Abstract
Touch is initiated by diverse somatosensory afferents that innervate the skin. The ability to manipulate and classify receptor subtypes is prerequisite for elucidating sensory mechanisms. Merkel cell–neurite complexes, which distinguish shapes and textures, are experimentally tractable mammalian touch receptors that mediate slowly adapting type I (SAI) responses. The assessment of SAI function in mutant mice has been hindered because previous studies did not distinguish SAI responses from slowly adapting type II (SAII) responses, which are thought to arise from different end organs, such as Ruffini endings. Thus we sought methods to discriminate these afferent types. We developed an epidermis-up ex vivo skin–nerve chamber to record action potentials from afferents while imaging Merkel cells in intact receptive fields. Using model-based cluster analysis, we found that two types of slowly adapting receptors were readily distinguished based on the regularity of touch-evoked firing patterns. We identified these clusters as SAI (coefficient of variation = 0.78 ± 0.09) and SAII responses (0.21 ± 0.09). The identity of SAI afferents was confirmed by recording from transgenic mice with green fluorescent protein–expressing Merkel cells. SAI receptive fields always contained fluorescent Merkel cells (n = 10), whereas SAII receptive fields lacked these cells (n = 5). Consistent with reports from other vertebrates, mouse SAI and SAII responses arise from afferents exhibiting similar conduction velocities, receptive field sizes, mechanical thresholds, and firing rates. These results demonstrate that mice, like other vertebrates, have two classes of slowly adapting light-touch receptors, identify a simple method to distinguish these populations, and extend the utility of skin–nerve recordings for genetic dissection of touch receptor mechanisms.
INTRODUCTION
In mammals, the sense of touch is initiated by more than a dozen morphologically and physiologically distinct sensory afferents in the skin. These somatosensory afferents encode a wide range of stimuli, including hair movement, light touch, vibration, texture, and pain (Halata 1993; Lumpkin and Caterina 2007; Perl 1992). Whether these disparate receptor subtypes share common mechanotransduction molecules remains unknown. Moreover, the developmental pathways underlying the physiological diversity of mammalian touch receptors are only now being uncovered (Bourane et al. 2009; Luo et al. 2009; Seal et al. 2009). The answer to these questions relies on the ability to selectively label, accurately classify, and isolate different receptors for molecular and physiological studies.
With only a few exceptions, physiologically identified responses have been linked to morphologically distinct cutaneous receptors largely through post hoc anatomical correlations (Chambers et al. 1968, 1972; Iggo and Muir 1969). The best characterized light-touch response is the slowly adapting type I (SAI), which was identified as arising from Merkel cell–neurite complexes through a painstaking combination of ex vivo recording, neuronal tracing, and post hoc histological analysis (Woodbury and Koerber 2007). Atoh1, which is specifically expressed in Merkel cells in the skin serves as a molecular marker of these complexes. Very recent molecular and histological studies have also established markers for low-threshold C-mechanoreceptors and rapidly adapting myelinated mechanoreceptors (Bourane et al. 2009; Luo et al. 2009; Seal et al. 2009); however, discrimination of individual subclasses within these groups remains elusive.
Cutaneous mechanosensitive afferents in vertebrate models have traditionally been divided primarily by conduction velocity in physiological assays (Gasser 1941). Fast, myelinated afferents, or A-afferents, are subdivided into Aβ- and Aδ-afferents, whereas unmyelinated afferents are dubbed C-fibers. In addition to conduction velocity, cutaneous afferents are often divided into touch receptors and nociceptors based on sensory threshold. Noxious levels of force primarily activate A-mechanonociceptor (AM) afferents and nociceptive classes of C-fibers, although some groups report that ≤20% of Aβ-afferents respond to these force levels (Djouhri and Lawson 2004). AM afferents are thinly myelinated, falling primarily into the Aδ conduction velocity range, and are responsible for quick, prickling pain sensations, whereas unmyelinated nociceptors convey slow, sustained responses (Lumpkin and Caterina 2007; Stucky et al. 2001). Low-threshold C-fibers, which represent a rare population of unmyelinated afferents, appear to be important for the onset of mechanical hypersensitivity during inflammation or injury (Loken et al. 2009). Low-threshold Aδ-afferents include down hair receptors (D-hairs or DHs) whose response properties and extreme sensitivity place them firmly in the category of light-touch receptors.
Most cutaneous Aβ-afferents are low-threshold mechanoreceptors that can be divided into slowly adapting and rapidly adapting (RA) categories, based on whether they maintain action potential discharge throughout a sustained mechanical stimulus. Based on their low mechanical thresholds and cutaneous location, it is likely these somatosensory afferents subserve the sensation of touch. RA afferents fire only in response to a changing stimulus, providing the brain with a neural image of moving or vibrating stimuli (Johnson 2001). Sensory structures associated with RA responses include hair-follicle afferents, Meissner's corpuscles, and Pacinian corpuscles, which are innervated by early Ret+ neurons (Bourane et al. 2009; Horch et al. 1977; Johnson 2001; Luo et al. 2009; Zimmermann et al. 2009).
Slowly adapting afferents maintain firing during sustained indentation and have been divided into two types in all vertebrate models except mice. SAI responses convey high-resolution spatial information to the brain and are thought to be responsible for our ability to discriminate texture, curvature, patterns such as Braille, and some component of proprioception (Edin 2001; Goodwin et al. 1997; Johansson and Flanagan 2009; Johnson and Lamb 1981; LaMotte and Srinivasan 1993; Phillips and Johnson 1981). The Merkel cell–neurite complexes that generate SAI responses are located in highly touch sensitive skin structures including finger tips, whisker follicles, and touch domes of hairy skin (Iggo and Muir 1969; Merkel 1875; Woodbury and Koerber 2007). SAII responses have been postulated to arise from Ruffini endings (Chambers et al. 1972); however, direct evidence supporting this correlation is still lacking.
Mice share similar classes of cutaneous mechanosensitive afferents with other mammals and have myriad genetic tools available to manipulate identified cell populations. Past characterization of touch receptors in mice, however, has differed somewhat from that of other mammals, given that prior studies have reported an absence of SAII responses or have not distinguished SAI from SAII afferents (Boada and Woodbury 2007; Cain et al. 2001; Kinkelin et al. 1999; Koerber and Woodbury 2002; McIlwrath et al. 2007; Wetzel et al. 2007; Woodbury and Koerber 2007; Woodbury et al. 2001). This limitation complicates the detailed study of either afferent type.
Most investigations of cutaneous mechanosensitive afferents in mice have been performed in ex vivo skin–nerve preparations, the majority of which mounted the epidermal surface facing down to allow superfusion of the dermis with oxygen-rich saline solution. In this configuration, mechanical stimuli are applied to the dermal surface, which does not mimic stimuli encountered by the living animal. This inverted configuration has been proposed to account for the lack of resolution of SAII from SAI responses (Lewin and Moshourab 2004). If so, multiple classes of slowly adapting afferents might be discerned in mice by stimulating touch receptors from the epidermal surface.
To test this possibility, we developed a novel chamber for ex vivo skin–nerve recording that allows the skin to be perfused from below, exposing a dry epidermal surface for stimulation and direct visualization of fluorescently labeled touch receptors. We used this preparation to assess conduction velocity, mechanical threshold, receptive field size, and response patterns to maintained stimuli from both the dermal and epidermal surfaces for direct comparison and for comparison with previous studies. We then used model-based multivariate cluster analysis to query the number of distinct slowly adapting afferent populations in mouse hairy skin, validating the results using genetic tools. The ability to resolve these cutaneous mechanosensitive afferents in the mouse is essential for future studies using genetic manipulations to investigate mechanotransduction mechanisms in these two receptors.
