cerebellum for simple and complex movements. A and B: activation during simple movements produced with either the fingers (red) or toes (blue) of the left (A) or right (B) side of the body. Activation is primarily restricted to the anterior lobe with little overlap between the 2 types of movements. C and D: activation during complex movements. A 2nd, map is now evident in the neocerebellum with toe activation posterior and lateral to the finger region, with a region of overlap. Statistical threshold is based on an alpha of P < 0.005 (uncorrected) at the group level (n = 12).
A somatotopic map has also been reported in the inferior aspect of the posterior lobe, spanning lobules VIIB–IX (Grodd et al. 2001; Habas et al. 2004a,b; Thickbroom et al. 2003). At the group level, we failed to observe significant activation in this region. This null result may reflect signal loss due to distortion artifacts at 4T (see Spencer et al. 2007) as well as individual variation in anatomy.
A previously unidentified somatotopically organized response was observed in lobules VI and VIIA (Crus I) of the neocerebellum. Interestingly, this pattern was only present in the complex movement condition. During complex toe movements, the activation was posterior and lateral to that evoked during complex finger movements (Fig. 1, C and D). In contrast to the ipsilateral activation patterns observed in the anterior lobe, the neocerebellum was activated during both ipsi- and contralateral movement. We quantify these effects in the subsequent sections.
ROI
Anatomical alignment methods have been developed to account for individual variation in the human cerebellum (Diedrichsen 2006). While these methods are ideal for regions surrounding the primary and intrabiventral fissures, they may not be sufficiently sensitive to account for individual variation within a given lobule of the cerebellar cortex, particularly as there are no reliable anatomical landmarks for normalization along the mediolateral axis. Given this concern, we devised an ROI strategy in which we used the SUIT atlas of the cerebellum (Diedrichsen et al. 2009) to identify anatomically specific regions. Because no activation was observed in the inferior posterior lobe (lobules VIII and IX), we restricted this analysis to the anterior lobe and neocerebellum.
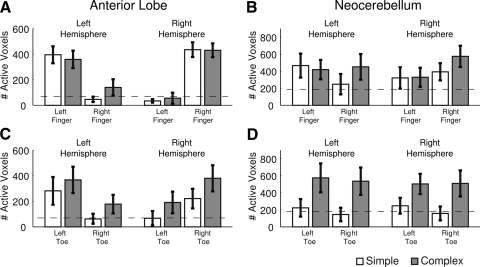
During finger movements, the number of activated voxels within the anterior lobe was strongly lateralized with greater recruitment on the side ipsilateral to the movement. This was confirmed by a highly significant main effect of limb (right vs. left) in the left [F(1,11) = 28.27, P < 0.001] and right [F(1,11) = 58.37, P < 0.001] anterior lobe ROIs (Fig. 2A). Neither ROI exhibited a main effect of task or an interaction between task and hand. Thus for finger movements, simple and complex movements produced similar patterns of activation in the ipsilateral anterior lobe.
Fig. 2.
Region of interest (ROI) analysis of task related activity. A and C: number of task-related voxels in the anterior lobe ROI during finger (A) and toe (C) movement conditions. - - -, the expected value given a false-discovery rate of 0.05. B and D: same plots for the neocerebellar ROIs during finger (B) and toe (C) movement conditions.
Both anterior lobe ROIs again exhibited a strong preference for ipsilateral toe movements [left anterior: F(1,11) = 13.41, P = 0.004; right anterior: F(1,11) = 12.12, P = 0.005]. Unlike finger movements, there also was a main effect of movement type with stronger activation during complex toe movements compared with simple toe movements. This effect was reliable for the right ROI [F(1,11) = 4.94, P = 0.048] and approached significance for the left ROI [F(1,11) = 4.14, P = 0.067]. In neither case did the interaction terms reach significance (all F's < 1).
In the neocerebellar ROI, the left hemisphere did not show a significant effect of hand or task (all F's < 1; Fig. 2B) nor was there a significant hand x task interaction [F(1,11) = 2.78, P = 0.123]. However, the number of activated voxels during left finger movements was greater than predicted by chance (dashed line, Fig. 2) for both simple [t(11) = 1.98, P = 0.037] and complex [t(11) = 2.02, P = 0.034] movements. A similar trend was observed in the left hemisphere ROI during complex movements with the contralateral (right) hand [t(11) = 1.75, P = 0.054]. The right hemisphere was more strongly activated during ipsilateral hand actions [F(1,11) = 9.43, P = 0.01], independent of the movement type [task: F(1,11) = 1.48, P = 0.247; task × hand: F(1,11) = 1.86, P = 0.20].
