Abstract
Accumulating evidence points to a map of visual regions encoding specific categories of objects. For example, a region in the human extrastriate visual cortex, the extrastriate body area (EBA), has been implicated in the visual processing of bodies and body parts. Although in the monkey, neurons selective for hands have been reported, in humans it is unclear whether areas selective for individual body parts such as the hand exist. Here, we conducted two functional MRI experiments to test for hand-preferring responses in the human extrastriate visual cortex. We found evidence for a hand-preferring region in left lateral occipitotemporal cortex in all 14 participants. This region, located in the lateral occipital sulcus, partially overlapped with left EBA, but could be functionally and anatomically dissociated from it. In experiment 2, we further investigated the functional profile of hand- and body-preferring regions by measuring responses to hands, fingers, feet, assorted body parts (arms, legs, torsos), and non-biological handlike stimuli such as robotic hands. The hand-preferring region responded most strongly to hands, followed by robotic hands, fingers, and feet, whereas its response to assorted body parts did not significantly differ from baseline. By contrast, EBA responded most strongly to body parts, followed by hands and feet, and did not significantly respond to robotic hands or fingers. Together, these results provide evidence for a representation of the hand in extrastriate visual cortex that is distinct from the representation of other body parts.
INTRODUCTION
Social interactions are at the core of our common everyday experience. Identifying other people based on their facial and bodily features and understanding their behaviors and intentions from their movements are highly complex yet mostly effortless tasks (Van Overwalle and Baetens 2009). Within the human cortex several visual areas have been found to be selective for static depictions of human physical features. While the fusiform face area (FFA; Kanwisher et al. 1997; Puce et al. 1996) in the lateral fusiform gyrus and the occipital face area (OFA; Rossion et al. 2003) in the ventral occipital lobe encode visual aspects of human faces, the extrastriate body area (EBA; Downing et al. 2001) in the lateral occipitotemporal cortex and the fusiform body area (FBA; Peelen and Downing 2005) in the lateral fusiform gyrus encode whole bodies and body parts. Converging evidence from lesion and transcranial magnetic stimulation studies indicate that ventrotemporal and lateral occipitotemporal cortices are indeed causally associated with face and body discrimination (Barton 2003; Damasio et al. 1982; Moro et al. 2008; Pitcher et al. 2009; Steeves et al. 2009; Urgesi et al. 2007).
One body part in particular, the hand, is of great significance in daily life. For example, while communicating, we use hands to emphasize our speech and to direct people's attention. Moreover, while learning how to use new tools, we closely observe how the model's hands manipulate the object. More crucially, in action execution, on-line visual control is applied to monitor and correct the direction of the hand, even when performing simple reach-to-grasp movements. Humans' outstanding manual dexterity is an important capability that sets humans apart from other species (Napier 1956). Considering the unique role played by the hand in our capacity to interact with the external world, the question arises as to whether the hand may be selectively represented in the human visual cortex.
Pioneering neurophysiological studies in the inferior temporal (IT) cortex of macaque monkeys (Desimone et al. 1984; Gross et al. 1969) have provided evidence for neurons selectively responding to the vision of the hand. In humans, evidence for hand-selective neural responses is limited, with studies mostly focusing on investigating the selectivity of assorted body parts or whole bodies rather than individual body parts (Peelen and Downing 2007). Nonetheless, initial evidence for hand selectivity comes from functional magnetic resonance imaging (fMRI; Op de Beeck et al. 2010) and event-related potential (ERP; McCarthy et al. 1999) studies demonstrating higher responses to hands compared with that to torsos or faces in restricted parts of the human occipitotemporal cortex. Furthermore, it was shown that distributed fMRI response patterns in multiple regions of visual cortex discriminated between hands and torsos (Op de Beeck et al. 2010). These results suggest the existence of hand-selective responses in human visual cortex, although further investigations that include additional body and nonbody control conditions are needed to strengthen the evidence for this hypothesis. Furthermore, it is unknown how putative hand-selective responses are related, both functionally and anatomically, to known body-selective responses in EBA. Finally, response properties of a putative human hand-selective region remain largely unknown.
Here, we present two experiments providing evidence for hand-preferring fMRI responses in human visual cortex. In experiment 1 we measured fMRI responses to hands, whole bodies, assorted body parts, chairs, and handheld tools. This experiment allowed us to test for hand-preferring responses relative to both body and nonbody control conditions. It also allowed for a direct comparison of hand-preferring and known body-preferring responses in EBA. In experiment 2 we further investigated the functional profile of hand- and body-preferring regions by measuring responses to hands, fingers, feet, assorted body parts (arms, legs, torsos), and nonbiological handlike stimuli, such as robotic hands. In addition, this experiment tested for response selectivity to other non-hand body parts (feet, fingers).
METHODS
Participants
Fifteen naive volunteers with no history of neurological diseases took part in experiment 1. Fourteen of the volunteers also took part in experiment 2. All subjects were right-handed as assessed by the Edinburgh Handedness Inventory (Oldfield 1971). Due to excessive head motion one subject was excluded from the data analyses. Participants gave informed consent and the study was approved by the Ethical Committees of The School of Psychology and Sport Sciences of Northumbria University and Newcastle Magnetic Resonance Centre, School of Clinical Medical Sciences, University of Newcastle-on-Tyne.