METHODS
Animals and dissection
All animal use was conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and the Department of Defense. When indicated, recordings were made from Atohl/nGFP transgenic mice expressing enhanced green fluorescent protein (eGFP) in Atoh1-expressing cells, including Merkel cells (Lumpkin et al. 2003). Mice were generated in a BDF1 or mixed genetic background. In a few cases, wild-type mice were injected with SynaptoGreen/FM1-43 (2–3 mg/kg; Biotium) from 16 to 72 h prior to recording to label Merkel cells (Maricich et al. 2009; Meyers et al. 2003).
Adult mice (≥6 wk of age) were sacrificed by inhalation of isoflurane followed by cervical dislocation. The posterior half of the animal was shaved and the remaining stubble was removed by applying a depilatory agent (SurgiCream; Ardell) for 10–15 min. The saphenous nerve and innervated skin of the hindlimb were dissected largely as previously described (Koltzenburg et al. 1997; Reeh 1986; Zimmermann et al. 2009). The plantar surface of the foot was attached to a wedge with double-sided tape to ease manipulation and stabilize the leg. To increase the length of accessible nerve, we removed the viscera along with skin and fascia proximal to the knee joint. The saphenous nerve and the trunk of the femoral nerve were dissected away from the femoral artery and underlying muscle, tied with nylon string at the level of the lumbar plexus, and severed. To facilitate fiber teasing, ≥2 cm of nerve was obtained beyond the point of attachment to the skin. An incision was made on the lateral side of the leg from knee to heel and the skin of the leg and the dorsal aspect of the hindpaw were removed. Care was taken to keep the connection between nerve and skin intact while dissecting between the underlying musculature and the fascia that contains the branching nerve fibers. The tissue was periodically washed with synthetic interstitial fluid (SIF, in mM: 108 NaCl, 3.5 KCl, 0.7 MgSO4, 26 NaHCO3, 1.7 NaH2PO4, 9.5 sodium gluconate, 5.5 glucose, 7.5 sucrose, and 1.5 CaCl2, saturated with 95% O2-5% CO2; pH 7.4) throughout the dissection to keep it moist and oxygenated and to desanguinate the dissection field if necessary. Additional sucrose (≤20 mM) was used to adjust the osmolality to a level comparable with that of mammalian interstitial fluid (290–305 mmol/kg). SIF ion concentrations were within 3% of those reported for subcutaneous interstitial fluid (Fogh-Andersen et al. 1995; Gilanyi et al. 1988).
The total time required by a trained experimentalist from the moment of animal sacrifice to the complete removal of both skin–nerve preparations was ≤2 h. The resulting skin–nerve preparations were maintained at 4°C in SIF until recording (≤6 h). No difference has been noted in either the viability of the two preparations or the populations of afferents detected. Fewer fibers overall have been recorded from the left-leg preparation, but this is largely due to the increased likelihood of damage to the preparation during dissection by right-handed experimenters, irrespective of dissection or recording order.
Recording chamber
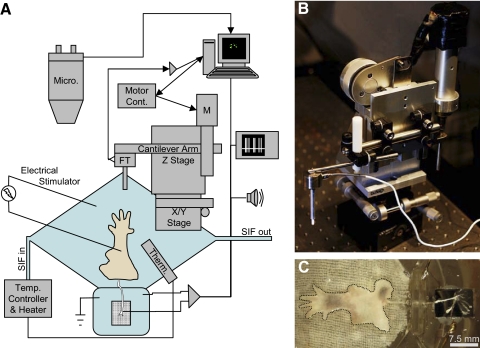
A custom recording chamber (Fig. 1A) was mounted on the base of a brain slice chamber containing a heating element (Model BSC-BU Base Unit; Harvard Apparatus). The perfusion chamber is rhomboid, with axes of 1.5 in. (38 mm) and 2.625 in. (67 mm) and a depth of 0.375 in. (9.5 mm). The smaller, ovoid recording chamber adjoins the perfusion chamber at one of the obtuse vertices, and is 0.625 × 1.25 in. (16 × 32 mm). The bottom of the perfusion chamber was coated with silicone-elastomer (Sylgard; Dow Corning), on top of which was pinned a nylon wick (L'eggs winter-weight pantyhose). The elasticity of the Sylgard and nylon wick substrates did not contribute significantly to the force adaptation of the system, as repeated stimuli at 30-s intervals to the substrate alone produced nonadapting force traces with highly repeatable amplitudes. During recording, the chamber contained about 3.5 mL of SIF and was perfused at a rate of approximately 4 mL/min with a microannular gear pump (Mikrosysteme mzr-2921). Bath temperature was maintained at 32°C with a temperature controller and reported by a bath thermistor (Model TC-202A; Harvard Apparatus).
Fig. 1.
Schematic of the epidermis-up ex vivo skin–nerve preparation (A). The hindlimb skin of a mouse is mounted in a perfusion chamber, with the attached saphenous nerve resting on a mirror in the adjoining recording chamber. The skin is perfused with synthetic interstitial fluid (SIF), saturated with 95% O2-5% CO2. Bath temperature is monitored by a thermistor (Therm) and maintained at 32°C. The preparation is visualized with a fluorescence-equipped stereomicroscope (Micro) connected to a camera and computer for image capture. The computer also controls electrical stimuli from a stimulus generator and mechanical stimuli via a motor controller. The motor controller drives a stepper motor (M) mounted to a rigid arm and an X/Y stage. A ceramic probe is mounted to the end of a cantilever arm with an in-line force transducer (FT). Force data are sent to the computer via an amplifier and captured along with differential recordings of extracellular potentials in a nerve bundle. Extracellular potentials are sent in parallel to an oscilloscope and a speaker for aural detection of action potentials. B: image of the mechanical stimulator. C: a skin–nerve preparation mounted in the recording chamber. The edge of the skin is denoted by a dotted black line. Pigmented areas of skin are in the active growth phase (anagen) of the hair cycle.
Electrophysiology and imaging
Ex vivo skin–nerve preparations were mounted with the epidermis facing up in the perfusion chamber and the nerve threaded into the adjacent recording chamber (Fig. 1, A and C). Mineral oil (cat. #M3516; Sigma–Aldrich) was layered over the SIF in the recording chamber, with the SIF–oil interface just below the surface of a raised platform (0.75 cm2). The severed end of the nerve was placed on the platform, stripped of its perineurium, and teased into isolated nerve bundles of up to a few tens of axons. Axon bundles were draped over a gold recording electrode and extracellular potentials were recorded via a differential amplifier (Model 1800; A-M Systems) while stimulating the skin. Receptive fields were located with a rounded glass probe and their size was estimated by touching the skin with a fine glass probe (diameter ≈ 0.5 mm). Mechanical threshold was defined as the lowest calibrated monofilament (von Frey hair) that elicited a response from a receptive field in ≥50% of trials. Von Frey hair forces ranged from 0.08 to 300 mN, although filaments >10 mN were not necessary for classifying low-threshold mechanosensitive afferents.
To estimate conduction velocity, biphasic electrical stimuli 0–35 V in amplitude and 100 μs in duration were delivered from a pulse stimulator (Model 2100; A-M Systems) to receptive fields via a tungsten electrode (World Precision Instruments). The latency of the action potential peak from the stimulus artifact was measured and the distance from the stimulating electrode to the recording electrode was measured with a calibrated eyepiece reticle. Conduction velocity was calculated as the quotient of distance to latency.
The preparation was visualized with a fluorescence stereomicroscope (Model SZX16; Olympus) equipped with ×0.5/0.075 numerical aperture (NA) and ×2.0/0.3 NA objective lenses, epifluorescence filters (Chroma), and a 300-W Xenon lamp (Sutter Instrument). Fluorescent images of Merkel cell–neurite complexes from Atoh1/nGFP mice (Lumpkin et al. 2003) were acquired with a charge-coupled device camera (DP-71 CCD; Olympus). In some cases, high-resolution images were captured postrecording by marking a grid around the touch dome with a waterproof marker for localization and then visualizing skin whole mounts with a confocal microscope equipped with a ×40/1.25 NA objective lens (DM IRBE; Leica). Images were processed in ImageJ (Abramoff 2004) with the Bio-Formats plugin (Linkert et al. 2009).