A strikingly different picture was observed in the neocerebellum during toe actions. These ROIs were only engaged during the complex movements [left neocerebellum: F(1,11) = 8.90, P = 0.013; right neocerebellum: F(1,11) = 5.90, P = 0.034]. Moreover, this activity was independent of which foot performed the movement (limb main effects and limb × task interactions were all F's < 1). Thus activation in the neocerebellar ROIs was limited to those conditions in which the participants produced complex sequential toe movements and was similar for both ipsi- and contralateral movement.
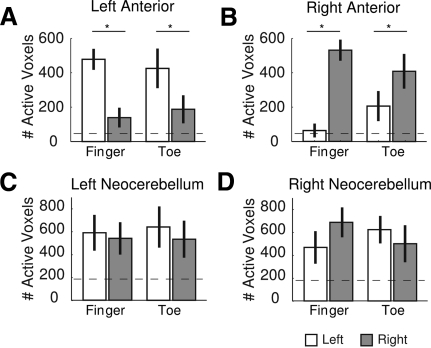
In summary, the activation maps (Fig. 1) and ROI analyses (Fig. 2) suggest that the anterior lobe and neocerebellum differ in the degree of lateralization of activation. Anterior lobe regions were selectively recruited during ipsilateral movements, whereas activation in the neocerebellum was generally bilateral, similar for both ipsi- and contralateral movement. This pattern was most evident when the data were combined across the two movement conditions.
To better illustrate this, we examined activation within the ROIs after combining the data for the simple and complex movement conditions (Fig. 3). In the anterior lobes, general movement-related activity was only observed during ipsilateral movements of the fingers [left anterior: t(11) = 4.96, P < 0.001; right anterior: t(11) = 7.00, P < 0.001] and toes [left anterior: t(11) = 3.71, P = 003; right anterior: t(11) = 3.03, P = 0.011]. In contrast, the only time the neocerebellum exhibited a similar preference for ipsilateral movements was during right hand finger movements [t(11) = 2.48, P = 0.031]. The asymmetry here bears some similarity to that observed in cortical motor areas: right hand movements produce a more lateralized pattern (adding contralateral) of activation than left hand movements, a pattern that generally holds for both left- and right-handers (Verstynen et al. 2005).
Fig. 3.
Laterality of evoked responses. A and B: the number of task-related voxels, collapsed across simple and complex movements, for the left (A) and right (B) anterior lobe. C and D: same plots for the left (C) and right (D) neocerebellar regions.
For the other three effectors (both feet and left hand), the response in the neocerebellum was similar for left and right side movements (all 2-sample t's < 1). However, the movement related activity was always greater than would be expected by chance (all 1-sample t-test P < 0.038). Thus the anterior lobe shows a strong selective lateralization for ipsilateral actions, whereas movement-related activity in the superior posterior lobe was comparable for both ipsi- and contralateral actions.
Spatial shifts in activity
As evident in Fig. 1, there appears to be a shift in the spatial extent of activity within the cerebellum for finger and toe movements. To quantify these shifts within each ROI, we used a center-of-mass approach that identified the location of highest density of task-related activity for each subject. The clusters of these peaks for ipsilateral limb movements are shown in Fig. 4A, collapsed across the simple and complex conditions. In the anterior lobes, the center of activity for toe movements shifted in a medial, anterior, and dorsal direction (mean values of 8.58 and 10.89 mm for left and right hemisphere, respectively) from that evoked during finger movements (see Tables 1 and 2). The direction of this shift is consistent with previous studies of the somatotopic organization in the anterior lobes of the cerebellum (Grodd et al. 2001; Rijntjes et al. 1999).
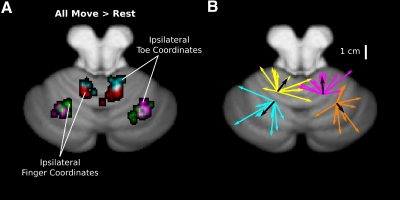
Fig. 4.
