Abstract
The central pattern generator can generate locomotor-like rhythmic activity in the spinal cord in the absence of descending and peripheral inputs, but the motor pattern is regulated by feedback from peripheral sensory inputs that adjust motor outputs to external stimuli. To elucidate the possible role of Hb9-expressing interneurons (Hb9 INs) in the locomotor circuitry, we investigated whether their induced oscillatory activity is modulated by low-threshold afferents in the isolated spinal cords of neonatal Hb9:eGFP transgenic mice. Low-intensity stimulation of segmental afferents generated short-latency, monosynaptic excitatory responses in 62% of Hb9 INs. These were associated with longer-latency (∼13 ms) excitatory postsynaptic currents that were evoked in all Hb9 INs, probably by slow conducting afferents that synapse directly onto them. Concomitant morphological analysis confirmed that afferent axons with immunoreactive expression of vesicular glutamate transporter-1 and parvalbumin, presumably from primary afferents, contacted somata and dendrites of all Hb9 INs. Most of the putative synaptic contacts were on distal dendrites that extended to an area with profuse afferent projections. We next examined whether low-threshold afferents in upper (flexor-related) and lower (extensor-related) lumbar segments altered the timing of neurochemically induced locomotor-like rhythms in Hb9 INs and motoneurons. Excitation of flexor-related afferents during the flexor phase delayed the onset of subsequent cycles in both Hb9 INs and segmental motoneurons while maintaining the phase relationship between them. The in-phase correlation between voltage oscillations in Hb9 INs and motor bursts also persisted during the two- to threefold increase in cycle period triggered by extensor-related afferents. Our findings that low-threshold, presumably muscle afferents, synapse directly onto these interneurons and perturb their induced locomotor-like membrane oscillations in a pattern that remains phase-locked with motor bursts support the hypothesis that Hb9 INs are part of the sensorimotor circuitry that regulates the pattern of locomotor rhythms in the isolated cord.
INTRODUCTION
In all walking vertebrates, the coordinated rhythmic activity of flexor and extensor motoneurons during locomotion is controlled by spinal circuits commonly referred to as the locomotor central pattern generators (CPGs). CPGs function in relative autonomy and can be neurochemically activated in the isolated rodent spinal cord in vitro (e.g., Cazalets et al. 1992; Cowley and Schmidt 1997; Kiehn and Kjaerulff 1998; Kudo and Yamada 1987; Smith and Feldman 1987; Whelan et al. 2000; reviewed by Goulding 2009; Kiehn 2006). Peripheral inputs that provide sensory feedback to the locomotor circuitry can alter the timing of rhythms produced by the generator (Burke et al. 2001; Pearson and Collins 1993; reviewed by Hultborn et al. 1998; McCrea 2001) and adjust locomotor patterns to external stimuli both in vivo (e.g., reviewed by McCrea and Rybak 2008; Pearson 2000) and in the isolated spinal cord (Iizuka et al. 1997; Kiehn et al. 1992). Stimulation of lumbar and sacrocaudal afferents can also trigger alternating locomotor-like motor bursts in the rat and mouse spinal cord in vitro (Bonnot et al. 2002; Gordon and Whelan 2006; Kwan et al. 2009; Lev-Tov et al. 2000; Marchetti et al. 2001; Smith et al. 1988; Zhong et al. 2007). The ability of peripheral inputs to either initiate or regulate left–right and flexor–extensor coordinated motor outputs indicates that sensory control of locomotor activity is exerted through the CPG circuitry (e.g., Gossard et al. 1994; Pearson 2004; Rossignol et al. 2006).
Afferent modulation of locomotor activity is often classified as resetting versus nonresetting actions on the timing of rhythmic activity. In the resetting paradigm, sensory inputs generate a permanent shift in the timing, so that subsequent locomotor cycles are advanced or delayed in their onset (reviewed by Hultborn et al. 1998). Three major forms of afferent perturbations of locomotor rhythms have been described in the adult cat. Perhaps the best-studied pathways involve low-threshold afferents, in particular the ankle extensor afferents that prolong the extensor phase (Conway et al. 1987; Duysens and Pearson 1980; Stencia et al. 2005). Afferents sensitive to hip position control the transition from flexor to extensor phase (Rossignol and Grillner 1978) and high-threshold cutaneous afferents can trigger nonresetting perturbations of the rhythms (Andersson et al. 1978; Schomburg et al. 1998). Much of what is known about afferent modulation of locomotor activity comes from studies in the adult cat and only a few studies have examined their role in regulating locomotor rhythms in neonatal rodents. The studies most relevant to our experiments are those of Kiehn et al. (1992) and Iizuka et al. (1997). Iizuka and colleagues have shown that low-intensity stimulation of the quadriceps nerve causes enhancement of the flexor phase (resetting) in the isolated spinal cord of postnatal day 1 (P1) to P3 rats, but as the result of a developmental switch, similar stimuli at P4–P6 inhibit the flexor phase and do not affect the extensor phase.
A few models have been proposed to explain the functional interaction between primary afferents and excitatory interneurons that are part of the CPG. Those include complex architectures with two- and three-level CPGs in which motoneurons are driven by excitatory interneurons that are part of the pattern formation network or last order interneurons (e.g., Burke et al. 2001; Endo and Kiehn 2008; Rybak et al. 2006; reviewed by McCrea and Rybak 2008). The prevailing hypothesis is that sensory inputs are capable of resetting the timing of rhythmic motor activity by acting directly on the rhythm generator. Nonresetting perturbations are thought to reflect the effects of peripheral afferents on other excitatory neurons in the locomotor circuitry.
One of the populations of neurons with probable function in the CPG is the cluster of excitatory interneurons that express the homeobox gene Hb9 (Hb9 INs) (reviewed by Brownstone and Wilson 2008; Goulding 2009; Kiehn 2006). These interneurons have several hallmark features of rhythmogenic neurons. First, neurochemically induced locomotor-like voltage oscillations and firing episodes in green fluorescent protein (GFP)–expressing Hb9 INs are in phase with flexor-related motor bursts (Han et al. 2007; Hinckley et al. 2005a). Second, the induced locomotor-like rhythms in Hb9 INs are independent of fast synaptic inputs (Han et al. 2007; Hinckley and Ziskind-Conhaim 2006; Tazerart et al. 2008; Wilson et al. 2005; Ziskind-Conhaim et al. 2008), raising the possibility that they are functional components of the rhythm-generating kernel of the CPG. Third, recurrent excitation through electrical coupling between clustered Hb9 INs (Hinckley and Ziskind-Conhaim 2006) and adjacent interneurons (Wilson et al. 2007) might contribute to the synchronization of oscillatory activity in the locomotor circuitry (Tresch and Kiehn 2000).
Hb9 INs are clustered in medial lamina VIII of predominantly flexor-related upper lumbar segments L1–L3 (Kiehn and Kjaerulff 1996) and are unlikely to serve as the sole rhythm generator for the numerous groups of motoneurons that control the coordinated excitation of all hindlimb flexor muscles. Indeed, a recent study has suggested that Hb9 INs cannot function as the only generator in the upper lumbar segments (Kwan et al. 2009). The conclusion is based on the findings that: 1) the onset of action potential firing in the majority of Hb9 INs lags behind ventral root bursts and 2) rhythmic activity triggered by stimulating the conus midualis of the cord is more variable in Hb9 INs than in the corresponding ipsilateral motoneurons. Several factors should be considered when discussing these interesting observations and their implication for our understanding of the possible role of Hb9 INs in spinal networks. Rhythmic activity recorded in L1–L3 ventral roots represents motor outputs from both medial and lateral motor columns and sympathetic preganglionic motoneurons in segment L1–L2 that might be active before rhythms are generated in Hb9 INs and somatic motoneurons. The lateral motoneurons innervate limb muscles and the medial motoneurons innervate primarily axial musculature and hip flexor muscles (Vanderhorst and Holstege 1997). It is conceivable that these populations are recruited at different times and Hb9 INs might provide excitatory drive to the group of motoneurons recruited at a later phase. Preliminary morphological observations suggest that Hb9 INs synapse onto the medial motoneurons (L. Ziskind-Conhaim, G. Z. Mentis, E. P. Wiesner, and D. J. Titus, unpublished data). Their role in driving motoneurons that innervate either axial muscle or different hindlimb muscles may be elucidated by simultaneous recordings from Hb9 INs and peripheral nerves that innervate various groups of axial and limb muscles.
The finding that stimulation of the conus midualis results in a more variable rhythmic activity in Hb9 INs than in the corresponding ipsilateral motoneurons might be related to its coexcitation of commissural and ipsilateral ascending/propriospinal pathways (Strauss and Lev-Tov 2003). Hb9 INs might receive their primary timing inputs from descending pathways and it remains to be determined whether the stimulation of the conus midualis activates the same CPGs that are controlled by descending/propriospinal networks (Cowley et al. 2008).
The role of interneuronal populations in motor circuits that underlie certain behaviors is partially determined by their synaptic inputs. Therefore the principal objective of this study was to examine whether low-threshold peripheral afferents synapse onto Hb9 INs and control the timing of their induced locomotor-like membrane oscillations in a pattern that remained phase-locked with motor activity.
A preliminary report of this study was previously published in abstract form (Hinckley and Ziskind-Conhaim 2005).
METHODS
Spinal cord preparation
Experiments were performed using the Hb9:eGFP transgenic mouse line (Wichterle et al. 2002), in which Hb9 INs express the reporter enhanced green fluorescent protein (eGFP). Newborn mice, 1 to 4 days old (P1–P4; birth is defined as P0) were anesthetized by hypothermia and decapitated; spinal cords were extracted in ice-cold oxygenated (95% O2-5% CO2) extracellular solution. The cord with dorsal and ventral roots attached was isolated and equilibrated at room temperature for 30 min. Following equilibration, the cord was transferred to a recording chamber, where it was continuously superfused with oxygenated extracellular solution at a rate of 2–5 ml/min at room temperature. Locomotor-like rhythms were triggered in the intact spinal cord using a “rhythmogenic cocktail,” a mixture of N-methyl- d-aspartic acid (NMA, 5 μM), 5-hydroxytryptamine creatinine sulfate complex (5-HT, 10 μM), and dopamine (50 μM) (Hinckley et al. 2005b). Once initiated, bouts of neurochemically induced locomotor activity continued for >4 h.