In some cases, high-resolution mapping of receptive fields and measurement of von Frey thresholds were carried out on both the epidermal and dermal surfaces for the same afferent. Receptive fields were mapped in these cases at ×20–50 magnification using a calibrated eyepiece reticle and fine forceps (Dumont #5). The working distance of this objective, about 2 cm, was insufficient for von Frey hairs or our mechanical indenter, but more than enough for mapping with handheld forceps. After receptive field mapping and threshold determination, the skin was carefully flipped over without disturbing the teased nerve fibers and the same procedure was carried out on the other surface for comparison. The order of mapping (dermis or epidermis first) was alternated to avoid a systematic change in sensitivity due to the passage of time or skin relaxation with subsequent manipulations. Action potential shape and the location of the receptive field center did not change when flipping the skin. When possible, the skin was flipped more than once to allow repeated measurements. Note that the working distance of the objective used for fluorescence (×2) was insufficient to accommodate our mechanical stimulator or von Frey hairs, meaning only the receptive field mapping is possible with fluorescent imaging.
Mechanical stimulation
Families of mechanical displacements were delivered using a custom-built indenter (Fig. 1B), with stimulus order randomized using atmospheric noise (Random.org). A 3.4-mm diameter (9.2 mm2) MACOR (Corning) filleted cylinder was mounted to a motorized Z-stage driven by a linear actuator (Ultra Motion model D-A.25AB-HT17-2-BR/4) that was wired in parallel to a stepper motor controller (Model 3540i; Applied Motion Products) configured for 2 × 104 steps per revolution. The indenter had a maximum travel of 50 mm and moved in 0.32-μm increments. Typical stimuli were ≤2 mm and were performed with accelerations ≤1.27 μm/ms2 and average velocities ≤40 μm/ms. Generated pressures under the probe tip ranged from 1 to 250 kPa, roughly matching the pressure generated by von Frey filaments of ≤10 mN (Supplemental Fig. S1).1 A digital signal from the motor controller was sampled to mark the onset and termination of probe movement.
During mechanical stimulation, the applied force was constantly monitored in real time by a load cell (Model 31; Honeywell) and amplified via an inline amplifier (Model 060-6827-02; Honeywell). The indenter was controlled via handheld remote or custom software. Displacement steps were 5 s in duration and were delivered at 30-s intervals. Displacement families were performed in 0.1- to 0.2-mm increments between the minimum displacement required to elicit a response and the maximum displacement with forces in the linear range of the force transducer (∼1.7 N). Up to three displacement families were delivered to each afferent's receptive field.
Data acquisition and analysis
Data were digitized via a PC data acquisition card (Model DT304; Data Translation) at the following sampling frequencies: extracellular potentials (12 kHz), applied force (250 Hz), stimulator movement (250 Hz), and bath temperature (30 Hz). Electrophysiological data were processed in Sciworks Experimenter software (DataWave Technologies). Action potentials were detected and sorted in real time to isolate single units. Captured action potential waveforms were compared with confirm spike identity between experiments. Off-line analysis was also carried out in Experimenter, with the resulting data exported into Microsoft Excel, Matlab (The MathWorks), R (www.rproject.org), or IGOR Pro (WaveMetrics) for further analysis, plotting, and statistics. Autocorrelation on extracted spike times was performed in Matlab.
For analysis of dynamic and static phases of touch-evoked responses, the point at which the indenter began its movement was treated as t = 0. The indenter typically reached its final position 40–50 ms into each stimulus. To ensure not only that any firing during stimulus onset but also that the initial rapidly adapting phase of stimulus maintenance were captured, the dynamic phase was defined as t = 0–200 ms, encompassing at least one time constant of rapid adaptation. Dynamic firing rates were calculated from the mean interspike interval (ISI) during the dynamic phase. The static phase was defined as the response between 2 and 4.5 s. Coefficient of variation (CoV) for each stimulus was calculated as the SD of static phase ISIs divided by mean static ISI.
Model-based cluster analysis was carried out in R using the MClust package (Fraley and Raftery 2002, 2006) to determine the number of classes of slowly adapting Aβ-afferent responses from mouse recordings. Univariate model possibilities include equal variance (E) or variable variance (V) Gaussians. Multivariate models tested by the software included: spherical clusters of equal or variable diameters or ellipsoid models with equal or variable size and shape, with orientations either aligned with the coordinate axes, aligned in the same orientation, or of variable orientation. Shorthand notations for each model indicate equal (E), variable (V), or inapplicable (I) variance for volume, shape, and orientation, in that order. Models tested were: EII (equal volume spheres), VII (variable volume spheres), EEI (equal volume and shape ellipsoids aligned with the coordinate axes), VEI (variable volume, equal shape ellipsoids along the coordinates), EVI (equal volume variable shape along the coordinates), VVI (variable volume and shape along the coordinates), EEE (equal volume, shape, and orientation), EEV (equal volume, shape with variable orientation), VEV (variable volume, equal shape, and variable orientation), and VVV (variable volume, shape, and orientation).
The independent variables used in these analyses were mean static ISI, minimum dynamic ISI, minimum static ISI, and the CoV of static ISIs. All combinations of these variables, as well as each individual variable, were tested. A hierarchical clustering method was used to determine the best description of each data set for one to nine clusters. For each combination of model, input data, and number of clusters, a Bayesian information criterion (BIC) was calculated as a measure of how well each model described the data, with a penalty for the number of free parameters. By comparing minimum BIC across all combinations, we determined the best model and number of clusters to describe the data. BIC was a useful metric for model comparison because it is an absolute value comparable across fits and models and it severely penalizes model complexity, which is important to protect against overfitting a small number of observations.
RESULTS
To distinguish subtypes of cutaneous mechanosensitive afferents in mice, we used a novel epidermis-up ex vivo skin–nerve recording chamber (Fig. 1A). This system uses a nylon wick to perfuse the skin from below, allowing mechanical stimuli to be applied directly to the dry surface of the epidermis, as occurs in vivo. We also designed a custom mechanical stimulator (Fig. 1B) to deliver rapid, well-defined displacement stimuli with on- and offset times of <200 ms. These stimuli provided compressive stresses, or pressures, of ≤200 kPa, which were monitored in real time via a force transducer mounted in series with the displacement probe. Rapid-onset stimuli inevitably produce some ringing, especially with a force transducer in the system. This ringing decays to within the noise well before the static phase of the response. Perfusion rates were adequate to collect data from healthy preparations for 4–6 h, but low enough that the skin rested securely on the perfusion wick (Fig. 1C). Under these conditions, slowly adapting low-threshold afferents were observed ≤4 h after transferring skin–nerve tissue from 4°C to the recording chamber.
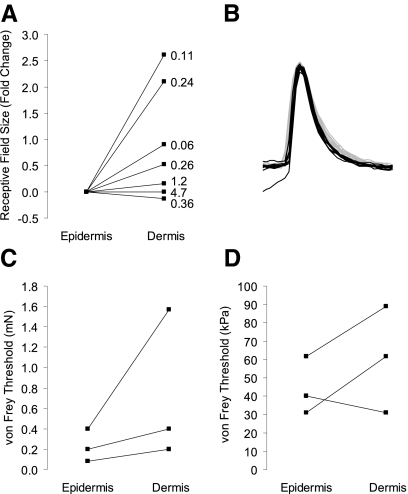
To determine whether the sensitivity of low-threshold touch receptors differed in this epidermis-up recording configuration compared with epidermis-down recordings, we directly compared receptive field size and von Frey thresholds in both configurations for the same afferents (Fig. 2). Consistent with our hypothesis, we observed significantly smaller receptive field areas and lower von Frey thresholds (P = 0.01, n = 7, Wilcoxon signed-rank test) when displacement stimuli were applied to the epidermal surface. Median receptive field areas were 0.17 mm2 when measured from the epidermal surface and 0.26 mm2 from the dermal side (Fig. 2A; P = 0.036, n = 7, paired Student's t-test). Mechanical thresholds (Fig. 2, C and D) measured from the epidermal surface were similar to those published by Woodbury and colleagues in the only other reports of an epidermis-up ex vivo preparation (Woodbury and Koerber 2007; Woodbury et al. 2001). These values are lower than those reported from epidermis-down reports (Table 1). Importantly, fluorescently labeled touch receptors could not be imaged from the dermal surface, making the epidermis-up configuration necessary for confirming afferent identity with genetically encoded fluorescent labels.