Somatotopic organization of activity. A: the clusters of center of mass during ipsilateral hand and foot movements collapsed over simple and complex movements. Red and cyan clusters show the distribution of peak activities in the anterior lobes for finger and toe activity, respectively. Purple and green clusters show peaks for the finger and toe conditions in the lateral hemispheres. B: vectors indicate individual (colored) and group averaged (black) shifts from finger to toe center of activation for the data shown in A. The origins are centered on the mean x, y, z position of activity for ipsilateral finger movements.
Table 1.
Hotelling's trace for ipsilateral actions
| Region of Interest | All Movements | Simple | Complex |
|---|---|---|---|
| Left interior lobe | 1.97** | 1.60** | 1.49** |
| Left lateral hemisphere | 1.64** | 2.50** | 1.30** |
| Right anterior lobe | 4.22** | 3.11** | 12.25** |
| Right lateral hemisphere | 0.59* | 0.45 | 0.38 |
(P < 0.025,
P < 0.001).
Table 2.
Vector shift for ipsilateral actions
| Region of Interest | All Movements | Simple | Complex |
|---|---|---|---|
| Left anterior lobe | (8.75, 11.75, 2.50) | (11.00, 8.25, 6.00) | (8.75, 10.00, 3.50) |
| Left lateral lobe | (−12.25, −13.75, −2.50) | (−6.00, −22.50, −4.50) | (−12.25, −12.75, −4.50) |
| Right anterior lobe | (−0.75, 20.75, −7.25) | (0.75, 21.00, −9.75) | (−2.50, 23.25, −5.50) |
| Right lateral lobe | (6.00, −7.50, −2.75) | (5.50, −9.00, −2.75) | (5.75, −4.50, −2.25) |
(3 mm voxels; positive values indicate right, anterior dorsal shift; negative values indicate left, posterior, ventral shifts)
Significant spatial shifts in activity were also observed in the neocerebellum, albeit in the opposite direction from that observed in the anterior lobe (Fig. 4B). In these ROIs, activity during toe movements was shifted in a lateral, dorsal, and posterior direction from the activity evoked during finger movements (mean values of 7.24 and 5.77 mm for left and right hemisphere, respectively; Tables 1 and 2). This effect was consistently observed when considering activity from all movement conditions (see Table 1); however, when restricted to either the simple or complex conditions alone, the effects are only marginally reliable (simple P = 0.056; complex = 0.083). These shifts point to a novel homunculus in lobules VI and VII that is distinct and inverted from that found in the anterior lobe (see Fig. 5).
DISCUSSION
The cerebellum has long been recognized to play a critical role in sensorimotor coordination. While previous imaging studies have highlighted two distinct somatotopic maps, corresponding to the anterior and inferior posterior regions (see Fig. 5), this form of topography has not been observed in more lateral cerebellar regions including the neocerebellum. Indeed the absence of a somatotopy, along with studies of anatomical and functional connectivity (Dum et al. 2002; Kelly and Strick 2003; Krienen and Buckner 2009; Middleton and Strick 2001) has motivated researchers to focus on nonmotor functions of the superior posterior lobe (e.g., Akshoomoff and Courchesne 1992; Desmond et al. 2005; Ravizza et al. 2006; Schmahmann and Caplan 2006; Strick et al. 2009; Stoodley and Schmahmann 2009).
However, the present results point to a previously unidentified somatotopic organization in lobules VI and VII of the cerebellar hemispheres (Table 2 and Fig. 4A). Two features of this new somatotopic map are noteworthy. First, activation in the neocerebellar ROIs was especially pronounced during complex movements, and it was during these movements that the somatotopic organization was evident. This suggests that the engagement of this region may be related to the coordination demands of the movements. The complexity dependency of the neocerebellum stands in contrast to that observed in the anterior lobe, where activation was similar for the simple and complex movement conditions.
Second, activation within the neocerebellar ROIs was similar for ipsilateral and contralateral movements (see also Rijntjes et al. 1999). Cortical motor areas also exhibit bilateral responses during complex finger movements (Hanakawa et al. 2005; Verstynen et al. 2005). The bilateral nature of this activation may reflect input from the neocortex for a higher-order level of movement planning that requires coordination across multiple effectors (Thach et al. 1992).