Whole cell and ventral root recordings
The procedures for simultaneous ventral root (VR) and whole cell recordings were similar to those described recently (Ziskind-Conhaim et al. 2008). Motor activity was recorded by drawing VRs into bipolar, tight-fitting suction pipettes. VR potentials were band-pass filtered between 300 Hz and 1 kHz and the output from the AC amplifier (DAM 50; World Precision Instruments) was acquired at a sampling rate of 2–3 kHz. The sampling rate was 10 kHz when VR and Hb9 IN potentials were recorded simultaneously. When quasi-DC recordings were performed the cutoff of the high-pass filter was reduced to 0.1 Hz. Electroneurograms of rhythmic motor activity alternating between left and right L2 VRs and ipsilateral L5 VRs confirmed that the induced rhythms were related to locomotor activity.
The spinal cord was then transected longitudinally along the midline and the hemicord was placed in a glass-bottom recording chamber with the medial side up, so that GFP-expressing Hb9 INs could be visually identified for targeted whole cell patch-clamp recordings. Hb9 INs are clustered in lamina VIII of predominantly flexor-related upper lumbar segments L1–L3 (Kiehn and Kjaerulff 1996). We used an epifluorescent microscope (BX50WI; Olympus) equipped with a 475 nm excitation filter, a 505 nm dichroic mirror, and a 535 nm peak emission filter (Omega Optics) to place the patch pipettes (tip resistances of 5–7 MΩ, P-97 multistage puller; Sutter Instrument) above the somata of Hb9 INs. The distinct spindle shape of the small Hb9 INs, their clustering in medial lamina VIII, and their visible primary ventral and dorsal dendrites facilitated their morphological identification (Hinckley et al. 2005a). The fluorescence was switched to infrared-differential interference contrast optics and videomicroscopy was used to obtain whole cell patch-clamp recording (Ziskind-Conhaim et al. 2003). Ectopic GFP expression in laminae VII–VIII interneurons required the use of electrophysiological criteria to unambiguously confirm the physiological identity of the recorded neurons as Hb9 INs (Ziskind-Conhaim et al. 2008). Intracellular potentials/currents were filtered at 3 kHz, sampled at 10–20 kHz (Multiclamp 700B amplifier; Molecular Devices), and recorded on a PC with Clampex software (v9.2). Interneurons were included in the study only if their resting membrane potential was more negative than −50 mV and action potential peak amplitude overshot zero. For voltage-clamp recordings whole cell capacitance transients were canceled on-line and monitored throughout the experiment. Series resistance was compensated to 60–80%. All recordings were corrected off-line for a liquid junction potential of 10 mV (Gao et al. 2001).
Dorsal root stimulation
One of the dorsal roots (DRs) in segments L1–L3 in the hemisected cord was drawn into tight-fitting suction electrodes and stimulated with tungsten electrodes. DR-evoked synaptic responses were recorded in Hb9 INs and their segmental VRs, except when L5 DR was stimulated. Threshold was determined by a single stimulus pulse at intensity that produced monosynaptic VR potentials in the majority of the trials. Primarily short-latency responses were generated at threshold. DR stimulus intensity was set to twofold threshold (2T) for monosynaptic reflex. The current for threshold ranged from 10 to 40 μA at 300 μs stimulus duration. We tested for current spread by placing the stimulating electrode at the same location but without drawing the dorsal root into the electrode. Currents intensities ≤800 μA did not evoke synaptic responses in either ventral roots or Hb9 INs.
In experiments examining the role of afferent axons in modulating locomotor activity, a train of four stimuli at 20 Hz was applied at approximately the midpoints of either the flexor (burst) or extensor (interburst) phases of the locomotor cycle. Each stimulus train was followed by a recovery period of 15 min to minimize the effect of synaptic depression in response to fast repetitive stimuli (Lev-Tov and Pinco 1992). Four to five trains were applied per burst and interburst phases during each recording in Hb9 IN. Prestimulus and second poststimulus cycle properties (Iizuka et al. 1997) were calculated from the average of four cycles. The average coefficient of variance for all prestimulus cycles was 0.27 and for the second poststimulus cycle, 0.29.
Tracing dorsal root projections in medial lamina VIII
Two complementary approaches were used to determine whether primary afferent terminals contacted Hb9 INs. In the first approach the fluorescent dye Texas Red dextran (Invitrogen) was used to trace L1 DR projections in the isolated spinal cords of P3 Hb9:eGFP mice (n = 3). Mice were initially deeply anesthetized (isoflurane 5% for 5 min) and decapitated; the spinal cord was isolated as described earlier. The cord was pinned down in a Sylgard base chamber and superfused with oxygenated extracellular solution (∼18°C). The dorsal root L1 was placed inside a suction electrode and backfilled with the Texas Red dextran (30–40 mM; MW 10,000). The labeling continued for about 24 h and the spinal cord was fixed in 4% paraformaldehyde (4 h) in 0.01 M phosphate-buffered saline (PBS; pH 7.4). The following day, the spinal cord was rinsed three times in 0.01 M PBS for 5 min each time. The cord was embedded in warm 5% agar and Vibratome sections (70–75 μm thick) were collected in multiwells (0.01 M PBS with 0.1% Triton X-100) and processed for immunohistochemistry using a “free-floating” method. The sections were initially exposed for 90 min to normal donkey serum (1:10; Millipore). Subsequently, they were incubated with the following primary antibodies: 1) the vesicular glutamate transporter 1 antibody (VGluT1; 1:1,000 dilution in PBS with 0.1% Triton, guinea pig polyclonal antibody; Synaptic Systems), 2) Hb9 antibody (gift from Dr. Thomas Jessell, 1:2,000 dilution in PBS with 0.1% Triton, rabbit polyclonal), 3) anti-GFP antibody (MAB3580, 1:500 dilution in PBS with 0.1% Triton, mouse monoclonal; Millipore) that amplified the endogenous GFP to better visualize the soma and the dendrites of the Hb9 INs. Following the primary antibody incubation, sections were washed six times in PBS-0.1%Triton (10 min each). Immunoreactive sites were revealed with donkey secondary antibodies (3 h; Jackson ImmunoResearch Laboratories) conjugated with: 1) Cy5 (donkey anti-guinea pig; 1:50) for VGluT1, 2) FITC (donkey anti-mouse; 1:50) for enhancing the endogenous GFP, and 3) AMCA (donkey anti-rabbit; 1:20) for the Hb9 epitope. At the end of the secondary antibody incubation, the sections were washed six times (10 min each time) and mounted on glass slides. Sections were covered with a PBS:glycerol (7:3) solution (antifading agent). Immunofluorescence was imaged with confocal microscopy (LSM510 Meta; Zeiss).
The GFP fluorochrome was excited with a 488 nm wavelength and detected with an internal photomultiplier tube, with an emission wavelength band of 505–550 nm. The fluorochrome AMCA was excited with a 405 nm wavelength and the emitted signal was acquired with an emission band filter of 420–480 nm. Texas Red dextran was excited with a 543 nm laser and acquired with an emission band filter of 560–615 nm. VGluT1 was visualized by using Cy5 as the fluorochrome for the secondary antibody, which was excited by a 633 nm laser and acquired with the long-pass emission filter LP650 nm.
Quantitative analysis of VGluT1 immunofluorescence in DR afferents was similar to that previously described (Mentis et al. 2006). Briefly, the analysis of VGluT1-immunoreactive (IR) boutons on the soma and the dendrites of Hb9 INs was performed in z-series of high magnification (×40) confocal optical images throughout the whole cell body and proximal dendrites of randomly sampled Hb9 INs. Two criteria were used to identify Hb9 INs: 1) their location in medial lamina VIII and 2) the coexpression of GFP and the Hb9 protein. Images were analyzed in LSM510 (Zeiss) software. For dendritic contacts, we performed only a linear density analysis (number of boutons per dendritic length).
In the second tracing approach Hb9 INs were filled with Neurobiotin (0.5–0.7%) during whole cell recordings. At the end of the recording period the patch electrode was withdrawn and the hemicords were perfused for an additional period of 15–30 min to allow Neurobiotin to better diffuse in the distal dendrites of Hb9 INs. The hemicord was fixed by immersion in 4% paraformaldehyde overnight and was then kept in PBS until sections were cut as described earlier. Neurobiotin was visualized using the ABC complex method. Rhodamine Avidin D (Vector Laboratories) was added (1:100 dilution in PBS and 0.1% Triton) at the same time as the primary antibodies VGluT1 (as described earlier) and parvalbumin (1:1,000 dilution in PBS with 0.1% Triton, rabbit polyclonal antibody; Swant). The latter is a protein that has been reported to be a marker for primary afferent fibers during early development (Alvarez et al. 2004). Analysis of VGluT1-IR boutons was performed as described earlier.
Data analysis
To facilitate the analysis of electroneurograms of VR rhythmic activity, extracellular recordings were rectified and smoothed using adjacent averaging over 400–800 points (Origin 6). These were used to calculate the cycle period, the time between the onsets of two consecutive VR bursts. The onset of each burst was assessed visually from baseline level. Histograms of normalized cycle periods were constructed by averaging four cycle periods before DR stimuli and the average was referred to as “prestimulus” cycle period. “Stimulus,” “1st poststimulus,” and “2nd poststimulus” cycle periods were compared and normalized to the “prestimulus” cycle period. Paired t-tests were used to determine the statistical significance (P < 0.05). All means are stated ±SE.
Circular statistics were used to analyze the phase relation between membrane voltage oscillations in Hb9 IN and VR bursts. The onset of membrane depolarization, rather than firing in Hb9 INs, was used for comparison because the onset of firing in Hb9 INs depends on several experimental factors including resting membrane potential and action potential threshold. Typically, at a membrane potential of −60 mV, action potential firing was initiated when voltage oscillations reached threshold potential of approximately −50 mV. Firing began without a delay compared with VR burst when membrane potentials were closer to threshold (see Hinckley et al. 2005; Fig. 5B). To obtain the phase, the onset of membrane depolarization was subtracted from the onset of VR burst and divided by the duration of cycle period. These values were plotted on a circle and the mean phase was represented with a vector. Values close to 0 indicated in-phase correlation, whereas 0.5 marked an out-of-phase correlation. The length of the vector is inversely proportional to the distribution of phase values around the mean. The significance of the mean is calculated from the length of the vector (r) using a Rayleigh test (Zar 1974), but critical values for this test are available only for sample size >6. The significance of the mean vector could not be calculated for stimulus modulated cycles in individual cells because only four to five DR train stimuli were given during flexor- and extensor-related phases in a given cell. The reason for that is that each stimulus was followed by a 15 min recovery period and smaller amplitude or failures of DR-evoked synaptic potentials/currents were apparent after four to five episodes of DR stimuli (in whole cell recordings lasting for >60 min).
Fig. 5.