Fig. 2.
Receptive field (RF) areas and von Frey thresholds are higher on the dermal surface than the epidermal surface. Measurements were made from both the epidermal and dermal surfaces on the same set of afferents by flipping the skin over during recording (n = 7). RF size was calculated as the area of an ellipsoid with measured diameters and had median values of 0.17 mm2 (epidermal) and 0.26 mm2 (dermal). A: fold change in RF area from epidermal to dermal surface. Each connected pair of points indicates a single afferent and shows the change in measured RF size due to skin orientation, with the absolute size of the dermal RF indicated at the right (mm2). B: superimposed action potentials from one of the afferents used in the epidermis vs. dermis comparisons. Ten action potentials are from an epidermal stimulus (black) and 10 are from a dermal stimulus (gray). C: von Frey force thresholds also increased when measured from the dermal surface (P = 0.01, Wilcoxon one-tailed signed-rank test). Only 3 pairs are visible due to overlap of multiple afferents (n = 7). D: the same data as shown in C, but with von Frey forces converted to minimum potential pressures, based on the assumption of a simple cylindrical probe. Because von Frey filaments do not monotonically increase in diameter, this conversion to pressure causes a spurious reversal of some trends in the data.
Table 1.
Reported properties of touch-sensitive afferents in various preparations
| Firing Rates, Hz |
||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Preparation | Conduction Velocity, m/s (Means) | Mechanical Threshold, mN (Medians) | Fraction of Afferents, % (of All Afferents) | Peak Dynamic | Mean Static | Sources | |
| Mouse | Epidermis-up (our preparation) | Aβ-SAI | 13 | 0.08 | 7 | <670 | <70 | Maricich et al. 2009 |
| Aβ-SAII | 12 | 0.3 | 8 | <650 | <70 | This study | ||
| Aβ-RA | 13 | 0.08 | 13 | <1,000 | ||||
| Aδ-DH | 6.7 | 0.08 | 31 | <1,100 | <50 | |||
| Aδ-AM | 4.4 | 6 | 12 | <100 | <100 | |||
| Mouse | Epidermis-up | Aβ-SA | ≤0.07–5.2 | <100 | <30 | Woodbury and Koerber 2007 | ||
| Aβ-RA | 14 | ≤0.07–7 | ∼200 | <5 | Mcllwrath et al. 2007 | |||
| Aδ-DH | 6.8 | 3 | >400 | <10 | Woodbury et al. 2001 | |||
| Aδ-AM | 7.4 | 30 | <50 | |||||
| (of myelinated afferents) | ||||||||
| Mouse | Epidermis-down | Aβ-SA | 14 | ≤1.4 | 23–24 | <10 | Wetzel et al. 2007 | |
| Aβ-RA | 14 | ≤1 | 17–23 | <10 | Koltzenburg et al. 1997 | |||
| Aδ-DH | 4.5 | ≤1 | 10–18 | <5 | ||||
| Aδ-AM | 5.3 | 2–5.6 | 23–33 | <10 | ||||
| Mouse | In vivo | Aβ-SA | 23–24 | 0.2–4 | 22 | Boada and Woodbury 2007 | ||
| Aβ-RA | 18–21 | 0.3–2 | 18 | Cain et al. 2001 | ||||
| Aδ-DH | 4.9–7.1 | ≤0.07–10 | 25 | |||||
| Aδ-AM | 7.8 | 5 | 35 | |||||
| (of all afferents) | ||||||||
| Rat | In vivo | Aβ-SAI | 39 | 0.04–14 | 0.5–5 | <20 | Leem et al. 1993a | |
| Aβ-SAII | 43 | 0.25–25 | 0.5–8 | <20 | Leem et al. 1993b | |||
| Aδ-RA | 45 | 0.8–14 | 0–7 | Lynn and Carpenter 1982 | ||||
| Ge and Khalsa 2002 | ||||||||
| (Range) | ||||||||
| Cat | In vivo | Aβ-SAI | 30–90 | >0.01 | 23 | >1,000 | Iggo and Muir 1969 | |
| Aβ-SAII | 30–90 | 9 | <800 | Chambers et al. 1972 | ||||
| Brown and Iggo 1967 | ||||||||
| Burgess et al. 1968 | ||||||||
| (Myelinated only) | ||||||||
| Human | In vivo | Aβ-SAI | 35–70 | 0.4 | 9–38 | Edin 2001 | ||
| Aβ-SAII | 35–70 | 1.0 | 27 | Vallbo et al. 1995 | ||||
| Aδ-RA | 35–70 | 0.1 | 22–48 | |||||
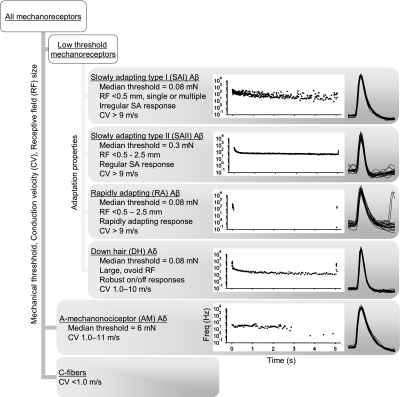
The epidermis-up ex vivo preparation enables precise classification of all previously identified classes of touch-sensitive afferents using conduction velocity, receptive field extent, von Frey threshold, and the response to a sustained stimulus (Fig. 3). Single-unit isolation was informed by action potential shape and receptive field. In agreement with published reports (McIlwrath et al. 2007; Wetzel et al. 2007; Woodbury and Koerber 2007), conduction velocity values were used to separate Aβ- (>9 m/s), Aδ- (1.0–11 m/s), and C-fibers (<1.0 m/s). Some units with response properties usually associated with Aβ-afferents (SAI, SAII, and RA) in our preparation had conduction velocities as low as 9.3 m/s and D-hair and AM fibers conducted as fast as 10.4 m/s. Due to overlapping conduction velocity ranges for these populations, fibers were assigned “Aβ” or “Aδ” designations based on their physiological response properties, instead of using a hard cutoff at 10 m/s. Mean conduction velocities (±SD) for Aβ-, Aδ-, and C-fibers were 12.5 ± 2.0 m/s (n = 27), 6.0 ± 2.3 m/s (n = 46), and 0.41 ± 0.08 m/s (n = 24), respectively. Receptive field extents matched previous reports (Table 1) and were an especially useful parameter in separating D-hair afferents, which had oblong receptive fields 3–4 mm in length and about 1 mm wide, with the long axis of the receptive field aligned with that of the leg. Other afferent types' receptive fields were more symmetrical and, for most types, varied from punctate (<0.5 mm) to several millimeters in diameter. Mechanical thresholds were useful in distinguishing AM afferents whose thresholds exceeded 1.5 mN (median = 6 mN, n = 19), consistent with reports from in vivo studies (Table 1). D-hair, RA and SA afferents had highly overlapping ranges of mechanical thresholds, with median thresholds for all three groups of 0.08 mN (n = 45, 14, and 32, respectively).
Fig. 3.
Flow chart for classifying touch receptors in the epidermis-up mouse skin–nerve preparation. After locating a mechanosensitive afferent with a mechanical search, A-afferents and C-fibers can easily be distinguished based on their conduction velocities. Aδ-fibers can usually be distinguished from Aβ-fibers by conduction velocity, but there is some overlap between the 2 populations. Slowly adapting (SA), rapidly adapting (RA), and any remaining A-mechanonociceptor (AM) or D-hair fibers can then be distinguished by mechanical thresholds, RF sizes, and adaptation properties. Slowly adapting types I and II (SAI and SAII, respectively) responses are differentiated by the regularity of their static-phase firing rates, which is quantified as the coefficient of variation (CoV) of interspike intervals (ISIs; see Figs. 4–6). Plots in each afferent-type box show a typical response, as instantaneous firing frequency vs. time, to a 5-s touch stimulus. Twenty mechanically evoked action potentials from the example fiber are superimposed to the right of each plot to show matching waveforms.