The hand areas within the anterior lobe and neocerebellum border the primary fissure. As such, this region might be treated as a single functional area that spans the fissure. However, by anatomically segmenting the cortex along the primary fissure, we observed robust functional differences in finger-related activation, suggesting by inference that these are, in fact, distinct finger representations. The hand area of the anterior lobe is only active during ipsilateral movement while the lateral hemisphere is recruited during movements produced by either side of the body. Importantly, this fissure is not properly aligned across subjects with all normalization methods (Diedrichsen 2006). With the introduction of refined atlases of the cerebellum (Diedrichsen et al. 2009), these subregions can be readily identified by defining anatomical borders, allowing for future functional comparisons. It should be noted that we did not observe any significant activation of lobule VIIA Crus II during either movement condition. This region of the neocerebellum may be specialized for nonmotor functions or, conversely, evoking a somatotopic map here may require further demands on movement coordination.
The somatotopy within the neocerebellar ROIs only became readily apparent by the inclusion of a complex sequential movement task. This was a difficult and certainly unnatural task. Given this, we have considered whether the activation observed in this condition is related to some other factor; for example, an increase in attentional focus or some sort of subvocalization strategy (Desmond et al. 2005; Feiz et al. 1996; Ravizza et al. 2006). However, the center of activity observed during the complex toe movements is much more medial than areas identified in previous imaging studies that involve verbal processing (Desmond et al. 2005; Feiz et al. 1996).
Similar to our study, Rijntjes et al. (1999) included two types of movements: one in which the toes made zigzagging movements and one in which the participants used their toes to write their signature. The zigzag movements are similar to the “simple” movements in the present investigation, involving coordinated abduction/adduction of the foot. The writing condition requires sequencing abduction/adduction and flexion/extension foot gestures and would thus appear to be similar to our “complex” condition. The activation pattern for the zigzag movements was in the anterior lobe and included an area shifted in the lateral, dorsal, and posterior direction from the finger movement condition (Rijntjes et al. 1999), consistent with our observations. Moreover, the writing condition led to move activation of neocerebellar regions although no somatotopic organization was reported. While this is at odds with the current results, it is also possible that this null result is a type II error, given recent advances in intersubject cerebellar alignment have increased statistical power at the group level (Diedrichsen 2006) or a number of task differences relative to the present study.
A long-standing debate in the cerebellar literature concerns the question of whether the functional emphasis for regions showing somatotopy should be on a contribution to the coordination of motor commands or a contribution to the generation and evaluation of sensory consequences related to these commands. Based on the current results, we cannot say whether one of these characterizations is more appropriate than the other with respect to this novel somatotopic map in the neocerebellum. Indeed, the same ambiguity persists for the anterior lobe map.
Nonetheless, we can gain some insight here by considering cortical activations during similar tasks. Complex finger movements produce activation along the medial surface of the left precentral gyrus, and this activation is similar for both left and right hand movements (Hanakawa et al. 2005; Verstynen et al. 2005). The anatomical, premotor location of this activity, coupled with the bilateral pattern of activation, favors a motor planning account. By analogy, we propose that the neocerebellar activity also reflects high level motor planning rather than the processing of sensory signals. While this hypothesis is admittedly speculative, the main point to be emphasized here is that the activation is somatotopic, arguing against functional hypotheses that are divorced from the sensorimotor domain.