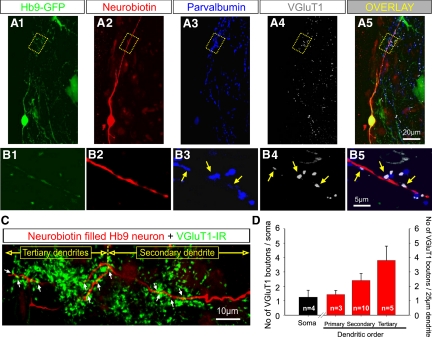
The majority of putative synaptic contacts between primary afferents and Hb9 INs were observed on distal dendrites. A1–A5: medium magnification images of z-stack projections in the transverse plane of a hemisected spinal cord in which an Hb9 IN was filled with Neurobiotin during whole cell recording. A1: GFP labeling was amplified by an anti-GFP antibody. A2: Neurobiotin-filled Hb9 IN visualized in red. A3: immunoreactivity against parvalbumin (in blue) to label primary afferents. A4: immunoreactivity using a VGluT1 antibody (in white), a specific marker for glutamatergic synaptic transmission. A5: an overlay image from A1 to A4 (scale bar shown in A5 applies to A1–A4). Note that the Neurobiotin-filled Hb9 IN was GFP+ (appearing yellow in the overlay, shown in A5). A large cluster of VGluT1+ boutons and parvalbumin immunoreactivity was apparent in close apposition to a distal dendrite (∼100 μm from the soma) (highlighted in the dotted box in A). The optical thickness of individual single scans was 0.58 μm. The total z-stack projection was 10.97 μm. The series of images B1–B5 are single optical scans of all the fluorochromes (B5 is the overlay) from the dotted box shown in the A1–A5 series. There are 3 contacts (arrows) of colocalized parvalbumin and VGluT1 immunoreactivity on distal dendrite of the Hb9 IN, indicating that these were putative synaptic boutons of primary afferents. C: a z-stack confocal image projection showing a 2nd order and 2 tertiary dendrites from the Hb9 IN that was previously recorded and subsequently filled with Neurobiotin (shown in red). VGluT1-IR synaptic boutons are shown in green. Putative synaptic appositions are marked by arrows. D: graphs showing the synaptic coverage on somata (black bar) and primary, secondary, and tertiary dendrites (red bars) from the 4 Hb9 INs. The number of putative VGluT1+ synaptic boutons on distal dendrites was threefold larger than that on primary dendrites (n is the number of dendrites analyzed).
Solutions and chemicals
Extracellular solution contained (in mM): NaCl 128, KCl 4, CaCl2 1.5, MgSO4 1, NaH2PO4 0.5, NaHCO3 21, and glucose 30. The solution was adjusted to pH 7.3 using NaOH and the osmolarity was 315–325 mOsm. Whole cell pipette solution contained (in mM): K gluconate 140, KCl 9, N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) 10, ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) 0.2, MgCl 1, Na-ATP 2, and Na-GTP 0.4. The solution was adjusted to pH 7.2 using KOH and the osmolarity was 290–305 mOsm. All chemicals were obtained from Sigma.
RESULTS
Properties of synaptic connections between dorsal root afferents and Hb9 INs
Whole cell patch-clamp recordings were carried out in flexor-related upper lumbar segments (L1–L3), where Hb9 INs could be readily visualized in longitudinally hemisected spinal cords of the Hb9:eGFP mouse line (Hinckley et al. 2005a). Specific electrophysiological criteria have been formulated to distinguish Hb9 INs from other GFP-expressing neurons in medial lamina VIII (Han et al. 2007; Hinckley et al. 2006; Willson et al. 2005; Ziskind-Conhaim et al. 2008). These include 1) high-input resistance (∼1–1.5 GΩ), 2) linear current–voltage (I–V) relationship without hyperpolarization-activated depolarization sags at potentials more negative than resting membrane potentials, 3) rebound action potentials, 4) spike frequency adaptation, and 5) lack of tonic firing that characterizes adjacent interneurons.
To examine whether DR projections synapse directly onto Hb9 INs, a brief stimulus (300 μs) was applied to measure the latencies of DR-evoked responses in Hb9 INs. Afferent volleys were not recorded because of the orientation of the hemicord in the recording chamber. To visualize Hb9 INs the hemicord was placed in the recording chamber with the medial side up, making it difficult to blindly place the extracellular recording electrode at the lateral site of DR entry to the cord. It has been suggested that low-intensity DR stimulation (up to twofold the time threshold [2T]) recruits primarily group I muscle afferents (Mentis et al. 2006; Shay et al. 2005), but we cannot rule out the possibility that group II and cutaneous afferents were activated as well. Spinal motoneurons of neonatal mice receive monosynaptic inputs from low-threshold afferents (e.g., Kudo and Yamada 1987; Pinco and Lev-Tov 1993; Seebach and Ziskind-Conhaim 1994; reviewed by Vinay et al. 2000); therefore we determined the current intensity that produced short-latency (<10 ms) VR potentials in the majority of the trials. Stimulus intensity was then set to 2T, <80 μA at 300 μs duration. At these intensities, the average latency of VR potentials in L1–L3 lumbar segments was 5.2 ± 0.6 ms (n = 11), ranging from 3.6 to 8.8 ms. VR responses consisted of a small, short-duration potential followed by a significantly larger and longer-lasting potential. This initial potential might represent the activity in a small subpopulation of motoneurons with faster conduction velocity or different intrinsic properties than those of the majority of segmental motoneurons. The latency was measured from the stimulus artifact to the onset of the small component (Fig. 1A2, arrowhead). VR latencies were not altered by increasing the stimulus intensities to three- and fourfold threshold (3T and 4T, respectively). Similar latencies were reported for monosynaptic responses in motoneurons of P2–P4 mice (e.g., 6.5 ± 0.8 ms; Mentis et al. 2006). Such low-intensity DR stimuli produced short-latency compound postsynaptic potentials/currents in 62% of Hb9 INs (n = 13/21). The average latency was 6.1 ± 0.3 ms, ranging from 5.0 to 7.6 ms (Fig. 1). This was similar to the latencies of the large VR responses, but it was delayed by about 1 ms compared with the onset of the initial small VR potential. This indicated that the latency of synaptic responses in Hb9 INs was similar to that generated in the majority of segmental motoneurons.
Fig. 1.
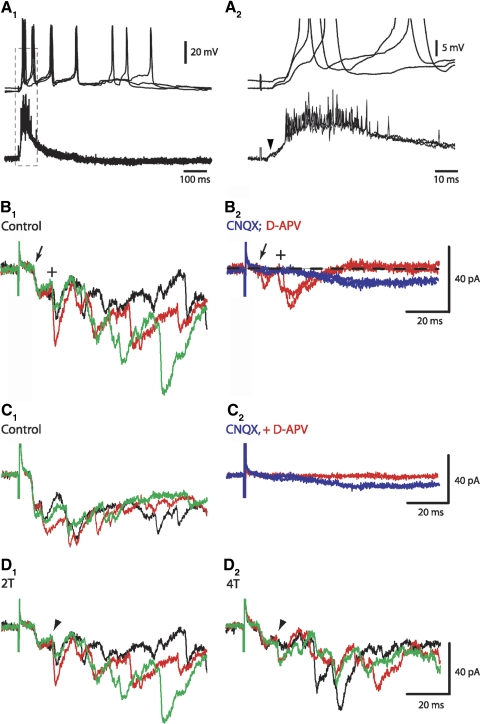
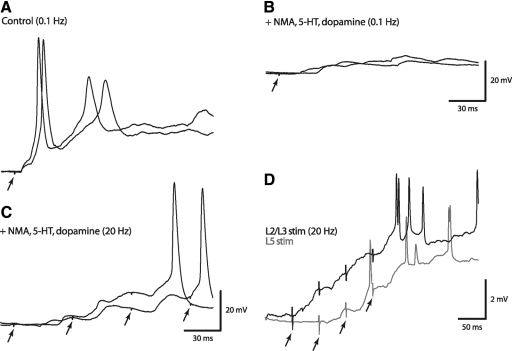
Low-intensity stimulation of segmental afferents generated short-latency excitatory postsynaptic potentials/currents (EPSPs/EPSCs) in homeobox gene Hb9-expressing interneurons (Hb9 INs). Unless otherwise stated, evoked responses were generated by stimulating dorsal roots at the same segment as Hb9 INs and recordings were carried out at postnatal day 2 (P2) to P3. A1: L2 dorsal root (DR) stimulation (twofold the threshold [2T] at 300 μs) produced short-latency excitatory potentials in a L2 Hb9 IN (top trace) and in segmental motoneurons (bottom trace). Ventral root (VR) potentials were recorded using a high-pass filter of 0.1 Hz (quasi-DC recording). A small VR potential (arrowhead) was recorded before the onset of the large potential. Superimposed are traces of 3 successive potentials generated at 0.1 Hz stimuli. DR-evoked membrane depolarizations and firing in Hb9 INs lasted significantly longer than the excitation of motoneuron population. A2: expanded timescale of traces shown in the boxed portion in A1. Resting membrane potential: −55 to −60 mV. B1: in a different Hb9 IN, L2 DR stimuli at 0.1 Hz produced compound EPSCs with stable short-latency (6.2 ms, arrow) and long-latency (14.6 ms, +) dual-component EPSCs (control). Superimposed are 3 successive evoked synaptic events in the order of black, red, and green traces. B2: exposure to CNQX (10 μM, blue trace) blocked the dual-component EPSCs, suggesting that they were mediated via non-NMDA receptors. A small CNQX-resistant component (∼9 pA) was recorded after a long latency (>20 ms). EPSCs were partially recovered within 20 min of CNQX removal (not shown). Exposure to d-APV (20 μM, red trace) did not suppress the dual-component EPSCs but it completely blocked the longer-latency EPSCs. Unless otherwise stated, voltage-clamp recordings were carried out at a holding potential of −60 mV. Baseline is marked by the dashed line. C1: dual-component and polysynaptic EPSCs in L2 Hb9 IN. C2: exposure to CNQX suppressed the dual-component EPSCs and the CNQX-resistant component was suppressed by adding d-APV to the CNQX-containing solution. D1: evoked EPSCs in the same L2 Hb9 IN shown in B. D2: higher-intensity stimuli (4T) did not change significantly the amplitude of the short-latency component but it reduced the amplitude of the 2nd component EPSC (arrowhead).
Voltage-clamp recordings revealed that the initial, short-latency excitatory postsynaptic currents (EPSCs) were always associated with a second inward current component (see Fig. 1B1, marked by +) with an average latency of 12.8 ± 0.6 ms (ranging from 9.7 to 16.4 ms). To determine whether the short- and longer-latency EPSCs (referred to as “dual-component” EPSCs) were mediated via glutamatergic transmission, we examined the effects of N-methyl-d-aspartate (NMDA) and non-NMDA receptor antagonists on these currents. The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM), suppressed the dual-component EPSC and most of the longer-latency (>20 ms) EPSCs (Fig. 1B2). A small, long-latency, CNQX-resistant component (5–12 pA) was blocked by d-2-amino-5-phosphonovaleric acid (d-APV, 20 μM), an NMDA receptor antagonist (Fig. 1, B and C). d-APV did not suppress the dual-component EPSCs (Fig. 1B2). These findings suggested that low-threshold afferents generated short-latency non-NMDA receptor-mediated EPSCs and a small fraction of NMDA receptor-activated polysynaptic EPSCs.