Because of the similar receptive field characteristics of most Aβ afferents and overlapping conduction velocity distributions of Aβ and Aδ afferents, controlled mechanical stimuli were often necessary to discriminate among mechanosensitive afferents with conduction velocities >9 m/s. D-hair afferents displayed robust rapidly adapting responses to very light touch stimuli (<0.08 mN), but maintained a low static firing rate throughout suprathreshold sustained stimuli, a behavior not seen in Aβ-RA afferents at any intensity level and consistent with other reports of D-hair properties in mice (Koltzenburg et al. 1997). AM afferents responded with low firing rates that adapted very slowly and lacked the robust on–off responses of D-hair receptors (Boada and Woodbury 2007; Koltzenburg et al. 1997). RA afferents fired exclusively during probe movement across force levels, making their classification relatively simple.
Slowly adapting afferents had little or no discharge at rest, fired rapidly during the onset of a mechanical stimulus, maintained firing throughout a sustained displacement, and sometimes produced a burst of action potentials at stimulus offset. We noted that some of these afferents responded to mechanical stimuli with a highly irregular sustained discharge of action potentials, whereas others displayed a more regular ISI during the adapted response (Fig. 3). This difference in firing regularity is readily detectable during recording by playing responses through a speaker (Supplemental Fig. S2). Such irregular firing is a hallmark of vertebrate SAI responses.
We next sought to determine whether mouse SAI responses could be reliably distinguished from other slowly adapting responses using the epidermis-up ex vivo preparation. To do so, we analyzed responses from 17 slowly adapting afferents, 9 of which were recorded from Atoh1/nGFP mice (or wild-type mice injected with FM1-43) to determine whether their receptive fields colocalized with Merkel cell–containing touch domes. As detailed in methods, we performed model-based cluster analysis and calculated BIC to assess how well each model described the data, with a penalty for increasing free parameters.
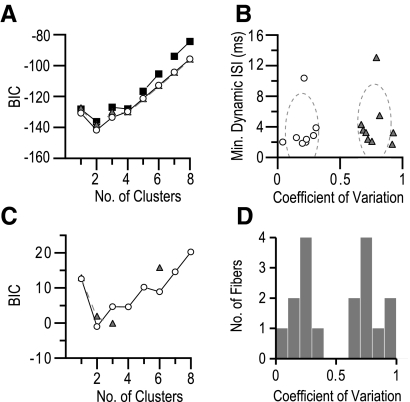
We found that several multivariate models yielded BIC < −100, the lowest of which was a five-cluster fit to static CoV of ISIs, minimum dynamic ISI, and mean static ISI. In this model, afferents that innervated touch domes failed to cluster together, indicating that the analysis was unsuitable for distinguishing SAI afferents.
By contrast, analysis of two variables (CoV and minimum dynamic ISI) identified two distinct clusters, one of which encompassed all fluorescently labeled touch-dome afferents. A model of two equal volume ellipsoid clusters oriented along the coordinate axes (EEI) yielded a minimized BIC of −142 for a two-cluster model (Fig. 4A). Other models, including equal-sized ellipsoids with freely oriented axes with the same orientation (EEE) or variable orientation (EEV) also predicted two clusters, but had minimum BIC > −142.
Fig. 4.
Two classes of SA mechanoreceptors exist in mice. Model-based cluster analyses on ≤4 variables including minimum dynamic ISI, mean static ISI, CoV of static ISIs, and minimum static ISI were performed on 17 SA fibers using the MClust package for R. A: the model and cluster number combination with the lowest Bayesian information criterion (BIC) that grouped together fluorescence microscopy-confirmed SAI fibers was equal size and shape ellipsoids, with their axes aligned with the coordinate axes (EEI, hollow circles). Minimum BIC with this model was −142 at 2 clusters. Other models shown are equal size and shape ellipsoids with either the same orientation (EEE, black squares) or freely oriented (EEV, gray triangles). Other models tested had minimum BIC above −100. B: all fibers used for cluster analysis plotted along the 2 discriminant variables. Fiber classification based on the best model chosen by the analysis from A is indicated by an open circle or gray triangle. Clusters for the best fit model were ellipsoids of the same volume with long axes perpendicular to the CoV coordinate. Clusters are indicated as dashed ellipses with radii of 3SDs centered around the mean of each cluster. C: univariate cluster analysis of CoV for all fibers yielded a higher BIC (−1), but categorized the fibers into the same clusters and provided the lowest BIC of any other single variable. The best fit was to an equal variance model (open circles) with 2 clusters. Results of variable variance model fitting are shown as gray triangles. D: mean CoV of interspike interval values plotted as a histogram of all fibers. For each afferent, values were calculated as the mean of the CoV of all stimuli. There is a bimodal distribution with maximal separation at 0.49. Afferents confirmed as SAI fibers by fluorescent microscopy lie within the cluster with CoV >0.49, leading to the designation of these 2 populations as SAI and SAII.
We noted that the two clusters identified by this analysis separated conspicuously along the CoV axis (Fig. 4B); therefore we reasoned that this coordinate alone might be sufficient to separate the clusters. Indeed, univariate analysis of CoV produced a minimum BIC for two equal-variance clusters. Despite a higher BIC (−1), the results from this classification were identical to those from the best multivariate model, yielding clusters with mean CoV values of 0.21 and 0.78 (SD = 0.09). The Gaussian distributions that describe these two clusters best separated at CoV = 0.49. As described in the following text, we designated these two clusters as SAI (CoV >0.49, n = 9) and SAII responses (CoV <0.49, n = 8), based on their firing properties and receptor morphologies.
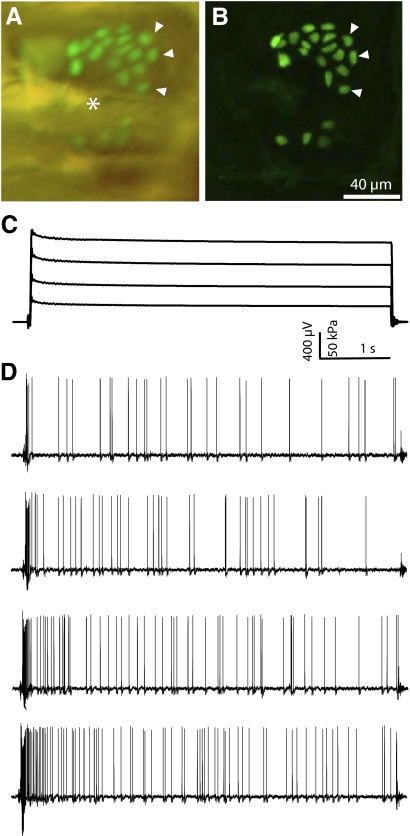
SAI afferents responded with irregular firing rates to sustained displacements of the skin (Fig. 5), as reported previously (Iggo and Muir 1969). Peak firing rates ≤668 Hz were observed for SAI responses (mean = 393 ± 177 Hz). Their punctate receptive fields encompassed only the extent of a touch dome and generally had von Frey thresholds <0.5 mN (median = 0.08 mN, n = 21). In two cases, we observed SAI afferents that innervated two touch domes. In Atoh1/nGFP or FM1-43-injected mice (Haeberle et al. 2004; Lumpkin et al. 2003), SAI receptive fields completely colocalized with groups of Merkel cells (Fig. 5, A and B; n = 10).
Fig. 5.