Material and apparatus
For each stimulus category we presented 65 different grayscale photographs on a white background. Stimulus presentation was controlled by a PC computer running Psychophysics Toolbox package (Brainard 1997) in Matlab (The MathWorks, Natick, MA). Pictures were projected (Canon Xeed SX6 projector) onto a screen located at the foot of the scanner bed and were viewed through a mirror mounted on the head coil.
Experimental design
Each experiment consisted of two runs lasting 7 min 14 s, corresponding to 217 functional volumes. Each functional run comprised 25 stimulus blocks and 6 fixation blocks. Each stimulus was presented only once within a run. Runs were organized into quasi-random sequences of five stimulus blocks (one for each stimulus category) followed by periods of fixation. In experiment 1 stimulus categories were: hands, whole bodies, body parts, tools, and chairs. In experiment 2 stimulus categories were: body parts, hands, fingers, feet, and robotic hands. Whole body stimuli did not have heads but they did have hands (this choice was made to keep the concept of whole body intact). Body-part stimuli did not include either feet or hands. Hand stimuli depicted both left and right hands in equal amounts and were captured in a resting position viewed from a third-person perspective. In only 4 of 65 pictures the hand posture could imply some sort of grasping pose. Robotic hands were recruited from Internet websites and only a subgroup showed grasping postures (18 of 65). Within each block, stimuli were presented for 800 ms, with a blank interstimulus interval (ISI) of 200 ms for a total block time of 14 s. Subjects were asked 1) to fixate toward the center of the screen during the whole scan and 2) to perform a one-back task by pressing a button with the index finger any time the same image was presented twice in a row (within each block one or two images were randomly repeated).
Imaging data acquisition
All images were acquired using a Phillips Achieva 3T scanner with a SENSE standard 8-channel birdcage head coil. The functional gradient-echo echoplanar T2*-weighted images (EPI) with BOLD (blood oxygenation level dependent) contrast were acquired using a repetition time (TR) of 2 s, echo time (TE) of 30 ms, film aperture (FA) of 90°, field of view (FOV) of 192, and a matrix size of 64 × 64 pixels. Each functional image consisted of 30 axial slices (4 mm thickness with no gap), which covered the whole cerebral cortex. T1-weighted anatomical scans were collected using TR of 9.6, TE of 4.6, FA of 8°, FOV 256, and a matrix of 256 × 208 pixels. We collected 180 slices of 1.0 mm thickness.
Imaging data: preprocessing and analysis
Imaging data for both experiments were analyzed using Brain Voyager QX (version 1.9; Brain Innovation, Maastricht, The Netherlands) using the following steps. Individual subject's imaging data underwent high-pass temporal frequency filtering to remove frequencies below three cycles per run, linear trend removal, three-dimensional motion correction, spatial smoothing (6 mm Gaussian kernel, for the group-average analysis only), and were transformed into Talairach stereotaxic space (Talairach and Tournoux 1988). Functional volumes were then aligned to the anatomical volumes, thereby transforming the functional data into a common stereotaxic space across subjects.
Data were analyzed using a general linear model (GLM) and a random-effect GLM was used for the group-average analysis. The model included five experimental predictors (one for each stimulus category in each experiment) and six motion correction predictors (x, y, z for translation and for rotation). The periods of fixation (14 s) were used as baseline. The experimental predictors were modeled as a transient (14 s) epoch where the square-wave function was convolved with the default Brain Voyager QX “two-gamma” function designed to estimate the hemodynamic response.
In the averaged voxelwise group analysis, statistical activation maps were set to reliable threshold levels and cluster volumes (P < 0.005, minimum cluster size = 27) using Monte Carlo simulations (performed using Brain Voyager QX) to verify that our regions of interest were unlikely to have arisen by chance as a consequence of multiple comparisons.
In single-subject analysis, activations were defined in each individual at a threshold of P < 0.05, Bonferroni corrected.
In experiment 1, for both the averaged voxelwise group analysis and the individual subject analysis we first identified the active brain areas with a given comparison of interest using odd runs only. Analyses on the percentage bold signal change (%BSC), which were performed on the individual subject data only, were extracted from the even runs only. The use of separate data sets to identify the statistical maps and to evaluate brain activity within them circumvents the problem of “circular analysis” (Kriegeskorte et al. 2009), where the independence of statistical tests for selective analysis is invalidated by the bias present in the comparisons used to select the areas (procedures also called “double dipping”). For each region of interest (ROI) we extracted the event-related time course from a 10 × 10 × 10 mm cube centered at peak activation and then we computed the %BSC for the peak response (by averaging 3 to 6 volumes after stimulus appearance). The exact number of volumes was chosen in consideration of the shape of the peak activation on the averaged data. %BSC was then analyzed by using repeated-measures ANOVAs and post hoc pairwise t-test comparisons (Šidák corrected).
The pattern of brain activity for the stimuli presented in experiment 2 was tested by localizing hand-selective voxels using odd and even runs of experiment 1 and extracting the %BSC by using a 10 × 10 × 10 mm cube centered at peak activation using odd and even runs from experiment 2. Data were then analyzed by averaging peak response (4–8 volumes) and by using ANOVA statistics as before. With respect to experiment 1, although brain activations related to a given comparison of interest were localized by using odd runs only, the pattern of brain activity was analyzed using even runs.