In the cat spinal cord, nerve stimuli at intensities >2T excite group II afferents (e.g., Perreault et al. 1995). It is conceivable that in our study, a fraction of higher-threshold afferents were activated at 2T to produce the EPSCs with average latencies of about 13 ms (marked by + in Fig. 1B and arrowheads in Fig. 1D). However, increasing the stimulus intensity to 4T did not increase the amplitude of the second component EPSCs. This observation does not rule out the possibility that group II contributed to the dual-component EPSCs because thresholds and conduction velocities of specific groups of afferents are not clearly defined in neonatal rodent, partly because of the ongoing process of myelination at that age; thus it is difficult to determine the role of specific afferents in generating the short- and long-latency EPSCs and we refer to axons stimulated at 2T as low-threshold afferents without classifying their group identity.
Our finding that the onset of short-latency postsynaptic potentials/currents in Hb9 INs was similar to that of presumed monosynaptic reflexes recorded simultaneously in segmental VRs suggested that these were generated by low-threshold afferents that synapsed directly onto Hb9 INs. One of the conventional criteria for monosynaptic responses is the stability of their onset at high-frequency stimulation. Indeed, the latency of short-latency EPSCs remained stable at frequencies of ≤5 Hz (Fig. 2A, n = 6). Higher stimulus frequencies were not examined on a regular basis because of the increased rate of EPSC failures at frequencies >5 Hz in immature neurons (also see Lev-Tov and Pinco 1992; Seebach and Ziskind-Conhaim 1994). The onset of the second component EPSCs was less consistent at 5 Hz, with variability of ≤0.3 ms between consecutive EPSCs (5 Hz, Fig. 2A). It is possible that those were also monosynaptic EPSCs generated by dorsal root afferents that were not yet myelinated in the neonatal mice (Ziskind-Conhaim 1988). The excitability of the slow conducting unmyelinated axons might vary at high-frequency stimuli. An alternative explanation is that the longer-latency EPSCs were produced by disynaptic inputs.
Fig. 2.
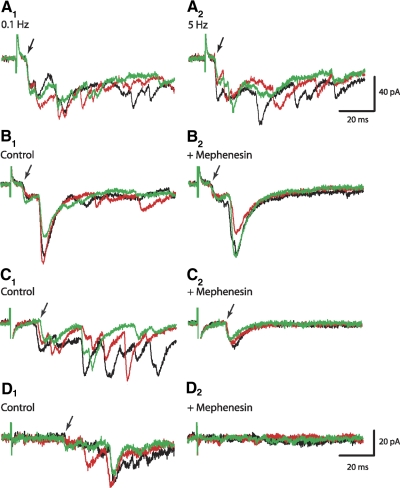
The onset of the dual-component EPSCs was stable at high-frequency DR stimulation and in the presence of mephenesin. Superimposed are 3 successive EPSCs in the order of black, red, and green traces. A1: dual-component EPSCs with latencies of 6 and 12 ms were generated by L2 DR stimuli at 0.1 Hz. A2: the onset of EPSCs did not change at higher-frequency stimulation (5 Hz). In this and all other panels, arrows point to the first evoked EPSCs. B1: DR-evoked EPSCs in L3 Hb9 IN. B2: exposure to mephenesin (1 mM) did not alter the onset of the dual-component EPSCs, but blocked all EPSCs with latencies >20 ms. C1: L1 DR stimuli at 0.1 Hz generated EPSC with latencies of 14.7 ± 1.2 ms. C2: EPSC onset became more consistent in the presence of mephenesin that blocked EPSCs with latencies >20 ms. D1: extensor-related, L5 DR stimulation evoked longer-latency EPSCs (33.5 ms) in L1 Hb9 IN. D2: L5 DR-evoked EPSCs were suppressed during exposure to mephenesin.
A second test that distinguishes mono- from polysynaptic connections is the effect of mephenesin, a barbiturate known to attenuate poly- but not monosynaptic transmission (Lev-Tov and Pinco 1992; Longo 1961; Ziskind-Conhaim 1990). Exposure to mephenesin (1 mM, >20 min) did not alter the onset of either the short- or long-latency dual-component EPSCs (Fig. 2B), but it blocked polysynaptic EPSCs with latencies >20 ms. This finding fits the hypothesis that the short-latency EPSCs were produced by fast conducting afferents, whereas the longer-latency excitatory postsynaptic potentials (EPSPs, <20 ms) were generated by slow conducting projections with monosynaptic inputs onto Hb9 INs. It is also conceivable that the longer-latency EPSPCs were evoked by disynaptic inputs that were not suppressed by mephenesin in neonatal spinal networks.
In 38% of Hb9 INs (n = 8/21), the average latency of the initial EPSCs was 13.8 ± 0.8 ms (ranging from 11.3 to 17.1 ms; Fig. 2C), not significantly different from the average of 12.8 ms calculated for the second of the dual-component EPSCs. In two of the interneurons, dual-component EPSCs with shorter-latency EPSCs were recorded initially but the shorter-latency component was not apparent after the first set of four stimuli at 0.1 Hz. This might be indicative of weak synaptic transmission between low-threshold afferents and Hb9 INs. Similar to the dual-component EPSCs, the long-latency EPSCs were not suppressed by mephenesin (Fig. 2C, n = 4) and their latency was relatively constant at high-frequency stimulation (n = 3, not shown). Our finding that mephenesin reversibly blocked EPSCs with latency >20 ms that were generated by excitation of extensor-related dorsal roots (L5, Fig. 2D) supported the assumption that the barbiturate suppressed polysynaptic responses in Hb9 INs. Based on these findings, the longer-latency EPSCs recorded in 38% of Hb9 INs were produced either by slow conducting unmyelinated afferent projections that synapsed onto Hb9 INs or by disynaptic inputs that were not blocked by mephenesin (see discussion).
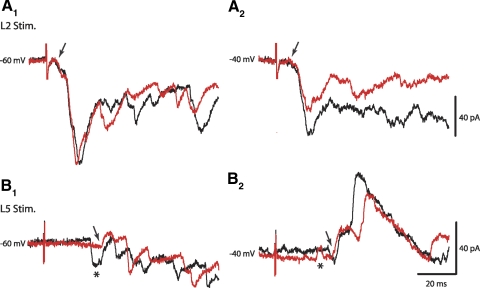
To determine whether inhibitory interneurons were part of the network activated by low-threshold, flexor-related afferents, voltage-clamp recordings were performed at various holding potentials to separate the inward inhibitory postsynaptic currents (IPSCs) from EPSCs. Spontaneous outward currents could be recorded at potentials more positive than −55 mV and those were blocked by a mixture of picrotoxin (15 μM) and strychnine (0.5 μM), γ-aminobutyric acid type A (GABAA), and glycine receptor antagonists, respectively. This observation suggested that the reversal potential for IPSCs is at potentials more positive than −55 mV. At a holding potential of −40 mV, the amplitude of dual-component inward currents generated by low-threshold L1–L3 afferents was reduced as expected for glutamatergic EPSCs (n = 4), but outward currents were not apparent at that potential (Fig. 3A). The amplitude of inward currents with latencies >20 ms slightly increased at this potential, possibly because of the activation of NMDA-mediated currents at depolarizing potentials (Fig. 2, B and C). Stimulation of extensor-related L5 afferents produced long-latency primarily inward currents at a holding potential of −60 mV (Figs. 2D and 3B). However, predominantly outward currents were recorded at −40 mV (Fig. 3B2). These observations suggested that at low-intensity stimulation, flexor-related L1–L3 afferents produced only mono- and polysynaptic EPSCs, whereas extensor-related L5 afferents generated polysynaptic EPSCs and IPSCs in Hb9 INs.
Fig. 3.
Low-threshold flexor-related afferents did not generate inhibitory postsynaptic currents in Hb9 INs. A1: low-threshold L2 afferents evoked dual-component and longer-latency inward currents. A2: the amplitude of dual-component EPSCs was reduced at a holding potential of −40 mV, but outward currents were not apparent during the first 100 ms. Black and gray traces are 2 consecutive recordings at 0.1 Hz stimuli. B1: low-threshold, extensor-related afferents (L5) produced longer-latency outward and inward currents. At holding potentials of −60 and −40 mV, the first outward currents were evoked after 29.7 ms (arrows). B2: outward currents were dominant at a holding potential of −40 mV. Asterisks mark possible spontaneous inward and outward currents. The latencies of the first inward current in B1 and the outward current in B2 were 23.4 and 20.5 ms, respectively.
Morphological and immunohistochemical identification of primary afferent contacts on Hb9 INs
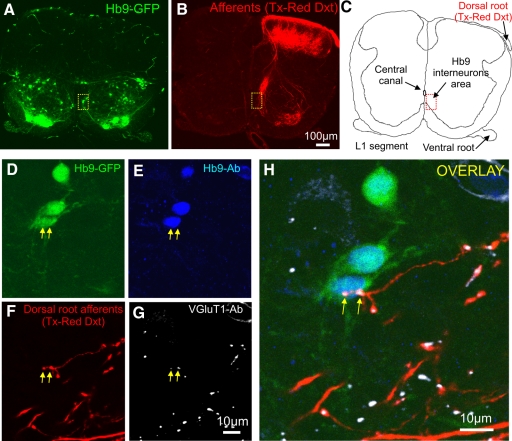
Morphological and immunohistochemical experiments were performed to provide additional evidence that primary afferent projections synapse onto Hb9 INs. In the first set of experiments, dorsal root axons were labeled orthogradely with Texas Red dextran and processed immunohistochemically with anti-VGluT1 antibody, a marker for primary afferent projections in the neonatal spinal cord (Alvarez et al. 2004; Oliveira et al. 2003). We then examined whether Texas Red dextran–filled axon terminals with immunoreactivity for VGluT1 were in close apposition to somata and dendrites of Hb9 INs (Fig. 4). L1 dorsal roots were labeled at P3 (n = 3) and a total of 39 Hb9 INs were scanned and analyzed (12 INs in the first spinal cord, 14 INs in the second, and 13 INs in the third spinal cord). There were no significant differences in the number of putative contacts from primary afferents on Hb9 INs across the three spinal cords examined. On average, 25.9% of Hb9 INs received putative synaptic contacts on the soma, 36.3% had contacts on proximal dendrites, whereas 45.7% had no contacts. Boutons were distributed at an average distance of 17.6, 17.9, and 27.6 μm from the center of the cell bodies of Hb9 INs (average 21.1 μm, n = 3 spinal cords), implying that putative synaptic contacts were predominantly on primary dendrites. It should be emphasized that this morphological approach underestimated the number of boutons on dendrites because the inability to clearly visualize GFP in secondary and tertiary order dendrites restricted the identification of VGluT1-expressing boutons to proximal dendrites.