GRANTS
This work was supported by funding from the National Institutes of Health Grants HD-060306 to R.B. Ivry and AG-29710 to R.M.C. Spencer and National Science Foundation Grant BCS0726685 R. B. Ivry.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- Akshoomoff N, Courchesne E. A new role for the cerebellum in cognitive operations. Behav Neurosci 106: 731–738, 1992 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learning Memory 4: 1–35, 1997 [DOI] [PubMed] [Google Scholar]
- Cramer S, Finklestein S, Schaechter J, Bush G, Rosen B. Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol 81: 383–387, 1999 [DOI] [PubMed] [Google Scholar]
- Desmond J, Chen S, Shieh P. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Ann Neurol 58: 553–560, 2005 [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JDE, Glover GH. Dissociation of frontal and cerebellar activity in a cognitive task: evidence for a distinction between selection and search. Neuroimage 7: 368–376, 1998 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 33: 127–138, 2006 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MRI atlas of the human cerebellum. Neuroimage 46: 39–46, 2009. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci 25: 9919–9931, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Li C, Strick PL. Motor and nonmotor domains in the monkey dentate. Ann NY Acad Sci 978: 289–301, 2002 [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci 16: 808–822, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878, 2002 [DOI] [PubMed] [Google Scholar]
- Greger B, Norris S, Thach W. Spike firing in the lateral cerebellar cortex correlated with movement and motor parameters irrespective of the effector limb. J Neurophysiol 91: 576–582, 2004 [DOI] [PubMed] [Google Scholar]
- Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13: 55–73, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Axelrad H, Cabanis E. The cerebellar second homunculus remains silent during passive bimanual movements. Neuroreport 15: 1571–1574, 2004a [DOI] [PubMed] [Google Scholar]
- Habas C, Axelrad H, Nguyen T, Cabanis E. Specific neocerebellar activation during out-of-phase bimanual movements. Neuroreport 15: 595–599, 2004b [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Parikh S, Bruno M, Hallett M. Finger and face representations in the ipsilateral precentral motor areas in humans. J Neurophysiol 93: 2950–2958, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JN, Kording KP, Howard IS, Wolpert DM. The statistics of natural hand movements. Exp Brain Res 188: 223–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23: 8432–8444, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19: 2485–2497, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni E, Petrosini LA. century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci 5: 241–249, 2004 [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 21: 700–712, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles OB, Cerminara NL, Marple-Horvat DE. Purkinje cells in the lateral cerebellum of the cat encode visual events and target motion during visually guided reaching. J Neurophysiol 571: 619–637, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke MF, Kleinschmidt A, Wessel K, Frahm J. Somatotopic motor representations in the human anterior cerebellum: a high-resolution functional MRI study. Brain 119: 1023–1029, 1996 [DOI] [PubMed] [Google Scholar]
- Ravizza S, Mccormick C, Schlerf J, Justus T, Ivry R, Fiez J. Cerebellar damage produces selective deficits in verbal working memory. Brain 129: 306–320, 2006 [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Buechel C, Kiebel S, Weiller C. Multiple somatotopic representations in the human cerebellum. Neuroreport 10: 3653–3658, 1999 [DOI] [PubMed] [Google Scholar]
- Santello M, Flanders M, Soechting JF. Postural hand synergies for tool use. J Neurosci 18: 10105–10115, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Flanders M, Soechting JF. Patterns of hand motion during grasping and the influence of sensory guidance. J Neurosci 22: 1426–1435, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: a defense of functional localizers. Neuroimage 30: 1088–1096, 2006. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Caplan D. Cognition, emotion and the cerebellum. Brain 129: 290–292, 2006 [DOI] [PubMed] [Google Scholar]
- Snider RS, Stowell A. Receiving areas of the tactile, auditory and visual systems in the cerebellum. J Neurophysiol 7: 331–357, 1944 [Google Scholar]
- Spencer RMC, Verstynen T, Brett M, Ivry RB. Cerebellar activation during discrete and not continuous timed movements: an fMRI study. Neuroimage 36: 378–387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann J. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44: 489–501, 2009 [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci 32: 413–434, 2009 [DOI] [PubMed] [Google Scholar]
- Thach W, Goodkin H, Keating J. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15: 403–442, 1992 [DOI] [PubMed] [Google Scholar]
- Thickbroom G, Byrnes M, Mastaglia F. Dual representation of the hand in the cerebellum: activation with voluntary and passive finger movement. Neuroimage 18: 670–674, 2003 [DOI] [PubMed] [Google Scholar]
- Thivierge JP, Marcus GF. The topographic brain: from neural connectivity to cognition. Trends in Neurosci 30: 251–259, 2007 [DOI] [PubMed] [Google Scholar]
- Todorov E, Ghahramani Z. Analysis of the synergies underlying complex hand manipulation. Conf Proc IEEE Eng Med Biol Soc 6: 4637–4640, 2004 [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry R. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol 93: 1209–1222, 2005 [DOI] [PubMed] [Google Scholar]
- Weiss EJ, Flanders M. Muscular and postural synergies of the human hand. J Neurophysiol 92: 523–535, 2004 [DOI] [PubMed] [Google Scholar]