SAI receptive field images and responses to touch stimuli. Micrographs (A and B) show the Merkel-cell cluster innervated by this SAI afferent. Arrowheads indicate the same 3 Merkel cells in both images. A: an epifluorescence micrograph of the touch dome demonstrating enhanced green fluorescent protein (eGFP, green) in Merkel cells in the living skin–nerve preparation. Asterisk (*) denotes the position of the guard hair. B: confocal z-series projection of the same touch dome shows 22 Merkel cells in the cluster. C: recorded force values during a family of 5-s displacements. D: voltage traces showing action potential trains for each stimulus. The SAI afferent responds to increased force with an increase in firing rate while maintaining its irregular firing pattern. Responses are shown from static-phase pressures of about 30–150 kPa. This particular afferent was chosen as an example of the hallmark irregularity of SAI responses, although its firing rate falls below the mean for SAI afferents. There is an RA unit present in this recording as well, visible at the end of the top 2 traces as an approximately 200-μV peak that was easily discriminated and did not interfere with data analysis.
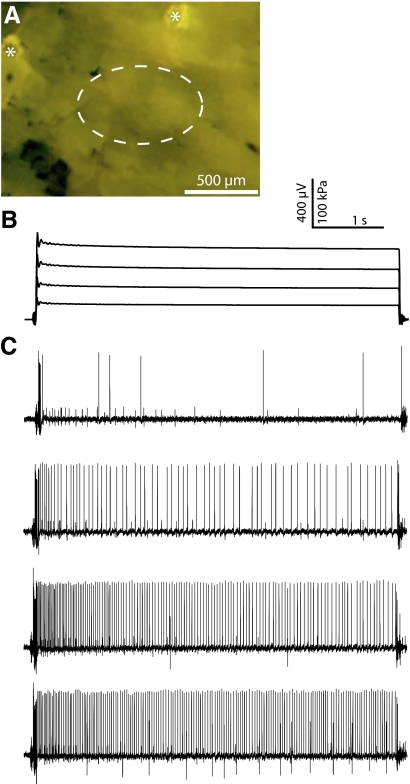
The slowly adapting Aβ afferents excluded from the SAI cluster displayed physiological properties consistent with those of SAII afferents in other species (Table 1). Most notably, they exhibited highly regular firing patterns during displacements that elicited a mean firing frequency ≥15 Hz (Fig. 6C), with peak firing frequencies of ≤633 Hz (mean = 380 ± 162 Hz). Additionally, their receptive fields ranged from punctate to 2 mm (median = 1 mm, n = 8) in diameter. Finally, they displayed mechanical thresholds of ≤4 mN (median = 0.3 mN, n = 14), suggesting that these afferents are slightly less sensitive to compressive stimuli than are SAI afferents. In recordings from mice with fluorescently labeled Merkel cells, SAII afferent receptive fields showed no overlap with fluorescent Merkel cells (Fig. 6A; n = 5), indicating that mouse SAII afferents do not innervate touch domes.
Fig. 6.
SAII receptive field image and responses to touch stimuli. A: micrograph of the skin of an FM1-43-injected mouse from which the recordings in B and C were taken. The receptive field of this afferent (dashed ellipse) does not overlap with 2 labeled touch domes (asterisks) visible in this field. B: force family for the voltage traces shown below. Each stimulus lasted for 5 s. Some mechanical ringing is visible at the onset of each stimulus, which decayed to below baseline well before the static phase. C: the SAII afferent responded with low, stochastic firing to very light stimuli (top trace), but more intense stimulation elicited robust and highly regular spike trains. When CoV values across stimulus intensity are averaged prior to afferent comparisons, the impact of low-firing rate responses is reduced. To avoid classification error, it is important to use stimuli sufficient to exceed this irregularity range (<15–20 Hz). Responses are shown from roughly 25- to 150-kPa static pressures. Two additional units are visible as low-amplitude action potentials, one at all stimulus intensities and one at only the highest 2 stimulus levels.
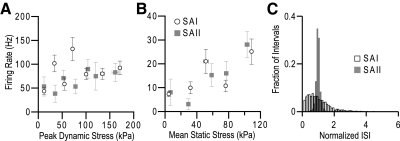
Stimulus–response plots revealed that the mean firing rates for SAI and SAII afferents were comparable during both dynamic and static response phases (Fig. 7, A and B). A histogram of normalized ISIs (Fig. 7C) illustrates the differences in ISI variability during static-phase responses. SAI intervals (n = 3,348 normalized intervals) encompass a much broader distribution than the tightly clustered peak for SAII intervals (n = 1,533 normalized intervals). This difference in ISI variability allows the use of CoV as a simple discriminant and is audibly detectible during recording (Supplemental Fig. S2).
Fig. 7.
Firing rate data for SAI responses (open circles) and SAII responses (gray squares) are shown in A and B (error bars denote ±SE). Data represent individual stimuli across afferents in 200-kPa bins, demonstrating that SAI and SAII afferents produce similar firing rates in response to displacement stimuli. A: mean firing rate during the dynamic phase (t = 0–200 ms, calculated as the inverse of the mean ISI) plotted against peak dynamic force. B: mean firing rate for the static phase (2–4.5 s) plotted against the mean static-phase force (n = 113 stimuli from 11 SAI afferents and 27 stimuli from 7 SAII afferents). C: histogram of normalized static-phase ISIs for SAI (black outline) and SAII (solid gray) responses. Note the wide dispersion of intervals in SAI responses relative to SAII. To allow comparison of intervals across stimulus intensities and afferents, each interval is normalized to the mean interval for their stimulus of origin (SAI, n = 3,348 intervals; SAII, n = 1,533 intervals).
DISCUSSION
Ex vivo skin–nerve preparations provide an expedient alternative to in vivo recordings and have been used for over two decades to investigate mechanisms of peripheral somatosensory transduction (Reeh 1986). Here—for the first time—we used a modified ex vivo skin–nerve preparation to discriminate classes of slowly adapting responses in mice. In this recording setup, the epidermis is dry and exposed to air, allowing direct stimulation of epidermal touch receptors on their physiologically relevant surface. The orientation of our preparation also allows direct imaging of fluorescently labeled end organs while recording. These capabilities represent a significant step toward experiments to rapidly identify, manipulate, and assess the function of sensory structures that transduce touch, mimicking in vivo mechanical thresholds and firing rates in an ex vivo preparation.
Most prior approaches to ex vivo recording used a “flipped” system, in which the epidermis lies against a substrate and the dermis is superfused with saline. This is an excellent method to ensure that the preparation stays well perfused, but may not be necessary, given a previous finding that epidermal exposure to air is sufficient to maintain SAI responsiveness in the absence of blood flow in vivo (Findlater et al. 1987). Additionally, stimulating the dermal surface of the skin has drawbacks for precise testing of light-touch responses. It is a nonnative stimulus for cutaneous receptors and provides slightly elevated values for receptive field area and mechanical threshold (Fig. 2), presumably attributable to force buffering and spreading in the dermis and the slipperiness of the wet corium surface (Lewin and Moshourab 2004). Consistent with published reports (Woodbury and Koerber 2007), we find that liquid on the receptive field can make accessing the receptive field difficult because the finest standard von Frey filaments (≤0.08 mN) are deflected away by surface tension or obscured by diffraction, making precise filament positioning and accurate threshold determination much more difficult.
Mechanical thresholds for identified sensory afferent types are consistent across species in vivo. Recordings of myelinated, touch-sensitive afferents in human hairy skin report the presence of SAI and SAII afferents with median mechanical thresholds of 0.45 and 1.3 mN, respectively (Vallbo et al. 1995). In the cat, minimum forces to elicit a response in SAI afferents were about 0.01 mN (Iggo and Muir 1969). Similarly, at least one study of rat in vivo recordings reported mechanical threshold values of <0.05 mN (Lynn and Carpenter 1982), although others reported thresholds of ≤14 mN (Leem et al. 1993a). All of the aforementioned models, including humans (Vallbo et al. 1995), cats, rabbits (Brown and Iggo 1967), and even reptiles (Kenton et al. 1971), have two distinct classes of slowly adapting touch receptors, making the two in vivo reports from mice a notable exception (Table 1). Neither study separated SAI from SAII responses, a feature they share with ex vivo characterizations of mouse cutaneous afferents.