Fig. 4.
Putative synaptic contacts between primary afferent projections and the somata of Hb9 IN in a transverse section of upper lumbar segment (L1). A: distribution of green fluorescent protein (GFP)–expressing neurons in the ventral horn. Anti-GFP antibody was used to amplify the GFP signal. The dotted box marks the location of Hb9 INs. B: in vitro labeling of L1 dorsal root projections by orthograde diffusion of Texas Red dextran (Tx-Red Dxt). Dense TX-Red Dxt–filled afferents projected laterally and ventrally where they surrounded motoneuron somata. Profuse afferents also projected medially, parallel to the midline. C: drawing of the transverse slice with the marked area of the location of Hb9 INs relative to the central canal. D–H: higher magnifications of 3 Hb9 INs shown in the dotted box in A. The images are short z-stack projections of the 4 different fluorochromes (D: GFP; E: Hb9 Ab; F: primary afferent fibers labeled with Tx-Red Dxt; G: VGluT1 Ab). H: merged image of D, E, F, and G. Immunoreactive boutons (arrows) with colocalization of Texas Red dextran (F, in red) and VGluT1 (G, in white) are in close apposition to the soma of one of the Hb9 INs. The single optical section thickness was 0.6 μm; the z-stack projection was constructed by superimposing 4 single optically scanned sections (total z-axis distance: 2.4 μm). The scale bar (G) is the same for D, E, and F.
A second morphological approach was used to determine whether primary afferents made putative synaptic contacts on distal dendrites of Neurobiotin-filled Hb9 INs. Hb9 INs were filled during whole cell recordings (Fig. 5, n = 7). Transverse sections of hemicords were processed immunohistochemically for VGluT1 and parvalbumin expression as markers for primary afferent boutons (Alvarez et al. 2004). Only four of the seven interneurons were recovered and adequately filled with Neurobiotin for quantitative morphological analysis. Whole cell recordings were stable enough in only two Hb9 INs and in those neurons DR stimulation produced short-latency EPSCs. The maximum transverse area of Hb9 INs was 121 ± 19 μm2 (n = 4 INs, range: 85–171 μm2). Hb9 INs possessed two primary dendrites, one projecting dorsally and the second one projecting ventrally. One of the Hb9 INs had two ventrally projecting primary dendrites. The average length of the primary dendrites was 20.2 ± 5.2 μm (range: 4.5–52.5 μm, n = 9). In all four Hb9 INs, the dorsally projecting primary dendrites bifurcated into secondary dendrites. In one of the Hb9 INs, a ventrally projecting dendrite also bifurcated into secondary dendrites. However, in the remaining three Hb9 INs, ventrally projecting dendrites could not be followed for distances >21.6 μm, possibly because they were cut during the sectioning. For the second order dendrites that could be clearly identified and traced, the average length was 79.6 ± 19.5 μm (range: 21.8 ± 249.8 μm, n = 10). In 3/8 secondary dendrites, a third order (tertiary) dendrite was observed. The average length of the tertiary dendrites was 39.0 ± 6.2 μm (range: 15.5–50.7 μm, n = 5). The majority of the secondary and tertiary dendrites (14/15) revealed in our method projected dorsally.
The number of VGluT1-expressing putative synaptic boutons was analyzed on the soma and dendrites of the 4 Hb9 INs. Boutons in close apposition to somata were observed in 3/4 Hb9 INs. The average number of boutons/soma was 1.25 ± 0.50 (Fig. 5, C and D). The linear dendritic VGluT1-expressing synaptic coverage (i.e., the number of VGluT1-expressing boutons per 25 μm of dendritic length) revealed that 3/9 primary dendrites received an average of 1.43 ± 0.29 boutons (6 primary dendrites had no synaptic contacts). Ten of the 11 secondary dendrites (90.9%) received on average 2.41 ± 0.48 boutons and all (5/5) tertiary dendrites received boutons with an average of 3.8 ± 0.99 boutons/25 μm of dendritic length (Fig. 5, C and D). It is interesting that most of the putative synaptic contacts were on distal dendrites that extended to an area with profusely distributed afferent projections (Fig. 4B).
Afferent modulation of induced locomotor-like membrane oscillations in Hb9 INs
Previous studies have reported that low-threshold afferents can reset the pattern of neurochemically induced locomotor-like activity in neonatal rats (Iizuka et al. 1997; Kiehn et al. 1992). One of the primary goals of this study was to determine whether similar low-intensity stimuli of flexor- and extensor-related dorsal roots can alter the timing of neurochemically induced locomotor-like rhythms in Hb9 INs while maintaining their in-phase relation with bouts of motor activity. As in our previous studies, coordinated rhythmic activity was triggered by a mixture of NMA (5 μM), 5-HT (10 μM), and dopamine (50 μM) (e.g., Hinckley et al. 2005b; Ziskind-Conhaim et al. 2008). Exposure to 5-HT significantly reduced the amplitude of DR-evoked monosynaptic potentials and their latency became irregular in immature motoneurons (Ziskind-Conhaim et al. 1993). A similar effect was reported for potentials generated by group II afferents in the cat intermediate zone interneurons (Jankowska et al. 2000). In addition to 5-HT, dopamine attenuates both short- and long-latency reflex responses in lumbar motoneurons in the isolated cord (Clemens and Hochman 2004). Presynaptic depression of Ia excitation is well documented during fictive locomotion in the cat (Gosgnach et al. 2000; Rossignol et al. 2006). Therefore we examined the effects of the rhythmogenic cocktail on the properties of afferent-evoked EPSCs/EPSPs. The average amplitude of afferent-evoked excitatory postsynaptic potentials (EPSPs) was reduced by 54% (ranging from 33 to 75%) and their latency was significantly prolonged to >20 ms in the presence of the cocktail (Fig. 6B, n = 4). Removal of the rhythmogenic cocktail reversed these effects (not shown). The cocktail did not alter the input resistance of Hb9 INs, which was 1.38 ± 0.07 GΩ (n = 16) before adding the cocktail and 1.29 ± 0.01 GΩ in its presence. Similar to previous reports, our observations imply that the mixture of neurotransmitter agonists triggered presynaptic inhibitory mechanisms that suppressed monosynaptic responses.
Fig. 6.
The rhythmogenic cocktail significantly reduced the amplitude and increased the latency of DR-evoked EPSPs in L2/L3 Hb9 INs. A: low-intensity L2 dorsal root stimuli (2T, 0.1 Hz) produced short-latency (5.2 ms) stable EPSPs that reached action potential threshold. The traces shown in all panels are sequential responses. Resting membrane potential: −53 to −55 mV. B: exposure to the rhythmogenic cocktail significantly reduced the amplitude and increased the latency of evoked EPSPs in the same neurons shown in A. This example demonstrates the most significant effects of the cocktail recorded in our experiments. Membrane depolarization of 3–5 mV (membrane potential was approximately −50 mV) was recorded in the presence of the cocktail. Location of traces in this panel does not reflect the actual membrane potential. C: in a different Hb9 IN, in the presence of the mixture of neurotransmitter agonists, action potentials were generated only after the summation of EPSCs during L3 dorsal root stimulation at high frequency (20 Hz, arrows). D: flexor- (L2/L3) and extensor- (L5) related DR stimuli during neurochemically induced rhythmic activity in Hb9 INs. The traces are the averaged responses of all Hb9 INs (n = 13) to a train of afferent stimuli (arrows, 4 stimuli at 20 Hz) during the interburst phase of the cycle period. L2/L3 stimuli (black, n = 6 neurons) generated shorter-latency EPSPs than those produced by L5 stimuli (gray, n = 7 neurons). Membrane potentials varied from −50 to −60 mV.
Characteristically, single stimuli of low-threshold afferents triggered action potentials in Hb9 INs (Figs. 1A and 6A), but similar stimuli failed to produce action potentials in the presence of neurotransmitter agonists (Fig. 6B). This suggested that during chemically induced locomotion, synaptic potentials generated by a single stimulation of low-threshold afferents could not contribute to suprathreshold excitation of Hb9 INs. A short train of repetitive stimuli (four stimuli at 20 Hz) at approximately 2T was required to generate action potentials during exposure to the rhythmogenic cocktail (Fig. 6C) and during neurochemically induced locomotor-like rhythms (Fig. 6D). Therefore a train of four low-intensity stimuli at 20 Hz, rather than that of a single stimulus, was used to determine the contribution of afferent inputs to the modulation of locomotor activity in Hb9 INs and motoneurons. Experiments were performed at P3–P4 to reduce development-related variability in afferent control of the locomotor pattern (Iizuka et al. 1997). The average cycle period of neurochemically induced locomotor-like rhythms recorded in these experiments was 6.0 ± 0.2 s (SD, n = 22), with an average burst duration of 2.3 s and interburst phase of 3.7 s. The frequency of induced locomotor-like voltage oscillations increased nearly twofold during exposure to a higher concentration of NMDA (20 μM; Ziskind-Conhaim et al. 2008). The experiments described in the following text were conducted using only a low concentration of NMA (5 μM).