RESULTS
Experiment 1
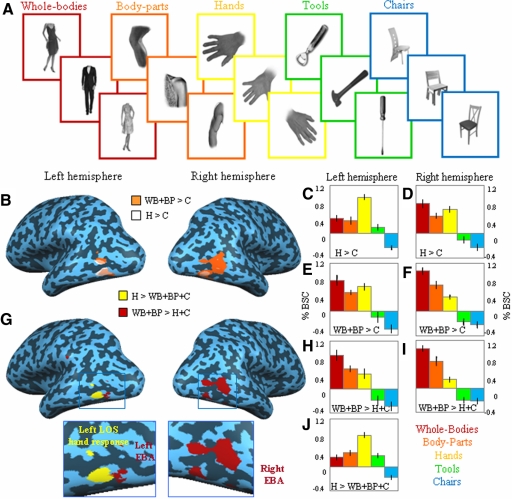
Experiment 1 of this fMRI study served to localize the selective neural substrate for visual coding of the human hand. In this experiment five different categories of visual stimuli were presented using a block design paradigm: whole bodies, body parts, human hands, tools, and chairs (Fig. 1A and methods). We first compared whole bodies and body parts versus chairs, given that it is the typical contrast used to localize body-selective voxels in the extrastriate visual cortex (e.g., EBA and FBA; Peelen and Downing 2007). To localize a selective neural response to hands, we similarly compared hands versus chairs. Figure 1B shows bilateral brain activations for both group average contrasts in the occipitotemporal cortex, demonstrating a large degree of overlap between regions activated in both contrasts: whole bodies and body parts versus chairs (body-preferring regions in EBA, depicted in orange) and hands versus chairs (hand-preferring regions, depicted in white; see also Table 1 for the Talairach coordinates of all activated areas). To investigate whether, despite a shared anatomical site, the hand-preferring regions and the body-preferring regions in the lateral occipitotemporal cortices are functionally dissociated, we analyzed the pattern of brain activity using percentage of bold signal change (%BSC; see methods) extracted from these regions localized in individual subjects. Brain activity underwent a 2 × 2 × 5 repeated-measures ANOVA using Hemisphere (left and right), region of interest ROI (EBA, hand-preferring region) and Stimulus type (whole bodies, body parts, hands, tools, and chairs) as within-subjects factors. ROIs were defined from independent data sets (see methods). Results revealed significant interactions for Hemisphere × Stimulus type [F(4,52) = 15.95, P < 0.0001] and for ROI × Stimulus type [F(4,52) = 24.97, P < 0.0001], suggesting that, despite an anatomical overlap, the different categories of stimuli activated each region of interest and the two hemispheres, to a different extent (Fig. 1, C–F). Post hoc pairwise comparisons on the %BSC revealed that, whereas hand-responsive voxels elicited higher activity for the hand stimuli compared with that for body parts [t(13) = 5.61, P < 0.001; see Fig. 1, C and D], body-responsive voxels in left and right EBA showed a higher response to whole bodies compared with that to hands [t(13) = 4.35, P < 0.008; see Fig. 1, E and F]. Interestingly, although the response to tools was statistically indistinguishable from that to chairs (P > 0.05) within the body-preferring region, it was significantly higher than that to chairs within the hand-preferring region [t(13) = 6.16, P < 0.0001]. In addition, we found a higher response to hand stimuli in the left hemisphere [hands > body parts, t(13) = 3.65, P < 0.028; see Fig. 1, C and E] and for body stimuli in the right hemisphere [whole bodies > hands, t(13) = 6.79, P < 0.0001; see Fig. 1, D and F]. These analyses suggest that, despite an anatomical overlap between body- and hand-preferring regions, a different hemispheric lateralization as well as a distinctive pattern of activation to the different stimulus categories could be observed for the two regions (Fig. 1, C–F).
Fig. 1.
Stimuli, averaged statistical maps and activation levels in experiment 1. A: exemplars of stimuli used in experiment 1: whole bodies, body parts, hands, tools, and chairs. B: group analysis results are shown in the right and left hemisphere of a single subject for the comparison of hands vs. chairs (H > C, shown in white) and for whole body and body parts vs. chairs (WB + BP > C, shown in orange). C–F: average peak activity (percentage bold signal change [%BSC]) for each stimulus category extracted from individual-subject brain areas functionally localized with the comparisons illustrated in B. Error bars represent SEs. G: group analysis results are shown in the right and left hemispheres of a single subject for the more selective comparison of hands vs. whole bodies, body parts, and chairs (H > WB + BP + C, shown in yellow) and for whole bodies and body parts vs. hands and chairs (WB + BP > H + C, shown in red). H–J: average peak activity (%BSC) for each stimulus category in the individual-subject brain areas localized with the comparisons illustrated in G. Error bars represent SEs.
Table 1.