We previously published an unbiased survey of mechanosensitive afferents in wild-type mice using our epidermis-up approach (Maricich et al. 2009). We found afferents with mechanical thresholds ranging from 0.08 to 254 mN (n = 97); however, the vast majority (n = 94/97) had thresholds ≤10 mN. We found 24 C-fibers (25.8%), 13 AM Aδ-afferents (14.0%), 33 D-hair Aδ-afferents (35.5%), 10 RA Aβ-afferents (10.8%), 6 SAI Aβ-afferents (6.5%), and 7 SAII Aβ-afferents (7.5%). These results agree with in vivo and ex vivo reports in mice (Table 1), with the exception that we separate slowly adapting afferent types I and II (Boada and Woodbury 2007; Cain et al. 2001; Koerber and Woodbury 2002; McIlwrath et al. 2007; Wetzel et al. 2007; Zimmermann et al. 2009). We did not observe fibers with unambiguously Aβ conduction velocities (>11 m/s) that had von Frey thresholds ≥6 mN, the cutoff for nociceptors used by Zimmerman et al. (2009).
It is worth noting that the bulk of the literature reports mechanical threshold as the approximate measured force of the smallest von Frey filament to consistently (≥50%) elicit a response, despite the finding that compressive stress/pressure is a more reliable predictor of firing rates in slowly adapting mechanoreceptors (Ge and Khalsa 2002). We follow this convention for mechanical threshold reporting for easier comparison with previous literature and because there is not a straightforward conversion of these forces into pressures. This is an inherent limitation of von Frey filaments because the bending of these filaments provides an irregular contact surface through which the force is applied. With that limitation in mind and assuming a simple cylinder perpendicular to the skin surface, the pressures applied by von Frey filaments in the light-touch range (<10 mN or ∼200 kPa) occupy the same pressure range as our mechanical indenter equipped with a filleted cylindrical probe (Supplemental Fig. S1).
We found that mouse SAI and SAII afferents can be reliably classified based on the regularity of firing, as measured by the CoV of static-phase ISIs. This parameter has previously been used to quantitatively discriminate slowly adapting units in hairy skin of the cat, with SAII response CoV generally <0.3 and SAI response CoV >0.5 (Chambers et al. 1972). CoV has also been used to discriminate slowly adapting responses in sinus hair follicles from rat and cat (Baumann et al. 1996; Cahusac and Mavulati 2009; Gottschaldt et al. 1973), although the value generally used in those studies to segregate whisker units (0.1 in rat, 0.2–0.5 in cat) was lower than the discriminant produced by our analysis (0.49). A few studies have reported regular responses arising from identified touch domes (Horch et al. 1974; Yasargil et al. 1988), but in both cases this required either deliberate damage to a portion of the touch dome or careful placement of very fine probes to stimulate specific regions of the touch dome. The relatively large size and perpendicular approach of our mechanical stimulus probe is designed to eliminate exactly these intratouch dome edge effects, minimizing the possibility of spuriously regular SAI responses.
Classifying slowly adapting afferents by their static-phase irregularity is not without complications because SAII responses lose much of their characteristic regularity at firing frequencies <15–20 Hz (Fig. 6C; Horch et al. 1974, 1977). We observe static firing rates of 20–40 Hz at about 100 kPa stimulus intensities (comparable to von Frey filaments <10 mN), above the minimum firing rate reported by Horch and colleagues to be necessary to distinguish SAI from SAII responses. At these stimulus intensities and response firing rates, the difference in regularity is audibly detectable when listening to neural responses during recording (Supplemental Fig. S2). Previously published ex vivo preparations typically reported SA firing rates below 10–20 Hz during the static phase, including the only previously published epidermis-up recording method, which is a likely explanation for the lack of afferent type discrimination (Kinkelin et al. 1999; Koerber and Woodbury 2002; McIlwrath et al. 2007).
It is possible that the responses we identify as SAII afferents do not share identity with the classically defined SAII response in the hairy skin of cat (Chambers et al. 1972), rat (Leem et al. 1993a,b; Lynn and Carpenter 1982; Reeh 1986), and macaque (Harrington and Merzenich 1970). Although their receptive field sizes, mechanical thresholds, and highly regular sustained firing rates closely match those of SAII afferents in other species, these afferents did not differ in stretch sensitivity or spontaneous firing rates compared with those of SAI afferents. This may explain some of the previous difficulty in separating these two light-touch responses in mice, given that SAII afferents have been consistently reported to be directionally stretch sensitive and to have higher spontaneous firing rates than those of SAI afferents in nonmurine species (Chambers et al. 1972; Zimmermann et al. 2009). A few publications have hypothesized the existence of a third SA light-touch receptor in humans (Edin 2001), although conclusive molecular, morphological, or statistical differentiation is lacking. Boada and Woodbury (2007) noted the presence of some regularly firing slowly adapting receptors in vivo, which they postulated to innervate musculature. In our ex vivo preparation, the major muscle mass is removed, leaving only small cutaneous muscles present. It is possible that our SAII afferents innervate this remnant cutaneous musculature, but because these sensors are exquisitely sensitive to epidermal touch, they function as cutaneous mechanoreceptors independent of the structure they innervate. Conclusive identification of SAII receptors awaits the discovery of a selective marker; however, our results clearly demonstrate that a significant proportion (∼50%) of slowly adapting Aβ-afferents in the hairy skin of mice arise from receptors other than Merkel–cell neurite complexes.