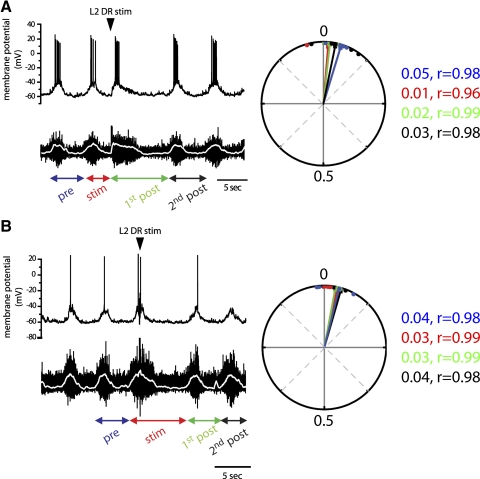
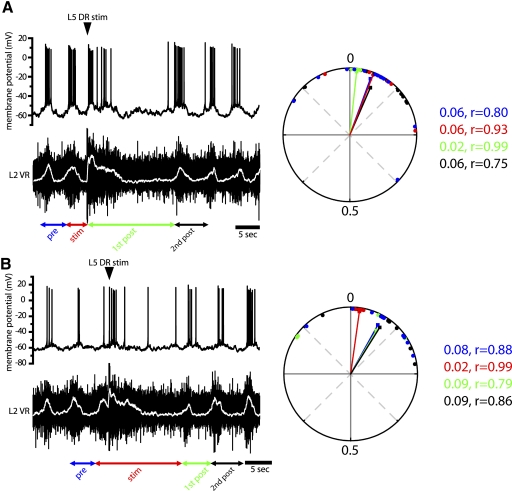
Stimulating L2/L3 low-threshold afferents (2T) during induced locomotor-like activity perturbed the cycle period in both Hb9 INs and flexor-related motoneurons, but the altered patterns remained phase-locked between the two neuronal populations (Fig. 7). The nature of afferent modulation differed depending on the timing of stimuli during the locomotor cycle. When afferent excitation occurred during the extensor phase (L2 interburst) it terminated the interburst phase and triggered an early burst in Hb9 INs and L2 VRs. This shortened the cycle period to roughly 60% of the control prestimulus cycle (0.6 ± 0.02 normalized value, n = 6) (Figs. 7A and 10). The shortening of the interburst phase was compensated by prolonging the subsequent cycle period (first poststimulus) to 160% of the prestimulus cycle (1.6 ± 0.1, normalized period). Therefore overall afferent inputs did not alter the timing of the onset of subsequent cycles (Fig. 10, top left). The longer first poststimulus cycle resulted from an increase in both burst and interburst durations so that the duty cycle, the fraction of the bursting phase during the cycle period, did not change in both the Hb9 INs and motoneurons. The periods of the second poststimulus cycle and all subsequent cycles were similar to prestimulus duration. Circular statistics demonstrated a strong in-phase relation between subthreshold membrane oscillations in Hb9 INs and motoneuron bursts during the stimulus and poststimulus cycles.
Fig. 7.
Low-intensity stimuli of upper lumbar afferents reset locomotor-like activity in Hb9 INs and segmental ventral roots only when applied during the flexor-related phase. A: stimulating L2 afferents during the extensor phase (arrowhead) triggered an early burst in both the Hb9 IN (top trace) and motoneurons as measured by electroneurograms of ventral root potentials (bottom trace). The shorter cycle period (red arrows, labeled as “stim”) was compensated by a longer subsequent cycle (green arrows, labeled as “1st post”). Therefore there was no shift in the timing of the 2nd poststimulus cycle. The periods of the 2nd poststimulus cycle and all subsequent cycles returned to prestimulus duration. Both subthreshold membrane oscillations and firing remained in phase with the ventral root bursts. Arrows denote the cycle periods in relation to the stimulus. Rectified and smoothed electroneurograms (white traces) were used to determine burst onset and cycle period. Right: circular plot of the phase relation between the Hb9 IN and VR shown on the left. Vectors with similar angles are indicative of the maintained phase relation between the 2 neuronal populations. Vectors are color coded according to the arrows below the traces. Points represent individual phase values. Average phase values varied from 0.01 to 0.05 and their distribution around the mean was small, as indicated by the vector lengths (r) that were close to 1 throughout the perturbations. The significance of the mean vector could not be calculated for stimulated and 1st post stimulated cycles for individual cells because of the small number of stimuli in each cell. B: stimulating L2 afferents during the flexor phase (arrowhead) prolonged the ongoing burst and the subsequent interburst quiescent phase (stim), resulting in a delay in the onset of the 1st poststimulus cycle. The periods of the 1st poststimulus cycle and all subsequent cycles returned to prestimulus duration. Only the burst duration is shown in the 2nd poststimulus cycle.
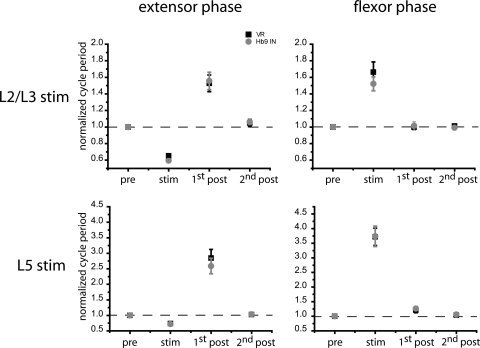
Fig. 10.
Summary histograms of perturbed cycle periods and their phase correlation with segmental motoneurons. Similar afferent modulation of cycle periods were evident in recordings from Hb9 INs (gray circles) and ventral roots (black squares). L5 stimulation resulted in significantly longer perturbations of the locomotor cycle. Note the different scales of y-axes for L2/L3 and L5 stimulation. Cycle periods are normalized to average values of prestimulus cycle periods. Sample sizes: L2/L3 afferent stimulation during the extensor phase, n = 6 neurons and the flexor phase, n = 5. L5 stimuli during the extensor/flexor phases, n = 7 cells.
In contrast, afferent stimuli during the flexor phase (L2 burst) prolonged the burst and interburst durations in both Hb9 INs and segmental motoneurons while maintaining the phase relation between them. The 150% increase in the cycle period (1.5 ± 0.1 of normalized value; Figs. 7B and 10, n = 5) delayed the onset of the first poststimulus cycle and all subsequent cycles. In all experiments, the periods of the first poststimulus cycle and all subsequent cycles were similar to prestimulus duration.
Similar to the stimulation of flexor-related afferents, a brief stimulus train (four stimuli at 20 Hz) of extensor-related DR afferents (segment L5) resulted in modulation of Hb9 IN cycle timing that remained in phase with motor activity. Changes in locomotor patterns were qualitatively similar to those triggered by L2/L3 afferents. However, L5 afferents generated significantly longer lasting perturbations of locomotor patterns than the excitation of upper lumbar sensorimotor pathways. When stimulated during the extensor phase, L5 afferents evoked an early burst in both Hb9 INs and flexor-related motoneurons, shortening the cycle period to 70% of prestimulus cycle period (0.7 ± 0.4 of normalized value, n = 7) (Figs. 8A and 10). This was similar to the 60% reduction in cycle period produced by L2/L3 afferents, but the first poststimulus cycle period increased by an average of 260% (normalized value: 2.6 ± 0.2), significantly longer than the average increase of 160% observed during activation of flexor-related afferents (Fig. 7A). The two- to threefold prolongation of the first poststimulus cycle resulted from an increase in the durations of both burst and interburst phases (Fig. 9) so that the duty cycles of Hb9 INs and segmental ventral roots were similar to control values (0.38 prestimulus and 0.39 first poststimulus). L5 afferent stimuli during the flexor phase prolonged the ongoing burst in both Hb9 INs and motoneurons to an average cycle period of 374% of control value (3.74 ± 0.35 of normalized value) (Figs. 8B and 10), significantly longer than the perturbed cycle period following L2/L3 stimulation. Cycle periods returned to prestimulus duration after the prolonged perturbation.
Fig. 8.
Stimulation of L5 lumbar afferents prolonged the cycle period in Hb9 INs and segmental motoneurons. A: stimulating L5 afferents during the extensor phase (arrowhead) terminated the interburst phase and triggered an early burst in both the Hb9 IN (top trace) and motoneurons. This shortened the cycle period (red arrows, stim) and prolonged the subsequent cycle (green arrows, 1st post). Cycle periods then returned to prestimulus values (2nd post). Both membrane voltage oscillations and firing remained in phase with the ventral root bursts. Arrows denote the cycle periods in relation to the stimulus. Right: circular plot of the phase relation between the Hb9 IN and VR shown on the left. Vectors with similar angles are indicative of the maintained phase relation of activities in Hb9 INs and motoneurons. Average phase values varied from 0.02 to 0.07 and their distribution around the mean (as measured by the vector lengths) was slightly larger than that calculated for L2 stimulation. DR stimuli increased the synchronization of rhythmic activity during the 1st poststimulus burst (green vector). B: stimulating L5 afferents during the flexor phase (arrowhead) prolonged the ongoing burst and the subsequent interburst phase (stim). Right: similar vector angles in the circular plots are indicative of the in-phase relation between Hb9 INs and motoneurons during modulated cycles. Prolongation of the stimulated cycle shifted the phase relation closer to zero (red vector).
Fig. 9.
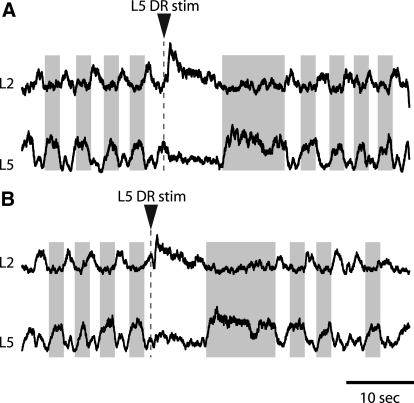
Neurochemically induced alternating pattern of L2–L5 motor bursts was maintained during L5 dorsal root stimuli in P2–P3 hemisected cords. Note that a small fraction of L5 VR burst was synchronous with L2 VR bursts and some L2 VR bursts were in phase with L5 bursts. This might reflect the mix of a small percentage of flexor-related motoneurons in L5 segment and extensor-related motoneurons in L2 segment (McHanwell and Biscoe 1981). A: L5 stimuli during L2 extensor phase (interburst) terminated the burst phase in L5 VR and in coordinated pattern triggered an early burst in L2 VR. During the subsequent prolonged L2 interburst phase, a long-lasting burst was recorded in L5 VR. B: DR stimuli during L2 flexor phase prolonged L5 interburst period and after a transient suppression (<400 ms) it prolonged the ongoing flexor burst. During the subsequent prolonged L2 quiescent period, a burst was apparent in L5 VR. Traces in A and B are rectified and smoothed for clarity. Gray shading highlights L2–L5 alternating activity before and after DR stimulation.
Stimulating extensor-related L5 afferents prolonged the locomotor cycle period in part by increasing the interburst duration in flexor-related L2 ventral roots. To determine whether this resulted from prolonging the burst phase in the extensor-related CPG circuitry, we recorded locomotor-like rhythms in L2 and L5 VRs during L5 DR stimulation. These experiments demonstrated that the coordinated alternating pattern between flexor- and extensor-related rhythmic activity persisted following L5 DR stimulation (Fig. 9, n = 4). Extensor-related afferents terminated an ongoing extensor burst while triggering a new flexor-related burst, thus switching rhythmic motor activity from extensor to flexor mode.