Main contrasts, brain areas, volume, Talairach coordinates, and t values for averaged group data in experiment 1
| Talairach Coordinates |
||||||
|---|---|---|---|---|---|---|
| Contrast | Brain Region | Volume, mm3 | x | y | z | t Value |
| WB + BP > C | Left occipitotemporal cortex | 3,970 | −46 | −67 | 1 | 4.2 |
| Left middle fusiform gyrus | 1,105 | −39 | −41 | −19 | 4.7 | |
| Right occipitotemporal cortex | 7,619 | 49 | −60 | 2 | 4.2 | |
| Right middle fusiform gyrus | 1,105 | 42 | −46 | −19 | 4.7 | |
| H > C | Left occipitotemporal cortex | 2,670 | −47 | −67 | −1 | 4.2 |
| Right occipitotemporal cortex | 1,632 | 49 | −65 | −1 | 4.2 | |
| WB + BP > H + C | Left occipitotemporal cortex | 1,539 | −47 | −72 | 3 | 3.3 |
| Left middle fusiform gyrus | 362 | −36 | −38 | −22 | 4.7 | |
| Right occipitotemporal cortex | 5,304 | 50 | −62 | 2 | 4.7 | |
| Right middle fusiform gyrus | 2,660 | 40 | −44 | −19 | 4.7 | |
| H > WB + BP + C | Left occipitotemporal cortex | 1,031 | −46 | −65 | −1 | 3.3 |
| H > BP | Left occipitotemporal cortex | 107 | −43 | −65 | 3 | 2.8 |
WH, whole bodies; BP, body parts; H, hands; C, chairs.
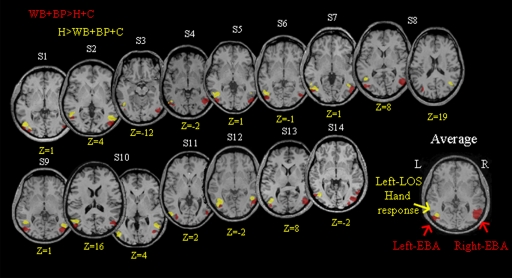
Next, we tested whether body-responsive and hand-responsive voxels can be anatomically dissociated by adopting more selective contrasts. To this end, we localized body-preferring voxels by comparing whole bodies and body parts versus hands and chairs, and hand-preferring voxels by comparing hands versus whole bodies, body parts, and chairs. The first contrast gave significant body-preferring activation in the right and left lateral occipitotemporal cortex and in the left and right fusiform gyrus (see Talairach coordinates in Table 1; also see Fig. 1G). The localization of the hand-preferring region in this analysis is now much more specific, with the contrast of hands versus whole bodies, body parts, and chairs showing a significant activation only in the left lateral occipitotemporal cortex (Talairach coordinates: x = −46, y = −65, z = −1; see also Fig. 1G). Furthermore, individual-subject analyses revealed that the hand-preferring region was evident in each participant (see Fig. 2, depicted in yellow and Supplemental Table S1).1 Moreover, hand- and body-preferring activations in the lateral occipitotemporal cortices were segregated from each other, with the hand-preferring response localized significantly more anterior (t-test for the y Talairach coordinates: P = 0.002) to the body-preferring response. Interestingly, the hand-preferring region was largely confined to an area along the left lateral occipital sulcus (Damasio 2005). For convenience, in the remainder of this study, we will refer to this highly robust hand-preferring response in the left lateral occipital sulcus using its anatomical location (left LOS, Fig. 2). The use of an anatomical landmark to label a functional activation has been previously applied—the lateral occipital cortex (LOC) for shape recognition (see Malach et al. 1995) and the middle temporal gyrus (MTG) for tool representation (see Chao et al. 1999)—and may also be appropriate here because functional characteristics of the region might change with future studies. Furthermore, this label is intended to describe rather than to identify a distinct functional brain area (see discussion). Interestingly, contrary to EBA, the hand response in LOS appears to be mainly lateralized in the left hemisphere, with only 6 of 14 subjects showing bilateral hand-preferring activation.
Fig. 2.
Individual statistical maps for hand-responsive and body-responsive voxels in experiment 1. The position of hand-selective voxels localized by comparing hands vs. whole bodies, body parts, and chairs (H > WB + BP + C, highlighted in yellow) and of body-selective voxels (extrastriate body area [EBA]) localized by comparing whole bodies and body parts vs. hands and chairs (WB + BP > H + C highlighted in red) in experiment 1 is shown in the clearest transversal slice of each participant. Averaged statistical maps (bottom right image) for the hand-responsive area within left lateral occipital sulcus (LOS) and the body-responsive area in right and left EBA are overlaid onto the slice of a single participant for clarity. As clearly shown, although the hand-related response in left LOS is mostly lateralized to the left (left LOS = 14/14 participants; right LOS = 6/14 participants), EBA is bilaterally represented (left and right EBA = 14/14). In addition, in most of the participants (11/14) left LOS is localized anterior to left EBA. L, left; R, right.