Widely used epidermis-down ex vivo systems have valuable advantages. The dermis is much more permeable to chemical agents than is the epidermis, making epidermis-down recording the preferred method for pharmacological studies in the intact skin (Kirchhoff et al. 1992; Steen and Reeh 1993). Nonetheless, the epidermis-up recording system may be preferable to epidermis-down recording for physiological studies of light-touch receptors because firing rates and mechanical thresholds more closely match those reported in vivo in mice and other mammals. Fine distinction of slowly adapting afferents in a genetically tractable model will allow exploitation of the full potential of mouse genetics to tease apart the mechanisms underlying touch sensation. Simply labeling Merkel cells by expressing eGFP in Atoh1-expressing cells, for example, allowed us to image the live end organs of SAI afferents during recording, confirming in living skin the observations of Iggo and Muir (1969) and Woodbury and Koerber (2007). This strategy can be implemented to easily identify other cutaneous receptors and to pave the way for genetic dissection of the cellular and molecular pathways that transduce touch.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-051219 to E. A. Lumpkin, Defense Advanced Research Projects Agency Grant HR0011-08-1-0072 to G. J. Gerling, National Library of Medicine Grant T15-LM-009462 to G. J. Gerling, and National Institute of General Medical Sciences Grant 2T32-GM-008507-16 to the Department of Neuroscience, Baylor College of Medicine.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Nelson for dissection assistance; K. Firozi for care and maintenance of the mouse colony; J. Howard and E. Martinson for assistance with chamber design and fabrication; and C. Cowen, D. Yatsenko, and members of the Lumpkin lab for insightful comments on the manuscript.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophot Int 11: 36–42, 2004 [Google Scholar]
- Baumann KI, Chan E, Halata Z, Senok SS, Yung WH. An isolated rat vibrissal preparation with stable responses of slowly adapting mechanoreceptors. Neurosci Lett 213: 1–4, 1996 [DOI] [PubMed] [Google Scholar]
- Boada MD, Woodbury CJ. Physiological properties of mouse skin sensory neurons recorded intracellularly in vivo: temperature effects on somal membrane properties. J Neurophysiol 98: 668–680, 2007 [DOI] [PubMed] [Google Scholar]
- Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S, Valmier J, Carroll P. Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron 64: 857–870, 2009 [DOI] [PubMed] [Google Scholar]
- Brown AG, Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol 193: 707–733, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PR, Petit D, Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol 31: 833–848, 1968 [DOI] [PubMed] [Google Scholar]
- Cahusac PM, Mavulati SC. Non-competitive metabotropic glutamate 1 receptor antagonists block activity of slowly adapting type I mechanoreceptor units in the rat sinus hair follicle. Neuroscience 163: 933–941, 2009 [DOI] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol 85: 1561–1574, 2001 [DOI] [PubMed] [Google Scholar]
- Chambers MR, Andres KH, von Duering M, Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci 57: 417–445, 1972 [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev 46: 131–145, 2004 [DOI] [PubMed] [Google Scholar]
- Edin B. Cutaneous afferents provide information about knee joint movements in humans. J Physiol 531: 289–297, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlater GS, Cooksey EJ, Anand A, Paintal AS, Iggo A. The effects of hypoxia on slowly adapting type I (SAI) cutaneous mechanoreceptors in the cat and rat. Somatosens Res 5: 1–17, 1987 [DOI] [PubMed] [Google Scholar]
- Fogh-Andersen N, Altura BM, Altura BT, Siggaard-Andersen O. Composition of interstitial fluid. Clin Chem 41: 1522–1525, 1995 [PubMed] [Google Scholar]
- Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc 97: 611–631, 2002 [Google Scholar]
- Fraley C, Raftery AE. MCLUST Version 3 for R: Normal Mixture Modeling and Model-based Clustering (Department of Statistics) Seattle, WA: Univ. of Washington Press; 2006 [Google Scholar]
- Gasser HS. The classification of nerve fibers. Ohio J Sci XLI: 145–159, 1941 [Google Scholar]
- Ge W, Khalsa PS. Encoding of compressive stress during indentation by slowly adapting type I mechanoreceptors in rat hairy skin. J Neurophysiol 87: 1686–1693, 2002 [DOI] [PubMed] [Google Scholar]
- Gilanyi M, Ikrenyi C, Fekete J, Ikrenyi K, Kovach AG. Ion concentrations in subcutaneous interstitial fluid: measured versus expected values. Am J Physiol 255: F513–F519, 1988 [DOI] [PubMed] [Google Scholar]
- Goodwin AW, Macefield VG, Bisley JW. Encoding of object curvature by tactile afferents from human fingers. J Neurophysiol 78: 2881–2888, 1997 [DOI] [PubMed] [Google Scholar]
- Gottschaldt KM, Iggo A, Young DW. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol 235: 287–315, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao M, Bechstedt S, Howard J, Lumpkin EA. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci USA 101: 14503–14508, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z. Sensory innervation of the hairy skin (light- and electronmicroscopic study. J Invest Dermatol 101: 75S–81S, 1993 [DOI] [PubMed] [Google Scholar]
- Harrington T, Merzenich MM. Neural coding in the sense of touch: human sensations of skin indentation compared with the responses of slowly adapting mechanoreceptive afferents innervating the hairy skin of monkeys. Exp Brain Res 10: 251–264, 1970 [DOI] [PubMed] [Google Scholar]
- Horch KW, Tuckett RP, Burgess PR. A key to the classification of cutaneous mechanoreceptors. J Invest Dermatol 69: 75–82, 1977 [DOI] [PubMed] [Google Scholar]
- Horch KW, Whitehorn D, Burgess PR. Impulse generation in type I cutaneous mechanoreceptors. J Neurophysiol 37: 267–281, 1974 [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 200: 763–796, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 10: 345–359, 2009 [DOI] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11: 455–461, 2001 [DOI] [PubMed] [Google Scholar]
- Johnson KO, Lamb GD. Neural mechanisms of spatial tactile discrimination: neural patterns evoked by Braille-like dot patterns in the monkey. J Physiol 310: 117–144, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenton B, Kruger L, Woo M. Two classes of slowly adapting mechanoreceptor fibres in reptile cutaneous nerve. J Physiol 212: 21–44, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkelin I, Stucky CL, Koltzenburg M. Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking the neurotrophin receptor p75. Eur J Neurosci 11: 3963–3969, 1999 [DOI] [PubMed] [Google Scholar]
- Kirchhoff C, Leah JD, Jung S, Reeh PW. Excitation of cutaneous sensory nerve endings in the rat by 4-aminopyridine and tetraethylammonium. J Neurophysiol 67: 125–131, 1992 [DOI] [PubMed] [Google Scholar]
- Koerber HR, Woodbury CJ. Comprehensive phenotyping of sensory neurons using an ex vivo somatosensory system. Physiol Behav 77: 589–594, 2002 [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol 78: 1841–1850, 1997 [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Srinivasan MA. Responses of cutaneous mechanoreceptors to the shape of objects applied to the primate fingerpad. Acta Psychol (Amst) 84: 41–51, 1993 [DOI] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol 69: 1684–1699, 1993a [DOI] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Weller SC, Chung JM. Differential activation and classification of cutaneous afferents in the rat. J Neurophysiol 70: 2411–2424, 1993b [DOI] [PubMed] [Google Scholar]
- Lewin GR, Moshourab R. Mechanosensation and pain. J Neurobiol 61: 30–44, 2004 [DOI] [PubMed] [Google Scholar]
- Linkert MR, Allan C, Kjellman E, Loranger B, Swedlow JR, Eliceiri KW. LOCI Bio-Formats Madison, WI: Univ. of Wisconsin–Madison, 2009 [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 12: 547–548, 2009 [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature 445: 858–865, 2007 [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns 3: 389–395, 2003 [DOI] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron 64: 841–856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B, Carpenter SE. Primary afferent units from the hairy skin of the rat hind limb. Brain Res 238: 29–43, 1982 [DOI] [PubMed] [Google Scholar]
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science 324: 1580–1582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwrath SL, Lawson JJ, Anderson CE, Albers KM, Koerber HR. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. Eur J Neurosci 26: 1801–1812, 2007 [DOI] [PubMed] [Google Scholar]
- Merkel F. Tastzellen und Tastkörperchen bei den Haustehieren und beim Menschen. Arch Mikrosk Anat 11: 636–652, 1875 [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci 23: 4054–4065, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl ER. Function of dorsal root ganglion neurons: an overview. In: Sensory Neurons: Diversity, Development, and Plasticity, edited by Scott SA. New York: Oxford Univ. Press, 1992, p. 3–23 [Google Scholar]
- Phillips JR, Johnson KO. Tactile spatial resolution. III. A continuum mechanics model of skin predicting mechanoreceptor responses to bars, edges, and gratings. J Neurophysiol 46: 1204–1225, 1981 [DOI] [PubMed] [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett 66: 141–146, 1986 [DOI] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462: 651–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen KH, Reeh PW. Actions of cholinergic agonists and antagonists on sensory nerve endings in rat skin, in vitro. J Neurophysiol 70: 397–405, 1993 [DOI] [PubMed] [Google Scholar]
- Stucky CL, Gold MS, Zhang X. Mechanisms of pain. Proc Natl Acad Sci USA 98: 11845–11846, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J, Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. J Physiol 483: 783–795, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, Erdmann B, Machelska H, Heppenstall PA, Lewin GR. A stomatin-domain protein essential for touch sensation in the mouse. Nature 445: 206–209, 2007 [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Central and peripheral anatomy of slowly adapting type I low-threshold mechanoreceptors innervating trunk skin of neonatal mice. J Comp Neurol 505: 547–561, 2007 [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, Koerber HR. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J Comp Neurol 436: 304–323, 2001 [PubMed] [Google Scholar]
- Yasargil GM, Macintyre L, Doucette R, Visheau B, Holmes M, Diamond J. Axonal domains within shared touch domes in the rat: a comparison of their fate during conditions favoring collateral sprouting and following axonal regeneration. J Comp Neurol 270: 301–312, 1988 [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Hein A, Hager U, Kaczmarek JS, Turnquist BP, Clapham DE, Reeh PW. Phenotyping sensory nerve endings in vitro in the mouse. Nat Protoc 4: 174–196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.