DISCUSSION
Recent advances in innovative molecular and genetic techniques facilitated the discovery of genetically defined neuronal populations in the mouse spinal cord (Jessell 2000), with probable functions in the locomotor CPG (reviewed by Goulding 2009; Goulding and Pfaff 2005; Grillner and Jessell 2009; Kiehn 2006). To gain insight into the function of a specific interneuronal population in the multicomponent architecture of the locomotor circuitry it is essential to determine the regulatory actions of peripheral sensory projections and descending inputs on their activity patterns. Peripheral inputs are capable of adjusting locomotor behaviors by modulating the activity of rhythmogenic spinal interneurons. Therefore to establish the role of a specific group of interneurons in controlling the timing of rhythmic motor activity, it is important to examine the influence of peripheral afferents on their oscillatory activity. This is the first report to demonstrate that afferent perturbations of locomotor-like activity in a distinct population of genetically identified rhythmogenic interneurons are correlated with changes in motor activity in the mouse spinal cord. Our finding that during the flexor phase peripheral sensory inputs delayed the onset of locomotor cycles while maintaining in-phase correlation with bouts of motor activity supports the suggestion that Hb9 INs are functional components of the sensorimotor circuitry that regulates the timing of locomotor-like activity in the spinal cord.
Properties of monosynaptic connections between low-threshold afferents and Hb9 INs
Brief stimulation of low-threshold afferents generated prolonged depolarizations (>600 ms) and repetitive firing in Hb9 INs. Hb9 INs are highly excitable cells and with input resistance of approximately 1 GΩ, even small amplitude EPSPs are likely to depolarize the membrane to action potential threshold. The prolonged membrane depolarization might be partially attributed to the component of DR-evoked polysynaptic EPSCs that is mediated via slowly inactivating NMDA receptors (Fig. 1). We did not attempt to investigate the cellular mechanisms that underlie the repetitive firing, but both our group and Tazerart and colleagues have recently shown that persistent sodium current (INaP) plays an important role in generating locomotor-like membrane oscillations in synaptically isolated Hb9 INs (Tazerart et al. 2008; Ziskind-Conhaim et al. 2008). The threshold for the slowly inactivating INaP is close to resting membrane potentials, making it a likely candidate to participate in DR-evoked prolonged repetitive firing.
Complementary electrophysiological and morphological approaches were used to determine whether low-threshold afferent, presumably muscle projections synapse directly onto Hb9 INs. In the majority of Hb9 INs, low-intensity stimulation generated EPSCs/EPSPs with latencies <10 ms, similar to values reported in embryonic and neonatal motoneurons (e.g., Pinco and Lev-Tov 1993; Ziskind-Conhaim 1990) and Renshaw cells (Mentis et al. 2006). One of the conventional criteria for monosynaptic inputs is the stability of short-latency responses during high-frequency stimulation. Stimuli at frequencies of ≤5 Hz did not affect the latency and amplitude of EPSCs in Hb9 INs. The effect of mephenesin was the second criterion used to distinguish between mono- and polysynaptic EPSCs. Mephenesin is a barbiturate known to suppress activity not only in polysynaptic pathways in the adult cat spinal cord (Longo 1961) but also in the isolated rat spinal cord (Lev-Tov and Pinco 1992; Ziskind-Conhaim 1990). Our observation that the drug did not significantly alter the amplitude of short-latency EPSCs provided additional evidence that these were generated by monosynaptic inputs.
The short-latency EPSCs were always associated with a second, longer-latency component (dual-component EPSCs). Moreover, the longer-latency component was evoked in all Hb9 INs independently of the existence of short-latency EPSCs. These synaptic components remained relatively stable at high-frequency stimulation and mephenesin did not suppress them, raising the possibility that they were produced by monosynaptic inputs from slowly conducting unmyelinated afferents in the neonatal spinal cord. Therefore it is conceivable that all Hb9 INs are directly innervated by low-threshold afferents. Similar observations were reported in trigeminal caudal neurons of P7–P10 rats in which short (10 ms) and long (16 ms) latency EPSCs remained constant during mandibular nerve stimuli at frequencies of ≤33 Hz (Onodera et al. 2000). It has been proposed that in that system the short- and long-latency EPSCs are generated by inputs from low-threshold myelinated afferents and high-threshold unmyelinated axons, respectively. Conduction velocity can be estimated by recording afferent volleys, but this was difficult to do in the hemicord that was placed in the recording chamber with the lateral side down, preventing direct access to record extracellular potentials from the short L1–L3 dorsal roots.
Our conclusion that low-threshold afferents form monosynaptic contacts on all Hb9 INs is supported by our morphological observations that boutons expressing VGluT1 are apparent on all tertiary dendrites of Hb9 INs. It has been previously reported that VGluT1-expressing boutons contact the somata or proximal dendrites of all Hb9 INs (Wilson et al. 2005). In view of our findings that low-threshold segmental afferents produced short-latency EPSCs in only roughly 60% of Hb9 INs, we used two complementary morphological approaches to carry out extensive quantitative analysis of the distribution of primary afferent contacts onto the somata and proximal and distal dendrites of Hb9 INs. In the first set of experiments, L1 dorsal roots were filled with Texas Red dextran and their association with boutons expressing VGluT1 was evident in 54% of Hb9 INs. Hb9 INs devoid of Texas Red–filled axons were also lacking VGluT1-expressing boutons. It is possible that this method underestimated the number of Hb9 INs contacted by primary afferents for two reasons. First, Texas Red might have not effectively filled all axonal terminals and, second, the boutons were preferentially located on distal dendrites that could not be detected because of their weak expression of GFP. Nevertheless, this approach provided us with an estimate of the extent of primary afferents that contacted Hb9 INs. A second technically more challenging approach was used to examine whether a significant number of primary afferents terminated on distal dendrites of Neurobiotin-filled Hb9 INs. In the few neurons that were analyzed in our study, the majority of VGluT1- and parvalbumin-IR boutons were in apposition to secondary and tertiary dendrites that extended into the area near the central canal with profuse afferent projections (Fig. 4B). Moreover, the density of VGluT1-expressing boutons was about fourfold higher on distal dendrites than that on the somata or proximal dendrites.
If indeed low-threshold afferents synapsed onto all Hb9 INs, our observation that dorsal root stimulation failed to produce short-latency EPSCs in 38% of Hb9 INs might be indicative of weak synaptic transmission or it reflects the state-dependent activity of afferent inputs. It is also conceivable that a lack of active conductances to boost evoked synaptic responses contributes to signal attenuation and the failures to record EPSCs.
Hb9 INs receive monosynaptic excitatory inputs from flexor-related segmental afferents and primarily polysynaptic inhibitory inputs from extensor-related L5 afferents. During induced locomotor activity this pattern changes. Repetitive stimuli of L2/L3 afferents, possibly via polysynaptic pathways, and stimulation of L5 afferents excite Hb9 INs and reduce cycle duration delivered during the extension phase and prolong the cycle period during the flexion phase. This observation suggests that distinct but potentially overlapping locomotor-related pathways can influence locomotor activity in Hb9 INs. The identity of the afferents producing these modulations is unknown.
Afferent modulation of locomotor-like activity
Our findings that during the flexor phase low-threshold afferents reset the timing of neurochemically induced locomotor-like activity in Hb9 INs while maintaining a phase-locked correlation with motor bursts indicated that this distinct neuronal population is an integral component of the sensorimotor circuitry that regulates locomotor activity. However, our study does not provide evidence that Hb9 INs drive the evoked phase shifts in segmental motoneurons. To further elucidate the role of Hb9 INs in the sensorimotor architecture of the locomotor circuitry it is important to determine the actions of afferent projections on locomotor rhythms generated by other methods such as brain stem stimulation and stimulation of sacrocaudal afferents. Such stimuli are likely to produce activity patterns different from the rhythms induced by neurotransmitter agonists.
Stimulating low-threshold afferents of either flexor- or extensor-related afferents triggered activity bursts during all phases of the locomotor cycle. Similar observations have been reported in the cat preparation in which excitation of flexor muscle group I afferents during the flexor phase (swing phase) of stepping increases the activity of flexor-related motoneurons (e.g., Stecina et al. 2005). In contrast to previous reports in the cat spinal cord (e.g., Duysens and Pearson 1980), our experiments demonstrated that extensor-related L5 stimulation did not enhance ongoing extensor activity but rather terminated it, as reported in the spinal cord of neonatal rats (Iizuka et al. 1997).
In our study, L2/L3 and L5 afferents had similar qualitative effects on modulating the cycle period and their stimulation during the flexor phase resulted in an increase in the duration of both the burst and interburst phases. The major difference between the regulatory actions of the upper and lower lumbar afferents was the duration of the perturbed cycles that followed afferent stimuli. Whether stimulated during the flexor or extensor phases, L5 afferents prolonged by almost threefold the period of the next cycle compared with the prestimulus cycle period. This was nearly twofold longer than the perturbed cycle period following L2/L3 afferent stimuli. L5 dorsal roots encompass a significantly larger number of afferent axons than L2/L3 dorsal roots and thus it is reasonable to assume that they excite a larger number of neurons in the locomotor circuitry. Indeed, low-threshold L5 afferents generate ventral root potentials that are almost twofold larger and significantly longer in duration than potentials evoked by L2 afferents (not shown). One caveat of our experimental approach using the longitudinally hemisected spinal cord preparation is the lack of commissural networks that play a key role in timing the alternating activity between the two sides of the cord. The balance between excitation and inhibition is undoubtedly affected in the absence of strong commissural inhibitory pathways. This might contribute to the robust excitation of flexor-related Hb9 INs and their segmental motoneurons in response to stimuli of extensor-related L5 afferents. Furthermore, descending inputs that are likely to have strong stabilizing effects on the rhythms are also missing in the isolated spinal cord preparation used in our study.
Similar to the findings of Iizuka and colleagues (1997), L5 dorsal root stimulation during the flexor phase reset locomotor activity by prolonging the flexor phase. However, unlike our observations low-threshold L5 afferents in the neonatal rat failed to reset the rhythms during the extensor phase. Resetting similar to what we have observed in the hemisected spinal cord was apparent only at high-intensity stimulation (5–25T). Although we cannot rule it out, it is unlikely that the 2T stimulus applied in our study (current intensity <80 μA) excited high-threshold afferents that produced short-latency responses in Hb9 INs. A possible explanation for the different results is that Iizuka and colleagues examined the effects of specific nerve (quadriceps) stimulation on the locomotor rhythms, whereas in our study dorsal roots probably comprised of a mix of extensor and flexor afferents were stimulated. This might also be the reason that the prolongation of the flexor burst recorded in our study was significantly longer than either the low- or high-intensity stimulation reported by Iizuka's group. In addition, it is possible that the loss of contralateral inputs in the hemisected cord affects the population of interneurons activated by a given stimulus so that low-threshold afferents excite interneurons normally inhibited by contralateral inputs during the extensor phase.