To demonstrate how EBA and left LOS, as defined with the more selective contrasts, functionally differ from each other, single subjects' %BSC underwent a 3 × 5 repeated-measures ANOVA using ROI (left-LOS, right-EBA, and left-EBA) and Stimulus type (whole bodies, body parts, hands, tools, and chairs) as within-subjects factors. The results revealed a main effect of Stimulus type [F(4,52) = 48.9, P < 0.0001] and, importantly, a significant interaction of Stimulus type × ROI [F(8,104) = 16.6, P < 0.0001], demonstrating that the functional properties of these regions differ from each other (Fig. 1, H–I). Whereas left LOS elicited a significantly higher response to hands compared with that to whole bodies [t(13) = 6.13, P < 0.0001], body parts [t(13) = 6.62, P < 0.0001], tools [t(13) = 7.06, P < 0.0001], and chairs [t(13) = 11.22, P < 0.0001], without distinguishing between whole bodies, body parts, and tools, activation in the right EBA was higher for whole bodies and body parts compared with that for hands [t(13) = 12.35, P < 0.0001; t(13) = 4.38, P < 0.001, respectively], tools [t(13) = 11.95, P < 0.0001; t(13) = 6.76, P < 0.0001, respectively], and chairs [t(13) = 12.26, P < 0.0001; t(13) = 7.75, P < 0.0001, respectively]. In addition, and in line with previous findings (Taylor et al. 2007), whole bodies elicited higher activation than body parts in the right EBA [t(13) = 3.65, P < 0.05]. Moreover, within right EBA, hands elicited a significantly higher response compared with nonbiological stimuli [tools: t(13) = 5.10, P < 0.002; chairs: t(13) = 5.33, P < 0.001]. Within left EBA, we found that hands, whole bodies, and body parts were statistically indistinguishable (P > 0.10) and showed similar significantly higher responses compared with the nonbiological stimuli represented by tools and chairs (for all comparisons, P < 0.05). Overall, the above-cited results indicate that the functional profile of left LOS differs from that of EBA, with left LOS being selective for the visual processing of the human hand compared with the rest of the body. Furthermore, whereas tool stimuli activated left LOS significantly above baseline and significantly more than chairs [t(13) = 6.56, P < 0.0001], both tools and chairs did not elicit any detectable activation in either right or left EBA (Fig. 1, H–J).
To confirm the hand versus nonhand body-part preference of left LOS, we computed the more direct and conservative comparison of hands versus body parts, both at group level and in individual subjects. In the group analysis, results revealed a hand-preferring activation in the left hemisphere only (x = −43, y = −65, z = 3; see also Table 1), overlapping with the left LOS region defined with the contrast hands versus whole body, body parts, and chairs. The same comparison was applied to single subject's data and, as shown in (Supplemental Fig. 1SA and Supplemental Table S1, the activated areas for hands versus body parts were localized in the left hemisphere in all participants (14/14 left hemisphere; 6/14 right hemisphere). Indeed, both contrasts (H > BP and H > WB + BP + C) activated highly similar regions, as indicated by nearly identical Talairach coordinates (Supplemental Table S1) that did not significantly differ (P > 0.34, for x, y, and z coordinates). Subsequently, the functional profile of the left LOS region defined by hands versus body parts was investigated. Single subjects' %BSC underwent a repeated-measures ANOVA using Stimulus type (whole bodies, body parts, hands, tools, and chairs) as within-subjects factor. The results (shown in Supplemental Fig. S1B) revealed a main effect of Stimulus type [F(4,52) = 30.20, P < 0.0001] and post hoc analyses confirmed previous results showing a significantly higher response to hands compared with that to whole bodies [t(13) = 5.25, P < 0.002], body parts [t(13) = 5.76, P < 0.001], tools [t(13) = 5.61, P < 0.001], and chairs [t(13) = 8.58, P < 0.0001]. Importantly, whole bodies and body parts were statistically indistinguishable when compared with each other (P = 1) and versus tools (for both comparisons, P > 0.925). Interestingly, the two nonbiological stimuli showed different responses in left LOS: whereas tools were significantly higher than chairs [t(13) = 5.35, P < 0.001], the latter were significantly inferior from baseline [t(13) = −5.28, P < 0.0001] and significantly lower than that of all the other categories of stimuli (for all comparisons, P < 0.003).
Experiment 2
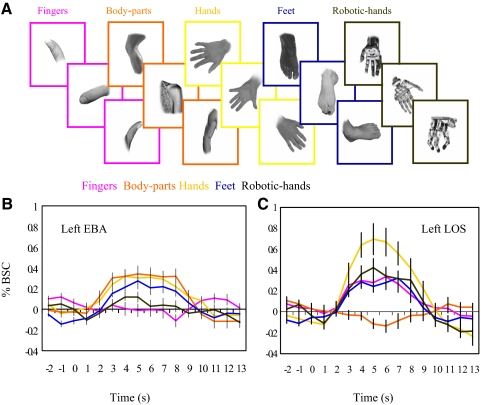
Questions arise as to the specific properties of the hand-preferring region in the left LOS. More in particular: 1) is the left LOS specifically responsive to hands or more generally to visually similar subcategories of body parts (e.g., feet); 2) does the left LOS respond to the hand as a whole or to a single part of it (e.g., the fingers); and 3) does the left LOS respond to nonbiological stimuli that resemble the hand in terms of their function (e.g., robotic hands)? To answer these questions in experiment 2 we measured the neural response of left LOS to images of not only fingers, feet, robotic hands, but also body parts and hands (Fig. 3A). Given the strong lateralization for the hand response, we focused our analyses in the left hemisphere and for each individual participant we first localized EBA and LOS in the left hemisphere as regions of interest using the main contrasts and data set of experiment 1 (hand contrast: hands vs. whole bodies, body parts, and chairs; body contrast: whole