Resetting of locomotor-like rhythms advance or delay the onset of subsequent cycles by regulating rhythm-generating networks in the locomotor CPG (reviewed by McCrea and Rybak 2008; Pearson 2004; Rossignol et al. 2006). Studies that explored the mechanisms underlying resetting and nonresetting modulations have used precise timing of muscle activations provided by electromyographic recordings (Iizuka et al. 1997; Rybak et al. 2006; Schomburg et al. 1998). This level of specificity cannot be applied when recording from ventral roots in the isolated rodent spinal cord. We have shown that 1) stimulation of low-threshold, flexor-related afferents during the flexor phase prolonged the cycle period and delayed the onset of subsequent cycles and 2) flexor-related stimuli during the extensor phase exhibit a pattern of nonresetting modulation. It shortened the cycle period, although the subsequent cycle was prolonged, potentially canceling out any phase advance caused by the early flexor burst. These observations are in agreement with a previous report demonstrating that in P1–P3 rats, stimulation of the quadriceps nerve reset the locomotor motor activity by prolonging the flexor phase, whereas its excitation during the extensor phase has no consistent action on the rhythms (Iizuka et al. 1997). Similar findings were reported in the cat spinal cord in which group I afferents reset the locomotor pattern during flexion but not extension (Rybak et al. 2006). It is tempting to hypothesize that Hb9 INs are partially responsible for afferent-induced bursting of flexor-related motoneurons, but it is also feasible that the synchronization of rhythmic activity is controlled by other interneurons that provide the drive in parallel to both Hb9 INs and motoneurons.
In summary, low-threshold afferents in flexor-related segments synapse onto Hb9 INs and produce robust excitation that lasts for several hundred milliseconds in the developing neonatal spinal cord. All lumbar dorsal root projections can alter the cycle period but the patterns of voltage oscillations in Hb9 INs remain phase-locked with segmental motor bursts. Various computational models of the spinal cord circuitry have been suggested to explain the interactions between peripheral sensory inputs and excitatory neurons at various levels of the CPG (McCrea and Rybak 2008). Based on the two- and three-level CPGs, low-threshold afferents can regulate the activity of rhythm-generating neurons as well as excitatory interneurons that are part of the pattern formation networks and last order interneurons. Our findings that low-threshold afferents are capable of resetting the timing of induced locomotor-like rhythms support the concept that Hb9 INs constitute a functional component of the rhythmogenic locomotor circuitry, although their synaptic integration in the complex hierarchy of locomotor networks remains unknown.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant NS-23808 to L. Ziskind-Conhaim and NINDS intramural funds to G. Z. Mentis.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
Present addresses: C. A. Hinckley, Gene Expression Laboratory and Howard Hughes Medical Institute, The Salk Institute, La Jolla, CA 92037; G. Z. Mentis, The Center for Motor Neuron Biology and Disease, Columbia University, P&S Building, Rm 4-450, 630 W 168th St., New York, NY, 10032.
REFERENCES
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004 [DOI] [PubMed] [Google Scholar]
- Andersson O, Forssberg H, Grillner S, Lindquist M. Phasic gain control the transmission in cutaneous reflex pathways to motoneurones during “fictive” locomotion. Brain Res 149: 503–507, 1978 [DOI] [PubMed] [Google Scholar]
- Bonnot A, Whelan PJ, Mentis GZ, O'Donovan MJ. Locomotor-like activity generated by the neonatal mouse spinal cord. Brain Res Brain Res Rev 40: 141–151, 2002 [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Rev 57: 64–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Degtyarenko AM, Simon ES. Patterns of locomotor drive to motoneurons and last-order interneurons: clues to the structure of the CPG. J Neurophysiol 86: 447–462, 2001 [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455: 187–204, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci 24: 11337–11345, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68: 643–656, 1987 [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol 77: 247–259, 1997 [DOI] [PubMed] [Google Scholar]
- Cowley KC, Zaporozhets E, Schmidt BJ. Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol 586: 1623–1635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187: 321–332, 1980 [DOI] [PubMed] [Google Scholar]
- Endo T, Kiehn O. Asymmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord. J Neurophysiol 100: 3043–3054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BX, Stricker C, Ziskind-Conhaim L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. J Neurophysiol 86: 492–502, 2001 [DOI] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Monoaminergic control of cauda-equina-evoked locomotion in the neonatal mouse spinal cord. J Neurophysiol 96: 3122–3129, 2006 [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Quevedo J, Fedrichuk B, McCrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol 526: 639–652, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994 [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr Opin Neurobiol 15: 14–20, 2005 [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 6: 572–586, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci 27: 13192–13204, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol 93: 1439–1449, 2005a [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Seebach B, Ziskind-Conhaim L. Distinct roles of glycinergic and GABAergic inhibition in coordinating locomotor-like rhythms in the neonatal mouse spinal cord. Neuroscience 131: 745–758, 2005b [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Ziskind-Conhaim L. Sensory modulation of locomotor-like rhythms in Hb9-expressing interneurons. Program No. 516.12. 2005 Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience, 2005. Online [Google Scholar]
- Hinckley CA, Ziskind-Conhaim L. Electrical coupling between locomotor-related excitatory interneurons in the mammalian spinal cord. J Neurosci 26: 8477–8483, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard JP, Brownstone R, Fedirchuk B, Schomburg ED, Enriquez-Denton M, Perreault MC. How do we approach the locomotor network in the mammalian spinal cord? Ann NY Acad Sci 860: 70–82, 1998 [DOI] [PubMed] [Google Scholar]
- Iizuka M, Kiehn O, Kudo N. Development in neonatal rats of the sensory resetting of the locomotor rhythm induced by NMDA and 5-HT. Exp Brain Res 114: 193–204, 1997 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci 12: 701–714, 2000 [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1: 20–29, 2000 [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Iizuka M, Kudo N. Resetting from low threshold afferents of N-methyl-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb muscles preparation from newborn rats. Neurosci Lett 148: 43–46, 1992 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75: 1472–1582, 1996 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann NY Acad Sci 860: 110–129, 1998 [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-Methyl-d,l-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett 75: 43–48, 1987 [DOI] [PubMed] [Google Scholar]
- Kwan AC, Dietz SB, Webb WW, Harris-Warrick RM. Activity of Hb9 interneurons during fictive locomotion in mouse spinal cord. J Neurosci 29: 11601–11612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Tov A, Delvolve I, Kremer E. Sacrocaudal afferents induce rhythmic efferent bursting in isolated spinal cords of neonatal rats. J Neurophysiol 83: 888–894, 2000 [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J Physiol 447: 149–169, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VG. Effects of mephenesin on the repetitive discharge of spinal cord interneurones. Arch Int Pharmacodyn Ther 132: 222–236, 1961 [PubMed] [Google Scholar]
- Marchetti C, Beato M, Nistri A. Alternating rhythmic activity induced by dorsal root stimulation in the neonatal rat spinal cord. J Physiol 530: 105–112, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol 533: 41–50, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Modeling the mammalian locomotor CPG: insights from mistakes and perturbations. Prog Brain Res 165: 235–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O'Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci 26: 13297–13310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse 50: 117–129, 2003 [DOI] [PubMed] [Google Scholar]
- Onodera K, Hamba M, Takahashi T. Primary afferent synaptic responses recorded from trigeminal caudal neurons in a mandibular nerve-brainstem preparation of neonatal rats. J Physiol 524: 503–512, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Neural adaptation in the generation of rhythmic behavior. Annu Rev Physiol 62: 723–753, 2000 [DOI] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004 [DOI] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70: 1009–1017, 1993 [DOI] [PubMed] [Google Scholar]
- Perreault M-C, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II muscle afferents during fictive locomotion. J Physiol 487: 211–220, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinco M, Lev-Tov A. Modulation of monosynaptic excitation in the neonatal rat spinal cord. J Neurophysiol 70: 1151–1158, 1993 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006 [DOI] [PubMed] [Google Scholar]
- Rybak IA, Stecina K, Shevtsova NA, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. J Physiol 577: 641–658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res 122: 339–350, 1998 [DOI] [PubMed] [Google Scholar]
- Seebach BS, Ziskind-Conhaim L. Formation of transient inappropriate sensorimotor synapses in developing rat spinal cords. J Neurosci 14: 4520–4528, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay BL, Sawchuk M, Machacek DW, Hochman S. Serotonin 5-HT2 receptors induce a long-lasting facilitation of spinal reflexes independent of ionotropic receptor activity. J Neurophysiol 94: 2867–2877, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods 21: 321–333, 1987 [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL, Schmidt BJ. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J 2: 2283–2288, 1988 [DOI] [PubMed] [Google Scholar]
- Stecina K, Quevedo J, McCrea DA. Parallel reflex pathways from flexor muscle afferents evoking resetting and flexion enhancement during fictive locomotion and scratch in the cat. J Physiol 569: 275–290, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss I, Lev-Tov A. Neural pathways between sacrocaudal afferents and lumbar pattern generators in neonatal rats. J Neurophysiol 89: 773–784, 2003 [DOI] [PubMed] [Google Scholar]
- Tazerart S, Vinay L, Brocard F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci 28: 8577–8589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Motor coordination without action potentials in the mammalian spinal cord. Nat Neurosci 6: 593–599, 2000 [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382: 46–76, 1997 [PubMed] [Google Scholar]
- Vinay L, Brocard F, Pflieger JF, Simeoni-Alias J, Clarac F. Perinatal development of lumbar motoneurons and their inputs in the rat. Brain Res Bull 53: 635–647, 2000 [DOI] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol 84: 2821–2833, 2000 [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell 110: 385–397, 2002 [DOI] [PubMed] [Google Scholar]
- Wilson JM, Cowan AI, Brownstone RM. Heterogeneous electrotonic coupling and synchronization of rhythmic bursting activity in mouse Hb9 interneurons. J Neurophysiol 98: 2370–2381, 2007 [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci 25: 5710–5719, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Circular distribution. In: Biostatistical Analysis, edited by McElroy WD, Swanson CP. Englewood Cliffs, NJ: Prentice Hall, 1974, p. 310–327 [Google Scholar]
- Zhong G, Masino MA, Harris-Warrick RM. Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. J Neurosci 27: 4507–4518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. Physiological and morphological changes in developing peripheral nerves of rat embryos. Brain Res 470: 15–28, 1988 [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J Neurosci 10: 125–135, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Gao BX, Hinckley C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J Neurophysiol 89: 806–813, 2003. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Seebach BS, Gao BX. Changes in serotonin-induced potentials during spinal cord development. J Neurophysiol 69: 1338–1349, 1993 [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Wu L, Wiesner EP. Persistent sodium current contributes to induced voltage oscillations in locomotor-related Hb9 interneurons in the mouse spinal cord. J Neurophysiol 100: 2254–2264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]