bodies and body parts vs. hands and chairs). The %BSC in individually defined left LOS and EBA underwent a 2 × 5 ANOVA with ROI (left LOS and left EBA) and Stimulus type (hands, feet, fingers, robotic hands, and body parts; see Fig. 3A) as within-subject factors. Results revealed a main effect of Stimulus type [F(4,48) = 9.66, P < 0.0001], further qualified by an interaction between ROI × Stimulus type [F(4,48) = 30.15, P < 0.0001], indicating that the two regions responded differently to the stimuli (see Fig. 3, B and C). Within left EBA, activation for body parts did not differ from that for feet and human hands, but was significantly higher than that for fingers [t(12) = 4.45, P < 0.01] and robotic hands [t(12) = 4.30, P = 0.01; see Fig. 3B]. Conversely, within left LOS brain activity for human hands was significantly greater than that for body parts [t(12) = 5.71, P = 0.001], feet [t(12) = 3.40, P < 0.05], and fingers [t(12) = 3.54, P < 0.05] and, despite showing a tendency, did not significantly differ from that for robotic hands [t(12) = 3.15, P = 0.079] (see Fig. 3C). For completeness, we repeated the analysis by localizing left LOS with the more conservative contrast of hand versus body parts. The %BSC for left LOS underwent a one-way repeated-measures ANOVA with Stimulus type (hands, feet, fingers, robotic hands, and body parts) as within-subjects factor. Results revealed a main effect of Stimulus type [F(4,48) = 20.11, P < 0.0001], indicating that in the left LOS brain activity for human hands was significantly greater than that for body parts [t(12) = 5.98, P = 0.001], feet [t(12) = 4.18, P < 0.013], and fingers [t(12) = 3.87, P < 0.023] and, despite showing a tendency, did not significantly differ from robotic hands [t(12) = 3.22, P = 0.068].
Fig. 3.
Stimuli and activation levels for experiment 2. A: exemplars of stimuli used in experiment 2: fingers, body parts, hands, feet, and robotic hands. B and C: average time course (%BSC) for each stimulus category in experiment 2 in left LOS, functionally localized in each individual by comparing hands vs. whole bodies, body parts, and chairs (H > WB + BP + C) and left EBA functionally localized in each individual by comparing whole bodies and body parts vs. hands and chairs (WB + BP > H + C) for odd and even runs of experiment 1. Error bars represent SEs.
In addition we tested whether, as found for hands, feet, and fingers may also activate a separate portion of the visual cortex. To do so, we compared feet versus body parts and fingers versus body parts using odd runs only. No significant activations were found for either contrasts at the chosen threshold (or at a more liberal one, for example, P = 0.05, uncorrected for multiple comparison). We also performed the comparison at the level of single subjects. Significant activation in left lateral occipitotemporal cortex was found in 9 of 13 participants for the contrast feet versus body parts and in 12 of 13 participants for the contrast fingers versus body parts. However, %BSC in both areas suggested a lack of selectivity for both feet and fingers stimuli showing that there was no significant difference between feet and hands and between fingers and hands (see Supplemental text and Supplemental Fig. S2, A and B). As expected, the contrast hands versus body parts again gave significant (P < 0.01) activation in left occipitotemporal cortex (Talairach coordinates: x = −43, y = −64, z = 2).
DISCUSSION
The present fMRI study provides evidence for a hand-preferring region in the human extrastriate visual cortex. Hand-preferring responses were strongest in left lateral occipitotemporal cortex. Two experiments demonstrated the functional preference of left LOS to hands relative to various control conditions, including feet and fingers, that share several features with hands (fingers are part of the hand and feet have five toes). The hand response was localized anterior and closely adjacent to non-hand body responses. Interestingly, contrary to EBA, the hand response in the LOS was strongly left lateralized. Indeed, all 14 participants showed a hand response in the left hemisphere, whereas only 6 participants showed a similar activation also in the right hemisphere. These findings are in line with previous evidence reporting the existence of 1) hand-selective cells in monkey IT (Desimone et al. 1984; Gross et al. 1969), 2) hand-responsive ERP sites in the human left temporal lobe (McCarthy et al. 1999), and 3) hand versus torso specificity in the human visual cortex using fMRI (Op de Beeck et al. 2010). Importantly, the present data provide the first evidence for a double dissociation in the lateral occipitotemporal cortex between the representations of a specific body part such as the hand in the left LOS (where activation for hands was higher than that for both whole bodies and body parts) and bodies in general in EBA (where activation for both whole body and body parts were higher than that for hands). Indeed, although previous work (Op de Beeck et al. 2010) showed higher activation for hands compared with that for torsos and faces in the lateral occipitotemporal cortex, it did not find significantly greater responses to torsos than to hands—a preferred response to torsos compared with that to hands was reported only in the right fusiform gyrus. The selective response to hands but not torsos is in agreement with our findings and suggests that hands, more than other body parts, could be overrepresented in the lateral occipitotemporal cortex.
What do our results suggest about the organization of body part representations in the human lateral occipitotemporal cortex? One possibility is that selective representations exist for some, or perhaps all, individual body parts, with the EBA being the collection of these separate representations. Alternatively, the hand, similar to the face, could be a “special” body part and distinctive among other body parts in eliciting selective responses in the human high-order visual cortex. Indeed, the way we interact with cospecifics and with the external world is continuously mediated by our hands, starting with learning how to intake food, following by pointing at objects to name them, continuing with learning how to count and write, and then developing into more complex abilities such as gesture communication and tool-use execution/understanding. In consideration of the special role played by hands in our daily experience it is therefore possible that hands, like faces, are overrepresented in visual cortex relative to other body parts. However, it should be pointed out that, despite the absence of a selective response to feet and fingers in the current study, we cannot rule out that a less conservative contrast (e.g., feet vs. chairs and fingers vs. chairs) would uncover such responses. Finally, future experiments using high-resolution fMRI (Schwarzlose et al. 2005) and fMRI adaptation will be useful to test for the existence of separate body part representations within EBA and the extrastriate visual cortex more generally.
Regardless of whether the hand response in left LOS is a unique functional region or should instead be considered a subregion of EBA, it clearly showed a distinct functional specialization in the present experiments. The left LOS, but not EBA, responded to non-human robotic hands and partially to nonbiological stimuli such as tools, but not to other objects such as chairs. This response profile may relate to recent findings suggesting a broader modulation of neural activity in visual areas from other modalities, such as semantics (Mahon et al. 2009), tactile information (Amedi et al. 2001; Burton et al. 2002), and motor action (Astafiev et al. 2004). In this respect, humans' unique manual abilities to manipulate external objects and tools might influence the information encoded within left LOS. The relevance of occipitotemporal regions in storing, not only visual object information, but also motion and associated action knowledge with it, gained support in a series of experiments by Beauchamp and colleagues (2002, 2003). These reports showed that although an area in the posterior part of the superior temporal sulcus (STS), anterior and superior to the motion area MT (Grossman and Blake 2002; Grossman et al. 2000), responds to body-related form and motion, a region within the posterior middle temporal gyrus (MTG), anterior and inferior to MT, responds to tool-associated form and motion (Beauchamp et al. 2002, 2003). Interestingly, anterior to left LOS in the left MTG a selective response to tools has been widely reported (Chao et al. 1999; Valyear and Culham 2009; Valyear et al. 2007), but also see the results report by Downing and colleagues (2006). A number of imaging studies support the role of left MTG in coding not only tool visual form but also conceptual knowledge and tool-associated actions (Binder et al. 2009; Devlin et al. 2002; Martin et al. 1995, 1996). In support of these findings, clinical studies (Tranel et al. 1997, 2003) showed that lesions involving left MTG cause impairments in action knowledge associated with objects. In this respect, the observation that left LOS, but not EBA, responds to tools may reflect a coding of motion information of hand actions associated with tool use.
Interestingly, in contrast to the predominantly right hemispheric lateralization of other visual body and face selective regions, such as FBA (Peelen and Downing 2005), FFA (Kanwisher et al. 1997), OFA (Puce et al. 1996), and EBA itself (Downing et al. 2001), the hand response in LOS showed a strong left hemispheric lateralization. Neural mechanisms encoding hands being primarily lateralized in the hemisphere dominant for human praxis may symbolize an advantageous structural organization. Indeed, a left lateralized network would allow fast interhemispheric connections between brain regions in left occipitotemporal cortex representing hand visual form, hand motion, and tool actions, and left frontoparietal regions subserving planning and guidance of hand actions (Goodale and Milner 1992; Rizzolatti et al. 1998). In line with this, clinical studies report that, although left frontoparietal and parietal lesions frequently induce postural and spatial temporal errors in gesture imitation and object usage (Buxbaum and Saffran 2002; Haaland et al. 2000), lesions within the left temporoparietal junction compromise the conceptual knowledge of the correct hand movements associated with a specific object (De Renzi and Lucchelli 1988). An interesting avenue for future research is to test the role of left LOS in the observation and execution of object-directed hand actions and how it is functionally connected with other regions implicated in these processes. Indeed, the body is not a passive entity but mediates our interaction with the external world. This bidirectional interactive experience is likely to be critical in modulating and shaping functional brain organization.
In conclusion, this report provides evidence for neural representation of the human hand in the left occipitotemporal cortex. EBA has generally been regarded as a uniform neural substrate dedicated to visual body processing. Our results indicate that segregated body-part representations may exist within lateral occipitotemporal cortex, with the hand possibly being overrepresented relative to other body parts. Future experiments are needed to address 1) the functional properties of the LOS hand response (e.g, the difference between hands in grasping and resting postures) and 2) how hand-preferring responses found within left LOS relate—functionally and anatomically—to other nearby functional areas encoding motion (MT; Tootell et al. 1995), biological motion (superior temporal sulcus [STS]; Hoffman and Haxby 2000), and tool action information (left MTG-TA; Chao et al. 1999).
GRANTS
This work was supported by internal funding from Northumbria University and UK Medical Research Council Grant G0401090.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Franz Mechsner and Dr. Quoc Vuong for inputs on earlier versions of the manuscript, L. Morris and C. Smith for assistance with fMRI data collection, and A. Bester for help in hardware development.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Amedi A, Malach R, Hendler T, Peled S, Zohary E. Visuo-haptic object-related activation in the ventral visual pathway. Nat Neurosci 4: 324–330, 2001 [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7: 542–548, 2004 [DOI] [PubMed] [Google Scholar]
- Barton JJ. Disorders of face perception and recognition. Neurol Clin 21: 521–548, 2003 [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron 34: 149–159, 2002 [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. J Cogn Neurosci 15: 991–1001, 2003 [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19: 2767–2796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Diamond JB, Raichle ME. Adaptive changes in early and late blind: a fMRI study of verb generation to heard nouns. J Neurophysiol 88: 3359–3371, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Saffran EM. Knowledge of object manipulation and object function: dissociations in apraxic and nonapraxic subjects. Brain Lang 82: 179–199, 2002 [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci 2: 913–919, 1999 [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology 32: 331–341, 1982 [DOI] [PubMed] [Google Scholar]
- Damasio H. Human Brain Anatomy in Computerized Images Oxford, UK: Oxford Univ, 2005 [Google Scholar]
- De Renzi E, Lucchelli F. Ideational apraxia. Brain 111: 1173–1185, 1988 [DOI] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci 4: 2051–2062, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Moore CJ, Mummery CJ, Gorno-Tempini ML, Phillips JA, Noppeney U, Frackowiak RS, Friston KJ, Price CJ. Anatomic constraints on cognitive theories of category specificity. NeuroImage 15: 675–685, 2002 [DOI] [PubMed] [Google Scholar]
- Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cereb Cortex 16: 1453–1461, 2006 [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science 293: 2470–2473, 2001 [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci 15: 20–25, 1992 [DOI] [PubMed] [Google Scholar]
- Gross CG, Bender DB, Rocha-Miranda CE. Visual receptive fields of neurons in inferotemporal cortex of the monkey. Science 166: 1303–1306, 1969 [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R. Brain areas involved in perception of biological motion. J Cogn Neurosci 12: 711–720, 2000 [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron 35: 1167–1175, 2002 [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain 123: 2306–2313, 2000 [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci 3: 80–84, 2000 [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 12: 535–540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A. Category-specific organization in the human brain does not require visual experience. Neuron 63: 397–405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA 92: 8135–8139, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science 270: 102–105, 1995 [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature 379: 649–652, 1996 [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Belger A, Allison T. Electrophysiological studies of human face perception. II: Response properties of face-specific potentials generated in occipitotemporal cortex. Cereb Cortex 9: 431–444, 1999 [DOI] [PubMed] [Google Scholar]
- Moro V, Urgesi C, Pernigo S, Lanteri P, Pazzaglia M, Aglioti SM. The neural basis of body form and body action agnosia. Neuron 60: 235–246, 2008 [DOI] [PubMed] [Google Scholar]
- Napier JR. The prehensile movements of the human hand. J Bone Joint Surg Br 38B: 902–913, 1956 [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971 [DOI] [PubMed] [Google Scholar]
- Op de Beeck HP, Brants M, Baeck A, Wagemans J. Distributed subordinate specificity for bodies, faces, and buildings in human ventral visual cortex. NeuroImage 49: 3414–3425, 2010 [DOI] [PubMed] [Google Scholar]
- Peelen MV, Downing PE. Selectivity for the human body in the fusiform gyrus. J Neurophysiol 93: 603–608, 2005 [DOI] [PubMed] [Google Scholar]
- Peelen MV, Downing PE. The neural basis of visual body perception. Nat Rev Neurosci 8: 636–648, 2007 [DOI] [PubMed] [Google Scholar]
- Pitcher D, Charles L, Devlin JT, Walsh V, Duchaine B. Triple dissociation of faces, bodies, and objects in extrastriate cortex. Curr Biol 19: 319–324, 2009 [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci 16: 5205–5215, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106: 283–296, 1998 [DOI] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Crommelinck M. The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. NeuroImage 19: 877–883, 2003 [DOI] [PubMed] [Google Scholar]
- Schwarzlose RF, Baker CI, Kanwisher N. Separate face and body selectivity on the fusiform gyrus. J Neurosci 25: 11055–11059, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves J, Dricot L, Goltz HC, Sorger B, Peters J, Milner AD, Goodale MA, Goebel R, Rossion B. Abnormal face identity coding in the middle fusiform gyrus of two brain-damaged prosopagnosic patients. Neuropsychologia 47: 2584–2592, 2009 [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain New York: Thieme Medical, 1988 [Google Scholar]
- Taylor JC, Wiggett AJ, Downing PE. Functional MRI analysis of body and body part representations in the extrastriate and fusiform body areas. J Neurophysiol 98: 1626–1633, 2007 [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15: 3215–3230, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia 35: 1319–1327, 1997 [DOI] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R, Damasio H, Damasio AR. Neural correlates of conceptual knowledge for actions. Cogn Neuropsychol 20: 409–432, 2003 [DOI] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Ionta S, Aglioti SM. Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nat Neurosci 10: 30–31, 2007 [DOI] [PubMed] [Google Scholar]
- Valyear KF, Cavina-Pratesi C, Stiglick AJ, Culham JC. Does tool-related fMRI activity within the intraparietal sulcus reflect the plan to grasp? NeuroImage 36, Suppl. 2: T94–T108, 2007 [DOI] [PubMed] [Google Scholar]
- Valyear KF, Culham JC. Observing learned object-specific functional grasps preferentially activates the ventral stream. J Cogn Neurosci 22: 970–984, 2009 [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage 48: 564–584, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.